Abstract
Background
Cholesterol embolism causes various organ dysfunctions, including skin, kidney, and gastrointestinal tract dysfunction, as well as immunological abnormalities, such as hypocomplementemia and eosinophilia. However, only a few cases of vasculitis accompanied by cholesterol embolism have been reported.
Case presentation
We present the case of an 82-year-old man with cholesterol embolism who also developed small-vessel vasculitis of the skin and muscles. The patient had a persistent fever, and blood tests showed eosinophilia and hypocomplementemia. Two months later, the patient developed a skin rash and myalgia in the thighs. Magnetic resonance imaging of the thighs revealed diffuse intramuscular hyperintensities on T2-weighted images and short tau inversion recovery sequences in the hamstrings and quadriceps femoris. Histological findings of the skin and muscle revealed small-vessel vasculitis, and random skin biopsy revealed cholesterol embolism. We diagnosed the patient with cholesterol embolism accompanied by small-vessel vasculitis of the skin and femoral muscles. Methylprednisolone was administered intravenously, and oral prednisolone was initiated. Muscle tenderness improved rapidly after the initiation of glucocorticoid therapy. However, he developed superior mesenteric artery embolization and died.
Conclusions
Our case demonstrates that cholesterol embolism can be accompanied by small-vessel vasculitis of the skin and muscles.
Keywords: Cholesterol embolism, Small-vessel vasculitis, Intramuscular vasculitis, Hypocomplementemia, Eosinophilia
Background
Cholesterol embolism is characterized by inflammation and occlusion of small arteries due to cholesterol crystals from disrupted atherosclerotic plaques [1]. It mainly occurs in patients with atherosclerosis after vascular surgery or endovascular procedures and sometimes arises spontaneously [2]. It develops in multiple organs, such as the skin, kidneys, and gastrointestinal tract. It causes various symptoms, such as fever, rash, acute kidney injury, and intestinal ischemia [1]. Cholesterol embolism evokes immunological reactions such as hypocomplementemia and eosinophilia; however, vasculitis associated with cholesterol embolism has rarely been reported [3]. Herein, we report the case of an older man with cholesterol embolism who developed small-vessel vasculitis of the skin and muscles, without a history of a preceding intravascular procedure.
Case presentation
An 82-year-old man with a 2-month history of fever was referred to our hospital. One month before admission, the patient visited another hospital because of persistent fever and weight loss. Blood test results revealed leukocytosis with eosinophilia, elevated C-reactive protein levels, and hypocomplementemia; however, blood cultures did not yield any bacteria, and contrast-enhanced computed tomography of the chest and abdomen did not reveal any findings that caused the fever. The cause of his fever was not clear, and the patient was prescribed acetaminophen and followed up. Three weeks before admission, the patient developed skin purpura on the lower extremities and myalgia in the thighs. He had a medical history of lacunar cerebral infarction and hypertension and was taking amlodipine and vonoprazan. The patient had no history of vascular surgery or endovascular procedures, smoking, or alcohol consumption.
