Abstract
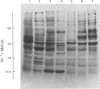
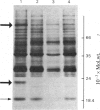
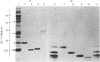
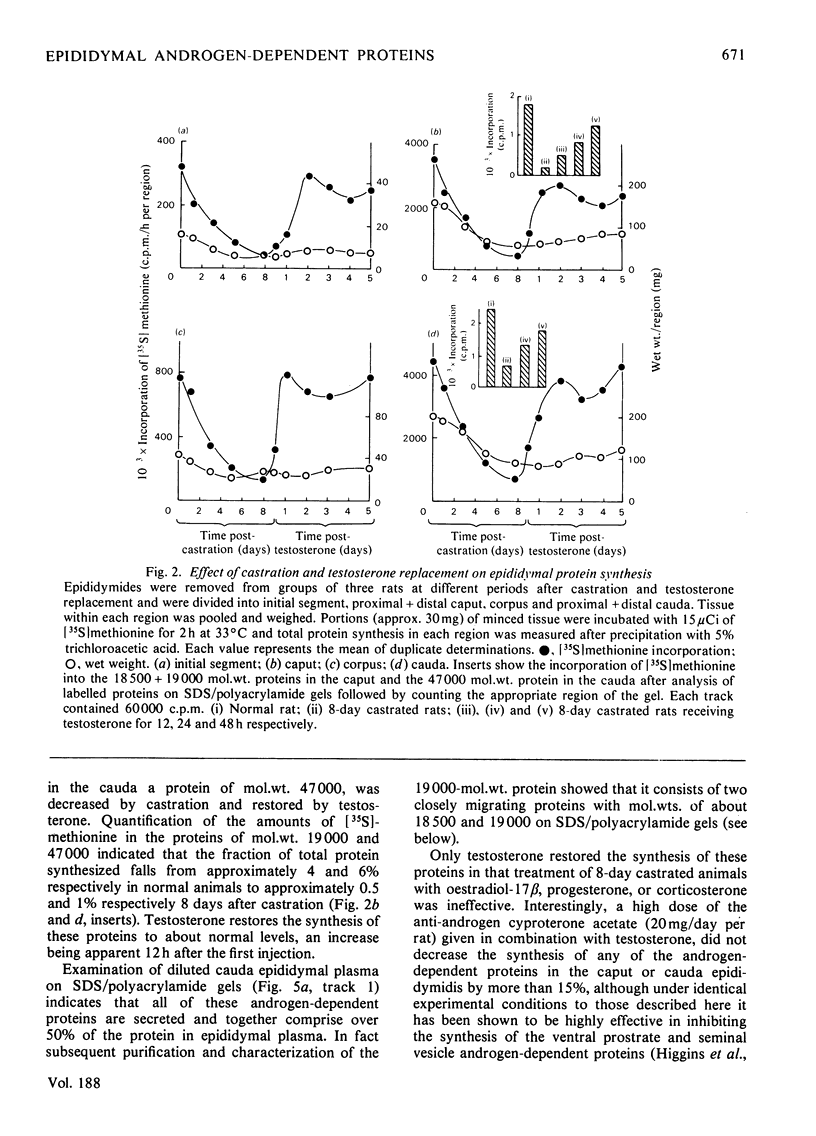
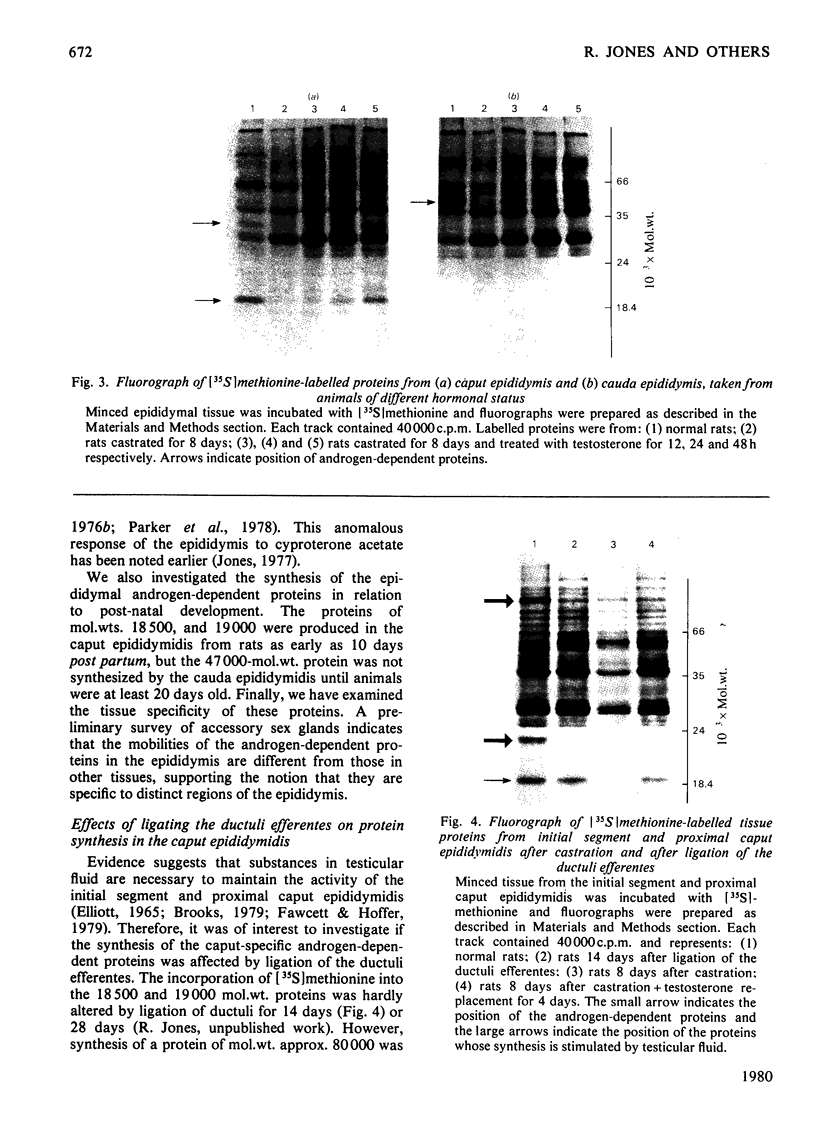
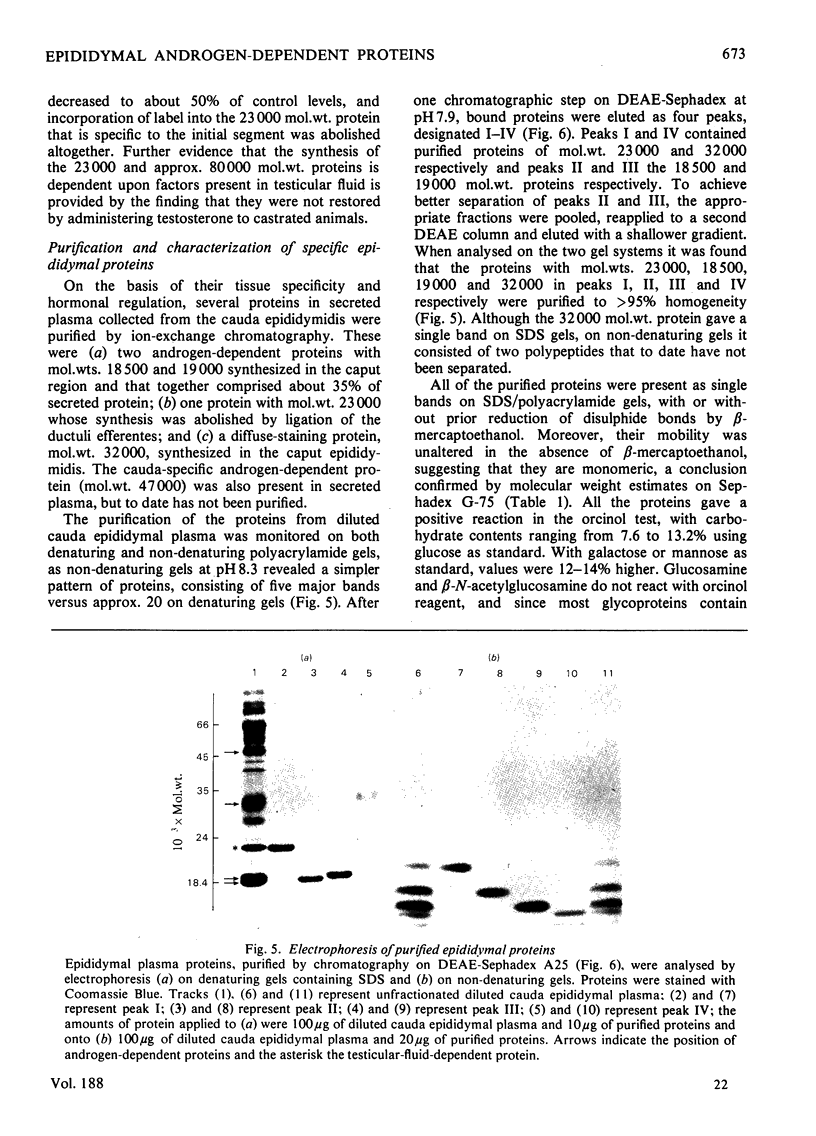
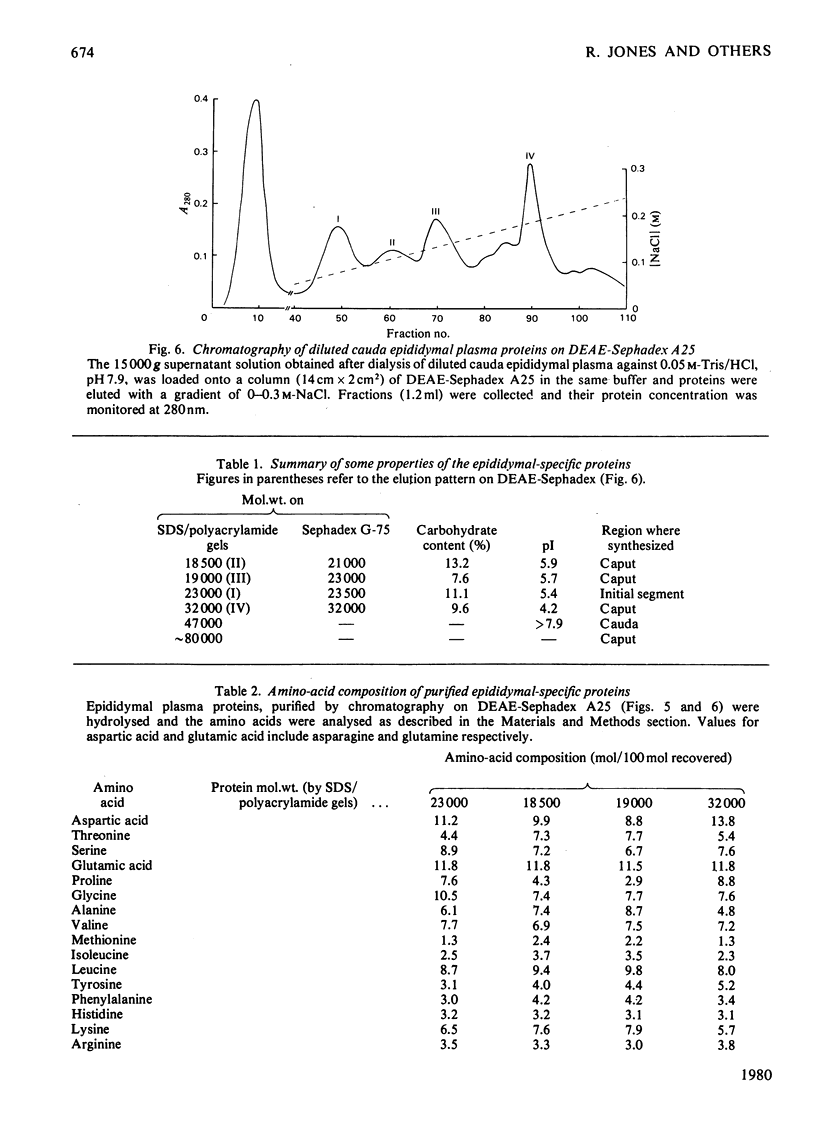
1. Protein synthesis has been investigated in different regions of the rat epididymis by measuring incorporation of [35S]methionine in tissue minces incubated in vitro followed by analysis of labelled proteins on polyacrylamide gels containing sodium dodecyl sulphate. Rates of synthesis were highest in the proximal cauda > distal cauda > initial segment > ductuli efferentes > corpus > distal caput > proximal caput. One protein (mol.wt. 23 000) characterized the initial segment, three proteins (mol.wts. 18 500, 19 000 and 32 000) the caput and one protein (mol.wt. 47 000) the cauda. 2. After castration, [35S]methionine incorporation in all regions of the epididymis was reduced to < 10% of that in normal animals but could be restored to control levels within 5 days by testosterone treatment. Other steroids (corticosterone, oestrogen or progesterone) were ineffective. 3. The synthesis of the 18 500, 19 000, and 32 000 mol.wt. proteins in the caput and the 47 000 mol.wt. protein in the cauda were preferentially regulated by androgens, whilst the synthesis of 23 000 and approx. 