Abstract
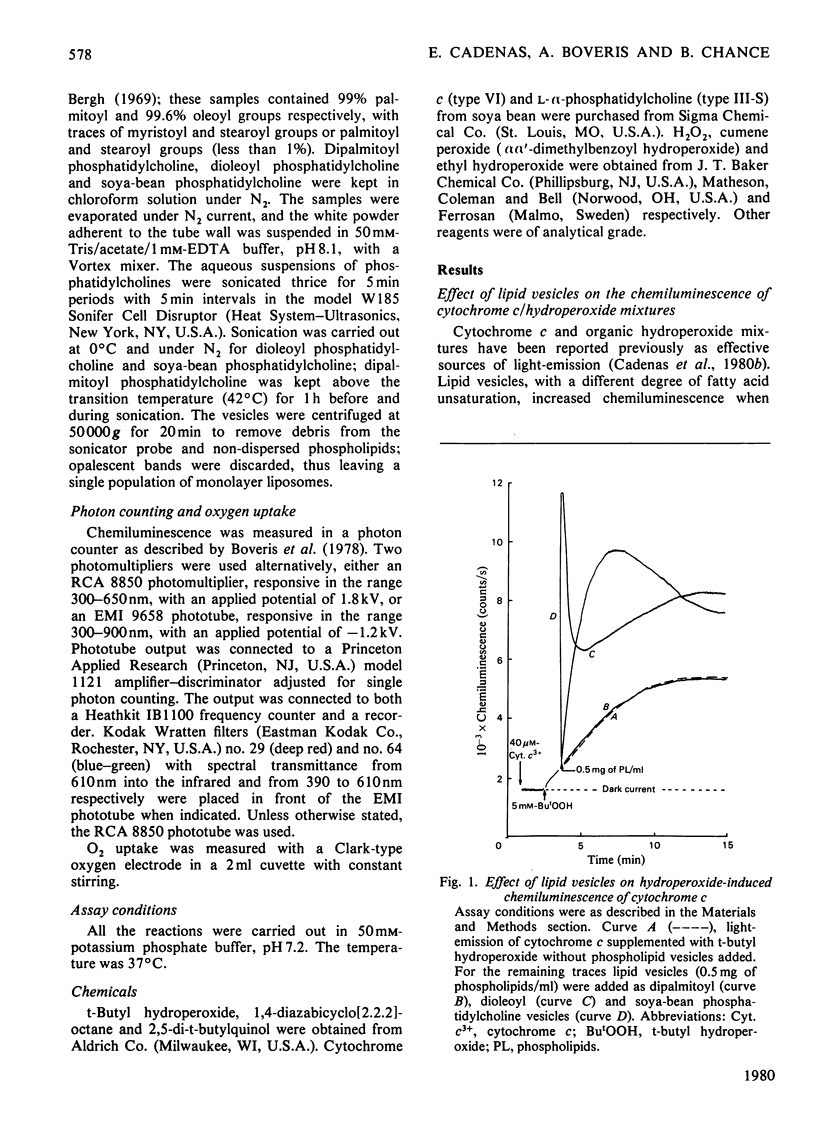
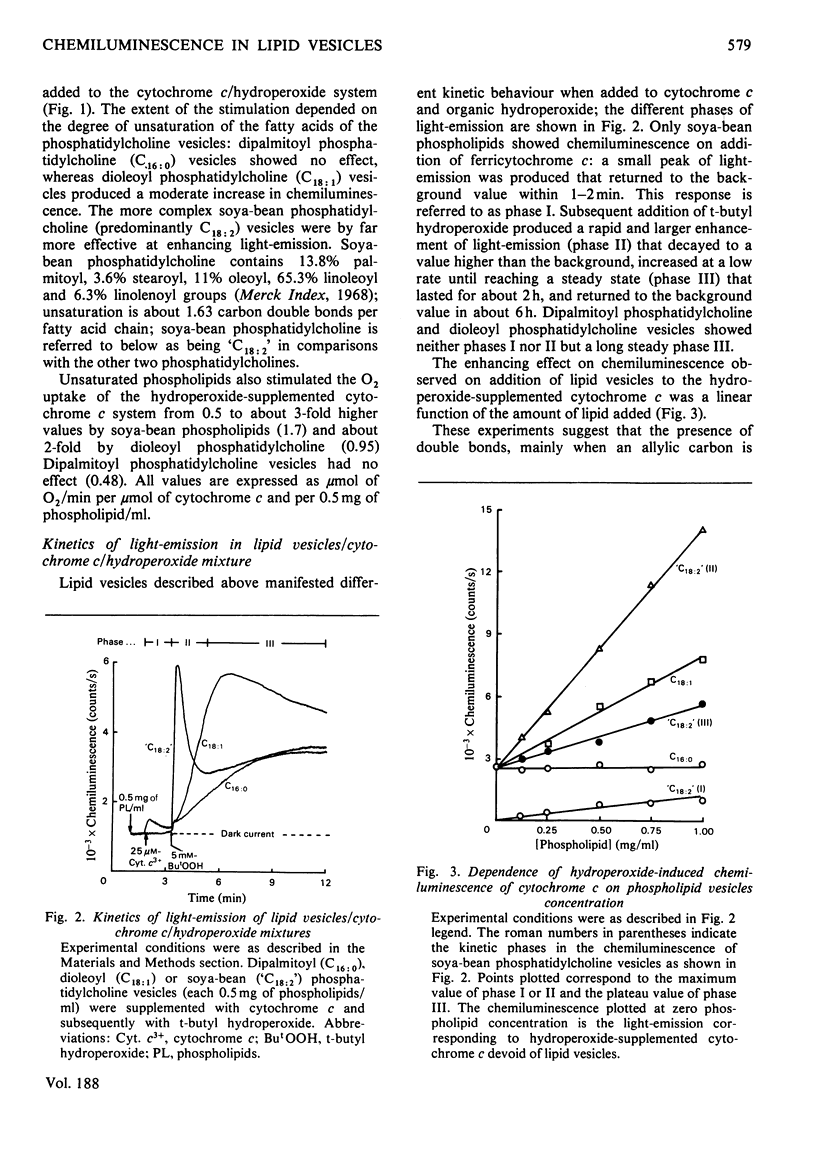
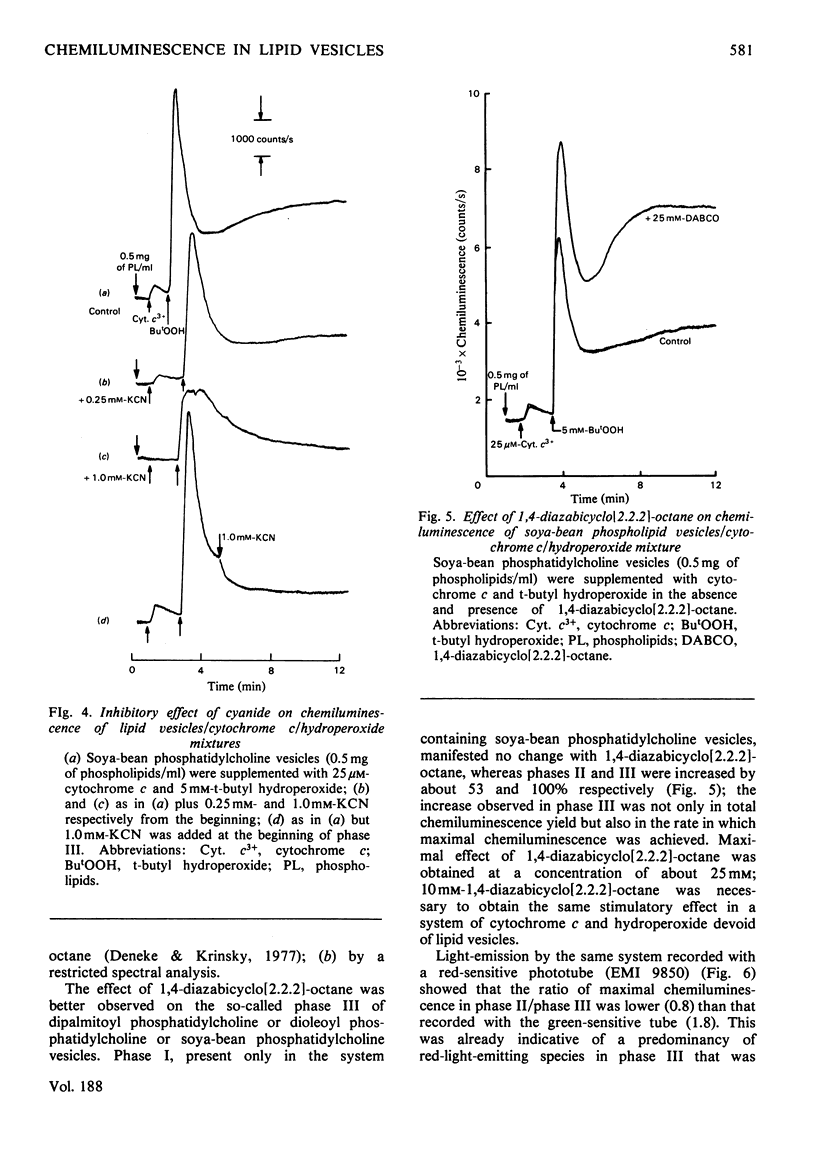
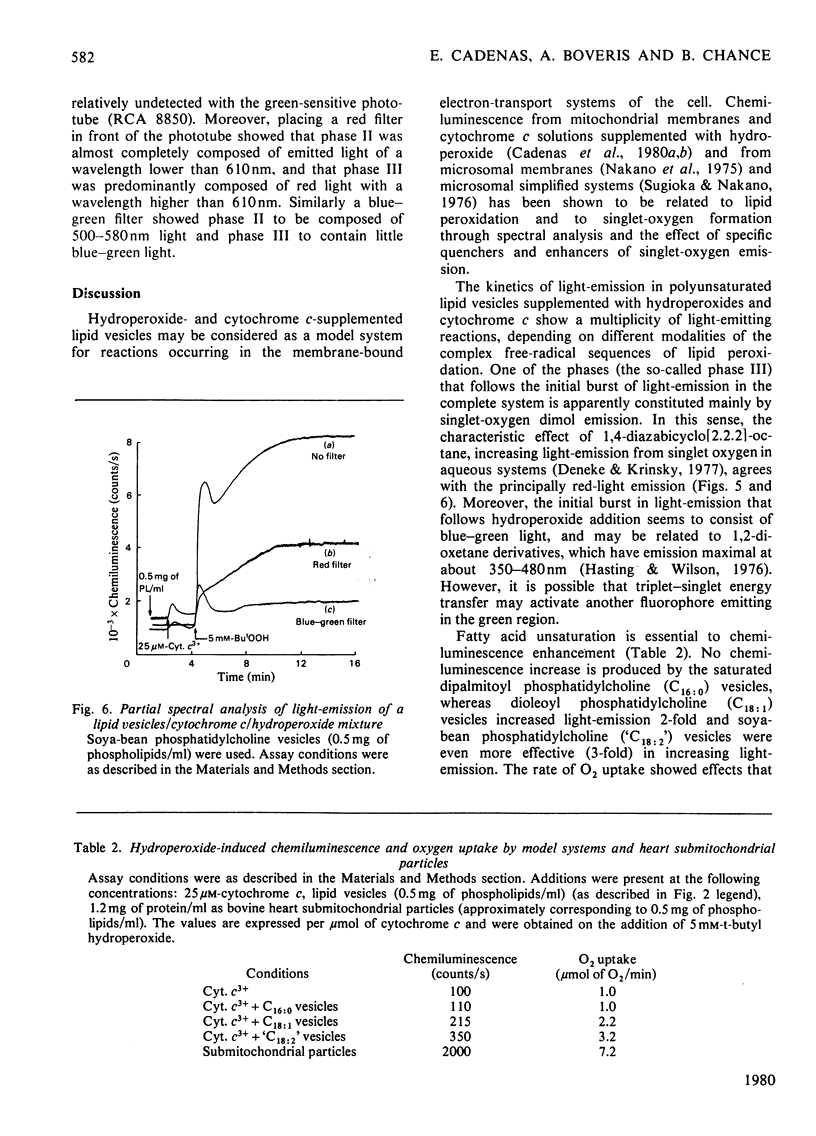
The increase in light emission of hydroperoxide-supplemented cytochrome c observed on addition of lipid vesicles was related to the degree of unsaturation of the fatty acids of the phospholipids: dipalmitoyl phosphatidylcholine was without effect, whereas dioleoyl phosphatidylcholine and soya-bean phosphatidylcholine enhanced chemiluminescence 2- and 3-fold respectively. Effects on light-emission were similar to those on O2 uptake. The chemiluminescence of the present system was sensitive to cyanide and to the radical trap 2,5-di-t-butylquinol, indicating a catlytic activity of cytochrome c and the presence of free-radical species respectively. Lipid-vesicle enhanced chemiluminescence showed different kinetic behaviours, apparently depending on unsaturation: three phases are described for soya-bean phosphatidylcholine, whereas only one phase was present in mixtures containing dipalmitoyl and dioleoyl phospholipids. Chemiluminescence of lipid vesicles supplemented with cytochrome c and hydroperoxide showed similar kinetic patterns with H2O2 and primary (ethyl) and tertiary (t-butyl and cumene) hydroperoxides. Participation of singlet molecular oxygen, mainly on the phase III of chemiluminescence, is suggested by the increase of light-emission by 1,4-diazabicyclo[2.2.2]-octane as well as by data from spectral analysis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bus J. S., Aust S. D., Gibson J. E. Superoxide- and singlet oxygen-catalyzed lipid peroxidation as a possible mechanism for paraquat (methyl viologen) toxicity. Biochem Biophys Res Commun. 1974 Jun 4;58(3):749–755. doi: 10.1016/s0006-291x(74)80481-x. [DOI] [PubMed] [Google Scholar]
- Cadenas E., Boveris A., Chance B. Low-level chemiluminescence of bovine heart submitochondrial particles. Biochem J. 1980 Mar 15;186(3):659–667. doi: 10.1042/bj1860659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E., Boveris A., Chance B. Low-level chemiluminescence of hydroperoxide-supplemented cytochrome c. Biochem J. 1980 Apr 1;187(1):131–140. doi: 10.1042/bj1870131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubero Robles E., van den Berg D. Synthesis of lecithins by acylation of O-(sn-glycero-3-phosphoryl) choline with fatty acid anhydrides. Biochim Biophys Acta. 1969 Dec 17;187(4):520–526. doi: 10.1016/0005-2760(69)90049-6. [DOI] [PubMed] [Google Scholar]