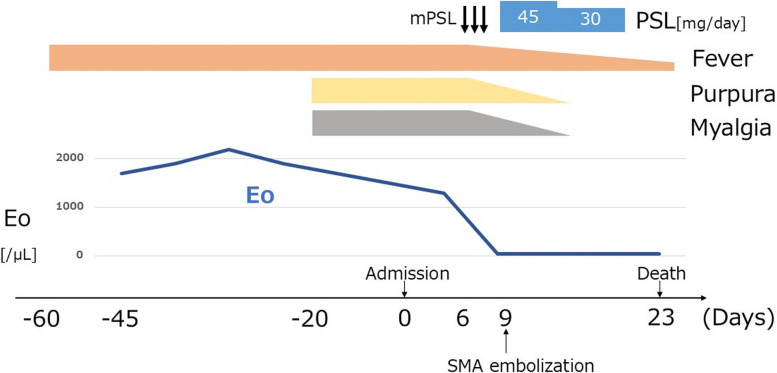
Physical examination revealed a blood pressure of 134/80 mmHg, pulse rate of 110/min, respiratory rate of 12 breaths/min, and body temperature of 37.3 ℃. The patient was alert and conscious. Both the thigh muscles were tender. On manual muscle testing, the upper limb muscles were all normal (right 5/5 and left 5/5), iliopsoas muscles right 4/5 and left 4/5, and quadriceps femoris right 4/5 and left 3/5. Palpable purpura was observed on the lower legs (Fig. 1a). None of the joints were swollen or tender. Laboratory findings revealed a white blood cell count of 18,200 /µL with eosinophilia (1965 /µL), C-reactive protein level of 11.9 mg/dL, rheumatoid factor of 1060 mg/dL, and hypocomplementemia with C3 of 45.3 mg/dL (reference range: 69 to 128) and C4 of 2.5 mg/dL (reference range: 14 to 36) (Table 1). The test results for anti-cyclic citrullinated peptide antibody, anti-nuclear antibody, myeloperoxidase anti-neutrophil cytoplasmic antibody (ANCA), proteinase-3-ANCA, cryoglobulin, and myositis-specific autoantibodies were negative. Blood cultures did not yield any organisms. Contrast-enhanced computed tomography of the chest and abdomen revealed no abnormalities other than aortic calcification with a plaque of 5 mm in size lacking free floating material (Fig. 1b). Transthoracic echocardiography showed no thickening or calcification of the heart valves; however, reveled the findings of Takotsubo cardiomyopathy and a 20 mm-sized thrombus in the left ventricle. Magnetic resonance imaging of the thighs revealed diffuse intramuscular hyperintensities on T2-weighted images and short tau inversion recovery sequences in the hamstrings and quadriceps femoris (Fig. 1c). Skin biopsy of the purpura on the right lower leg revealed perivascular inflammatory cell infiltration with hemorrhage and nuclear dust, indicating leukocytoclastic vasculitis (Fig. 1d). Muscle biopsy of the left vastus lateralis also revealed intramuscular vasculitis with lymphocyte and macrophage infiltration, without fibrinoid necrosis (Fig. 1e). No apparent necrotic fibers were observed, and direct immunohistochemical staining revealed no signs of inflammatory myopathy. Random skin biopsy was performed to investigate the underlying cause of prolonged fever in the patient. Histological examination revealed cholesterol crystal clefts within small vessels of the superficial dermis (Fig. 1f). We diagnosed the patient with cholesterol embolism accompanied by small-vessel vasculitis of the skin and femoral muscles. On day 6, methylprednisolone (1000 mg/day) was administered intravenously for 3 days, and on day 9, oral prednisolone (45 mg/day: 1 mg/kg/day) was initiated. Muscle tenderness improved rapidly after the initiation of glucocorticoid therapy. Although we also initiated continuous heparin infusion for intraventricular thrombus from day 1 of admission, the patient developed superior mesenteric artery embolism on day 9. Emergency abdominal surgery was performed, and the necrotic small intestine was removed from the point 80 cm from the Treitz ligament to 270 cm towards the anus, and 15 cm of the necrotic transverse colon was also resected. However, the patient developed pan-peritonitis after surgery and died on day 23. Pathological findings of the necrotic bowels revealed thrombus in the mesenteric arteries, but no cholesterol embolus was found. The thrombus was thought to be from the intraventricular thrombus or due to thrombogenic effect of high corticosteroid dose. (Fig. 2).
Fig. 1.