80 000 mol.wt. proteins in the initial segment was dependent upon factors present in testicular fluid. 4. The androgen-dependent and testicular fluid-dependent proteins were major components of epididymal secretion. Purification and characterization of the 18 500, 19 000, 23 000 and 32 000 mol.wt. proteins showed them to be acidic glycoproteins with a carbohydrate content of 7.6-13.2%. The 47 000 mol.wt. protein, on the other hand, is highly basic. 5. A possible role for these proteins in the acquisition of motility, fertilizing capacity and storage of spermatozoa in the epididymis is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acott T. S., Hoskins D. D. Bovine sperm forward motility protein. Partial purification and characterization. J Biol Chem. 1978 Oct 10;253(19):6744–6750. [PubMed] [Google Scholar]
- Acott T. S., Johnson D. J., Brandt H., Hoskins D. D. Sperm forward motility protein: tissue distribution and species cross reactivity. Biol Reprod. 1979 Mar;20(2):247–252. doi: 10.1095/biolreprod20.2.247. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boursnell J. C., Hartree E. F., Briggs P. A. Studies of the bulbo-urethral (Cowper's)-gland mucin and seminal gel of the boar. Biochem J. 1970 May;117(5):981–988. doi: 10.1042/bj1170981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks D. E. Influence of testicular secretions on tissue weight and on metabolic and enzyme activities in the epididymis of the rat. J Endocrinol. 