- Demopoulos H. B. The basis of free radical pathology. Fed Proc. 1973 Aug;32(8):1859–1861. [PubMed] [Google Scholar]
- Hastings J. W., Wilson T. Bioluminescence and chemiluminescence. Photochem Photobiol. 1976 Jun;23(6):461–473. doi: 10.1111/j.1751-1097.1976.tb07282.x. [DOI] [PubMed] [Google Scholar]
- Hawco F. J., O'Brien P. J. Singlet oxygen formation during hemoprotein catalyzed lipid peroxide decomposition. Biochem Biophys Res Commun. 1976 May 23;76(2):354–361. doi: 10.1016/0006-291x(77)90732-x. [DOI] [PubMed] [Google Scholar]
- Kakinuma K., Cadenas E., Boveris A., Chance B. Low level chemiluminescence of intact polymorphonuclear leukocytes. FEBS Lett. 1979 Jun 1;102(1):38–42. doi: 10.1016/0014-5793(79)80923-0. [DOI] [PubMed] [Google Scholar]
- Kaschnitz R. M., Hatefi Y. Lipid oxidation in biological membranes. Electron transfer proteins as initiators of lipid autoxidation. Arch Biochem Biophys. 1975 Nov;171(1):292–304. doi: 10.1016/0003-9861(75)90036-3. [DOI] [PubMed] [Google Scholar]
- Keenan T. W., Awasthi Y. C., Crane F. L. Cardiolipin from beef heart mitochondria: fatty acid positioning an molecular species distribution. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1102–1109. doi: 10.1016/0006-291x(70)90908-3. [DOI] [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem. 1977 Oct 10;252(19):6721–6728. [PubMed] [Google Scholar]