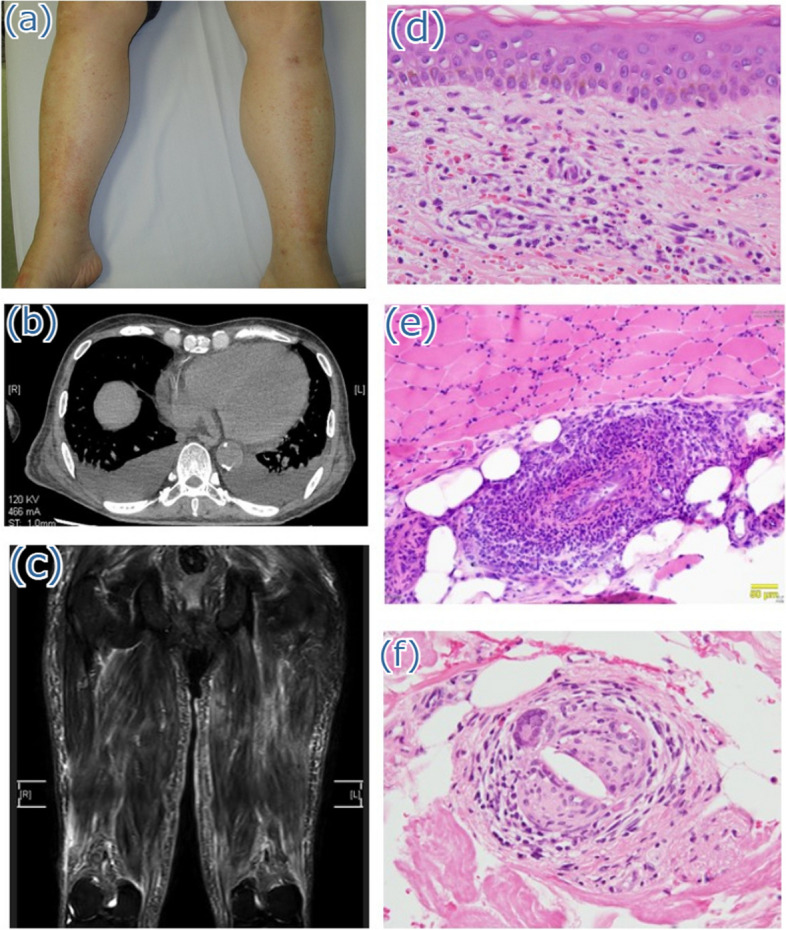
a Physical examination findings. Purpura on the lower legs is observed. b Contrast-enhanced computed tomography of the chest and abdomen reveals calcification of the descending aorta. c Magnetic resonance imaging of the thighs reveals diffuse intramuscular hyperintensities on T2-weighted images and short tau inversion recovery sequences in the hamstrings and quadriceps femoris. d Histological findings of the purpura on the lower leg demonstrate perivascular inflammatory cell infiltration with hemorrhage and nuclear dust. e Histological examination of the left vastus lateralis muscle reveals two vessels surrounded by lymphocytes and macrophages, with no evidence of fibrinoid necrosis. f Random skin biopsy shows the presence of crystal clefts within small blood vessels in the superficial dermal layer
Table 1.
Laboratory findings on the day of admission
| Complete blood count | Biochemical blood test | Immunological test results | |||||
|---|---|---|---|---|---|---|---|
| WBC | 18,200/μL | TP | 5.4 g/dL | RF | 1,060 mg/dL | ARS ab | negative |
| Neut | 73.4% | AST | 80 U/L | C3 | 45.3 mg/dL | Jo-1 ab | negative |
| Lymph | 12.7% | ALT | 39 U/L | C4 | 2.5 mg/dL | Mi-2 ab | negative |
| Eos | 10.8% | LDH | 276 U/L | ANA | 1:40 | MDA-5 ab | negative |
| Hb | 9.9 g/dL | CK | 1,630 U/L | SS-A ab | < 1.0 U/mL | cryoglobulin | negative |
| Plt | 53.5 10^4/μL | BUN | 34.7 mg/dL | SS-B ab | 1 U/mL | HBs-Ag | negative |
| Cre | 1.16 mg/dL | CCP ab | 0.6 U/mL | HCV ab | negative | ||
| CRP | 11.9 mg/dL | MPO-ANCA | 1 U/mL | TPHA | negative | ||
| PR3-ANCA | 1 U/mL | HIV ab | negative | ||||
WBC White blood cells, Neut Neutrophils, Lymph Lymphocytes, Eos Eosinophils, Hb Hemoglobin, Plt Platelet, TP Total protein, AST Aspartate aminotransferase, ALT Alanine aminotransferase, LDH Lactate dehydrogenase, CK Creatinine kinase, BUN Blood urea nitrogen, Cre Creatinine, CRP C-reactive protein, RF Rheumatoid factor, ANA Antinuclear antibody, CCP Ab Anti-cyclic citrullinated peptide antibody, MPO-ANCA Anti-myeloperoxidase anti-neutrophil cytoplasmic antibody, PR3-ANCA Anti-proteinase 3 anti-neutrophil cytoplasmic antibody, ARSab Anti-aminoacyl-tRNA synthetase antibody, MDA-5 ab Anti-melanoma differentiation-associated gene 5 antibody, TPHA Treponema pallidum hemagglutination test
Fig. 2.