1979 Aug;82(2):305–313. doi: 10.1677/joe.0.0820305. [DOI] [PubMed] [Google Scholar]
- Cameo M. S., Blaquier J. A. Androgen-controlled specific proteins in rat epididymis. J Endocrinol. 1976 Apr;69(1):47–55. doi: 10.1677/joe.0.0690047. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., FREEMAN G. Plaque production by the polyoma virus. Virology. 1959 Jul;8(3):396–397. doi: 10.1016/0042-6822(59)90043-1. [DOI] [PubMed] [Google Scholar]
- Danzo B. J., Eller B. C. Androgen metabolism by and binding to mature rabbit epididymal tissue: studies on cytosol. J Steroid Biochem. 1978 Mar;9(3):209–217. doi: 10.1016/0022-4731(78)90151-6. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., Hoffer A. P. Failure of exogenous androgen to prevent regression of the initial segments of the rat epididymis after efferent duct ligation or orchidectomy. Biol Reprod. 1979 Mar;20(2):162–181. doi: 10.1095/biolreprod20.2.162. [DOI] [PubMed] [Google Scholar]
- Fournier-Delpech S., Bayard F., Boulard C. Contribution à l'étude de la maturation du sperme. Etude d'une protéine acide de l'épididyme chez le rat. Dépendance androgène, relation avec l'acide sialique. C R Seances Soc Biol Fil. 1973;167(12):1989–1996. [PubMed] [Google Scholar]