- Kellogg E. W., 3rd, Fridovich I. Superoxide, hydrogen peroxide, and singlet oxygen in lipid peroxidation by a xanthine oxidase system. J Biol Chem. 1975 Nov 25;250(22):8812–8817. [PubMed] [Google Scholar]
- King M. M., Lai E. K., McCay P. B. Singlet oxygen production associated with enzyme-catalyzed lipid peroxidation in liver microsomes. J Biol Chem. 1975 Aug 25;250(16):6496–6502. [PubMed] [Google Scholar]
- Lloyd D., Boveris A., Reiter R., Filipkowski M., Chance B. Chemiluminescence of Acanthamoeba castellanii. Biochem J. 1979 Oct 15;184(1):149–156. doi: 10.1042/bj1840149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano M., Noguchi T., Sugioka K., Fukuyama H., Sato M. Spectroscopic evidence for the generation of singlet oxygen in the reduced nicotinamide adenine dinucleotide phosphate-dependent microsomal lipid peroxidation system. J Biol Chem. 1975 Mar 25;250(6):2404–2406. [PubMed] [Google Scholar]
- Oliveira O. M., Haun M., Durán N., O'Brien P. J., O'Brien C. R., Bechara E. J., Cilento G. Enzyme-generated electronically excited carbonyl compounds, Acetone phosphorescence during the peroxidase-catalyzed aerobic oxidation of isobutanal. J Biol Chem. 1978 Jul 10;253(13):4707–4712. [PubMed] [Google Scholar]