Clinical course of the patient. mPSL, methylprednisolone; PSL, prednisolone; CRP, C-reactive protein; Eo, eosinophil
Discussion and conclusions
The patient’s clinical course highlights two important observations. First, cholesterol embolism can be accompanied by small-vessel vasculitis with muscle involvement. Second, hypocomplementemia and eosinophilia can be diagnostic indicators of vasculitis caused by cholesterol embolism.
Although cholesterol embolism is known to involve various organs [4], vasculitis complications are rare, and only 11 cases have been reported [3, 5–12] (Table 2). All the patients had small-vessel vasculitis. Seven patients lacked a history of preceding intravascular procedures, and five patients were ANCA-positive. In most cases, the skin and kidneys were involved, and two autopsy cases demonstrated vasculitis of the muscles, lungs, and central nervous system [6, 11]. Our case is rare, wherein the patient demonstrated muscle involvement with small-vessel vasculitis. Intramuscular vasculitis is generally uncommon. ANCA-associated vasculitis and polyarteritis nodosa sometimes result in muscle vasculitis [13]; however, other vasculitis rarely involves the muscle, with only a few reports of giant cell arteritis, IgA vasculitis, systemic lupus erythematosus, and rheumatoid arthritis vasculitis [13]. Although the mechanism underlying the onset of vasculitis associated with cholesterol embolism remains unclear, immune responses are presumed to be involved. Cholesterol crystals obstruct the distal artery, with diameters ranging from 100 to 200 µm [14]. Cholesterol embolism causes ischemic injury to the distal tissues, subsequently inducing an inflammatory reaction. Macrophages and neutrophils are recruited to cholesterol crystals in the vessel walls, releasing enzymes and reactive oxygen species [15]. Cholesterol crystals activate the nucleotide-binding domain, leucine-rich repeat protein-3 inflammasome, and interleukin-1 production; they also stimulate immune cells and endothelial dysfunction, contributing to vascular inflammation [16]. This cascade triggers complement activation and influences the release of mast cells and eosinophils, thereby perpetuating endothelial injury [14]. Thus, inflammation of the blood vessels caused by cholesterol embolism and the subsequent immune response may trigger small-vessel vasculitis.
Table 2.
Review of previous reports on vasculitis associated with cholesterol embolism
| References | Sex, age | Past history | Endovascular surgery | Involved organ | ANCA | Eosinophillia | Hypocomplementemia | Pathology | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Goldman M et al.1985 [4] | M, 75 | COPD, CAD, PAD | carotid angiography | kidney | N/S | N/S | N/S | kidney; necrotizing glomerulonephritis, cholesterol craft | HD | died 6 month later |
| Peat DS et al. 1996 [5] | M, 73 | HT, CAD | - | kidney | + | + | N/S | kidney; cholesterol craft | PSL, CY | died due to HF |
| Sijpkens Y et al. 1997 [6] | M, 62 | N/S | - | skin, muscle, lung, CNS | - | - | + | (autopsy) muscle, kidney, pleura, brain; cholesterol clefts and necrotizing vasculitis | - | died |
| Kaplan Pavlovcic S et al. 1998 [7] | M, 63 | HT, CAD | - | skin, kidney | + | - | - | skin; vasculitis, cholesterol crystal clefts | PSL | HD |
| kidney; cholesterol crystal clefts | ||||||||||
| Kaplan Pavlovcic S et al. 1998 [7] | F, 69 | HT, DL, | iv streptokinase for MI | skin, kidney | + | + | - |
skin; infarction and haemorrhages kidney; cholesterol crystal clefts |
antiplatelet | N/S |
| therapy | ||||||||||
| Ballesteros AL et al. 1999 [8] | M, 69 | PAD, CI, PM, DL | aortic-bifemoral graft | skin, kidney | - | - | + | skin; LCV, kidney; necrotizing vasculitis, (autopsy) cholesterol crystal on skin, gastrointestinal tract, liver | PSL, CY | died |