- French F. S., Ritzén E. M. A high-affinity androgen-binding protein (ABP) in rat testis: evidence for secretion into efferent duct fluid and absorption by epididymis. Endocrinology. 1973 Jul;93(1):88–95. doi: 10.1210/endo-93-1-88. [DOI] [PubMed] [Google Scholar]
- Garberi J. C., Kohane A. C., Cameo M. S., Blaquier J. A. Isolation and characterization of specific rat epididymal proteins. Mol Cell Endocrinol. 1979 Jan;13(1):73–82. doi: 10.1016/0303-7207(79)90077-7. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M., Mainwaring W. I. Androgen-dependent synthesis of basic secretory proteins by the rat seminal vesicle. Biochem J. 1976 Aug 15;158(2):271–282. doi: 10.1042/bj1580271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. J., Burchell J. M., Mainwaring W. I. Testosterone control of nucleic acid content and proliferation of epithelium and stroma in rat seminal vesicles. Biochem J. 1976 Oct 15;160(1):43–48. doi: 10.1042/bj1600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. F., Johnson A. D. Comparative study of protein pattern of epididymal plasma of mouse, rat, rabbit and sheep. Comp Biochem Physiol B. 1975 Jul 15;51(3):337–341. doi: 10.1016/0305-0491(75)90017-6. [DOI] [PubMed] [Google Scholar]