- Pederson T. C., Aust S. D. The role of superoxide and singlet oxygen in lipid peroxidation promoted by xanthine oxidase. Biochem Biophys Res Commun. 1973 Jun 8;52(3):1071–1078. doi: 10.1016/0006-291x(73)91047-4. [DOI] [PubMed] [Google Scholar]
- Pryor W. A. The formation of free radicals and the consequences of their reactions in vivo. Photochem Photobiol. 1978 Oct-Nov;28(4-5):787–801. doi: 10.1111/j.1751-1097.1978.tb07020.x. [DOI] [PubMed] [Google Scholar]
- Roubal W. T., Tappel A. L. Damage to proteins, enzymes, and amino acids by peroxidizing lipids. Arch Biochem Biophys. 1966 Jan;113(1):5–8. doi: 10.1016/0003-9861(66)90150-0. [DOI] [PubMed] [Google Scholar]
- Sugioka K., Nakano M. A possible mechanism of the generation of singlet molecular oxygen in nadph-dependent microsomal lipid peroxidation. Biochim Biophys Acta. 1976 Feb 16;423(2):203–216. doi: 10.1016/0005-2728(76)90179-1. [DOI] [PubMed] [Google Scholar]
- TAPPEL A. L. Unsaturated lipide oxidation catalyzed by hematin compounds. J Biol Chem. 1955 Dec;217(2):721–733. [PubMed] [Google Scholar]
- Tappel A. L. Lipid peroxidation damage to cell components. Fed Proc. 1973 Aug;32(8):1870–1874. [PubMed] [Google Scholar]