| Sugimoto T et al. 2006 [9] | M, 75 | CAD | coronary artery bypass | skin, kidney | + | + | N/S | skin; cholesterol crystal cleft LCV | PSL | survived |
| Maejima et al. 2010 [10] | M, 76 | CAD | - | skin | + | - | - | skin; LCV, cholesterol crystal cleft | PSL | died |
| Moriya M et al. 2015 [11] | M, 70 | CAD | - | skin, CNS, lung | - | - | + | autopsy; vasculitis of alveolar capillary, | PSL | died |
| leptomenigial necrotizing vasculitis | ||||||||||
| Bajić et al. 2017 [3] | M, 67 | HT, psoriasis | - | skin, testis | - | - | - | skin; necrotizing vasculitis, cholesterol crystal cleft | PSL | recovered |
| Kojima R et al. 2020 [12] | F, 76 | HT | - | skin, kidney | - | + | - | skin&kidney; cholesterol clefts | PSL | survived |
| kidney; whole-layer vasculitis | ||||||||||
| Our patient | M, 82 | HT, CI | - | skin, muscle | - | + | + | skin; LCV, cholesterol clefts | PSL | died |
| muscle; small vessel vasculitis |
M Male, F Female, ANCA Anti-neutrophil cytoplasmic antibody, COPD Chronic obstructive pulmonary disease, CAD Coronary artery disease, PAD Peripheral artery disease, HD Hemodialysis, HT Hypertension, PSL Prednisolone, CY Cyclophosphamide, CNS Central nervous system, DL Dyslipidemia, MI Myocardial infarction, CI Cerebral infarction, PM Pacemaker implantation, LCV Leukocytoclastic vasculitis, N/S Not specified
Vasculitis accompanied by hypocomplementemia occurs in patients with systemic lupus erythematosus, Sjögren’s syndrome, rheumatoid vasculitis, and cryoglobulinemia. Vasculitis accompanied by eosinophilia develops in patients with eosinophilic granulomatosis with polyangiitis. However, vasculitis accompanied by hypocomplementemia and eosinophilia is rare [17]. Although patients with cholesterol embolism-associated vasculitis do not always present with hypocomplementemia and eosinophilia, they may serve as diagnostic indicators of the underlying conditions of vasculitis. Early diagnosis of cholesterol embolism-associated vasculitis is important because immunosuppressive therapies such as prednisolone have been reported to be effective [3, 5–12, 18].
In summary, our case demonstrates that cholesterol embolism can be accompanied by small-vessel vasculitis of the skin and muscles. When patients present with intramuscular small-vessel vasculitis accompanied by hypocomplementemia or eosinophilia, physicians should consider cholesterol embolism-associated vasculitis as a differential diagnosis and investigate the presence of cholesterol crystals in small arteries.
Acknowledgements
This study was supported in part by an Intramural Research Grant (5-6) for Neurological and Psychiatric Disorders from the NCNP and Matsumoto Allergic Disease Research Grant from Kobe City Medical Center General Hospital. We would like to thank Editage (www.editage.jp) for English language editing.
Clinical trial number
Not applicable.
Abbreviation
- ANCA
Anti-neutrophil cytoplasmic antibody
Authors’ contributions
DU collected data and wrote the first draft of the manuscript. IN and DY provided pathological data and drafted the manuscript. DU, NI, and HN reviewed the literature. HN coordinated the study and edited the manuscript. All the authors have read and approved the final manuscript.
Funding
This study was supported in part by an Intramural Research Grant (5–6) for Neurological and Psychiatric Disorders from the NCNP and Matsumoto Allergic Disease Research Grant from Kobe City Medical Center General Hospital.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
This work obtained a waiver of approval from the Institutional Review Board of Kobe City Medical Center General Hospital.