- Jones R. Effects of testosterone, testosterone metabolites and anti-androgens on the function of the male accessory glands in the rabbit and rat. J Endocrinol. 1977 Jul;74(1):75–88. doi: 10.1677/joe.0.0740075. [DOI] [PubMed] [Google Scholar]
- Koskimies A. I., Kormano M. Proteins in fluids from different segments of the rat epididymis. J Reprod Fertil. 1975 May;43(2):345–348. doi: 10.1530/jrf.0.0430345. [DOI] [PubMed] [Google Scholar]
- Main S. J., Davies R. V., Setchell B. P. The evidence that inhibin must exist. J Reprod Fertil Suppl. 1979;(26):3–14. [PubMed] [Google Scholar]
- Olson G. E., Hamilton D. W. Characterization of the surface glycoproteins of rat spermatozoa. Biol Reprod. 1978 Aug;19(1):26–35. doi: 10.1095/biolreprod19.1.26. [DOI] [PubMed] [Google Scholar]
- Parker M. G., Scrace G. T., Mainwaring W. I. Testosterone regulates the synthesis of major proteins in rat ventral prostate. Biochem J. 1978 Jan 15;170(1):115–121. doi: 10.1042/bj1700115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie P. S., Symes E. K., Mainwaring W. J. The androgenic regulation of the activities of enzymes engaged in the synthesis of deoxyribonucleic acid in rat ventral prostate gland. Biochem J. 1975 Oct;152(1):1–16. doi: 10.1042/bj1520001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Wilson J. D. Sexual differentiation. Annu Rev Physiol. 1978;40:279–306. doi: 10.1146/annurev.ph.40.030178.001431. [DOI] [PubMed] [Google Scholar]