Consent for publication
Consent to publish the article was obtained from the patient.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kronzon I, Saric M. Cholesterol embolization syndrome. Circulation. 2010;122:631–41. [DOI] [PubMed] [Google Scholar]
- 2.Scolari F, Ravani P, Gaggi R, Santostefano M, Rollino C, Stabellini N, et al. The challenge of diagnosing atheroembolic renal disease: clinical features and prognostic factors. Circulation. 2007;116:298–304. [DOI] [PubMed] [Google Scholar]
- 3.Bajić M, Hočevar A, Rotar Ž, Praprotnik S, Jurčić V, Tomšič M. The role of statins on the outcomes on the cholesterol embolism syndrome with secondary vasculitis - a case report and review of the literature. JSM Arthritis. 2017;2:1022. [Google Scholar]
- 4.Goldman M, Thoua Y, Dhaene M, Toussaint C. Necrotising glomerulonephritis associated with cholesterol microemboli. Br Med J (Clin Res Ed). 1985;290:205–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peat DS, Mathieson PW. Cholesterol emboli may mimic systemic vasculitis. BMJ. 1996;313:546–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sijpkens Y, Westendorp R, van Kemenade F, van Duinen S, Breedveld F. Vasculitis due to cholesterol embolism. Am J Med. 1997;102:302–3. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan-Pavlovcic S, Vizjak A, Vene N, Ferluga D. Antineutrophil cytoplasmic autoantibodies in atheroembolic disease. Nephrol Dial Transplant. 1998;13:985–7. [DOI] [PubMed] [Google Scholar]
- 8.Ballesteros AL, Bromsoms J, Vallés M, Llistosella E, Garijo G, Bernadó L, et al. Vasculitis look-alikes: variants of renal atheroembolic disease. Nephrol Dial Transplant. 1999;14:430–3. [DOI] [PubMed] [Google Scholar]
- 9.Sugimoto T, Morita Y, Yokomaku Y, Isshiki K, Kanasaki K, Eguchi Y, et al. Systemic cholesterol embolization syndrome associated with myeloperoxidase-anti-neutrophil cytoplasmic antibody. Intern Med. 2006;45:557–61. [DOI] [PubMed] [Google Scholar]
- 10.Maejima H, Noguchi T, Tanei R. Cholesterol embolism associated with MPO-ANCA. Eur J Dermatol. 2010;20:539–40. [DOI] [PubMed] [Google Scholar]
- 11.Moriya M, Naba I, Nakano M, Tatsumi C, Inoue K, Fujimura H. A case of cholesterol embolization syndrome with cognitive impairment and pulmonary hemorrhage. Rinsho Shinkeigaku. 2015;55:823–7. [DOI] [PubMed] [Google Scholar]
- 12.Kojima R, Harada M, Yamaguchi A, Hashimoto K, Kamijo Y. Cholesterol emboli co-existing with anti-neutrophil cytoplasmic antibody-associated vasculitis in a 76-year-old woman. Tohoku J Exp Med. 2020;251:61–8. [DOI] [PubMed] [Google Scholar]
- 13.Conticini E, d’Alessandro M, Al Khayyat SG, D’Alessandro R, D’Ignazio E, Pata AP, et al. Inflammatory muscle involvement in systemic vasculitis: a systematic review. Autoimmun Rev. 2022;21:103029. [DOI] [PubMed] [Google Scholar]
- 14.Ozkok A. Cholesterol-embolization syndrome: current perspectives. Vasc Health Risk Manag. 2019;15:209–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Bayliss G, Zhuang S. Cholesterol crystal embolism and chronic kidney disease. Int J Mol Sci. 2017;18:1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jennette JC, Falk RJ, Bacon PA, Basu N, Cid MC, Ferrario F, et al. 2012 revised international chapel hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2013;65:1–11. [DOI] [PubMed] [Google Scholar]
- 18.Maningding E, Kermani TA. Mimics of vasculitis. Rheumatology (Oxford). 2021;60:34–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.