ABSTRACT.
More than 1,000 children under 5 years of age die every day from malaria. Cerebral malaria (CM) is the most severe and deadly manifestation of the disease. The occurrence of multiple organ dysfunction syndrome (MODS) has been associated with increased mortality in adult patients with CM. However, little is known about the frequency and severity of MODS in children with CM. This was a retrospective study of 199 pediatric patients with CM admitted to a referral hospital in Blantyre, Malawi, between January 2019 and May 2023. Data were abstracted from charts to calculate scores using four established scoring systems: Pediatric Logistic Organ Dysfunction-2 (PELOD-2), Pediatric Sequential Organ Failure Assessment (pSOFA), Signs of Inflammation in Children that Can Kill (SICK), and Lambaréné Organ Dysfunction Score (LODS). Mortality was 16% (n = 32). All four scoring systems were predictive of mortality, but the PELOD-2 and pSOFA scores outperformed the others with area under the curve values of 0.75 and 0.67, respectively. Multiple organ dysfunction syndrome was diagnosed in 182 patients (91%) using the PELOD-2 score, 172 patients (86%) using the pSOFA score, 99 patients (50%) using the SICK score, and 30 patients (15%) using the LODS. The PELOD-2 and pSOFA identify MODS in children with CM but require laboratory-based testing that is often unavailable in malaria-endemic areas. Furthermore, these scoring systems may identify primary malarial disease pathology rather than true organ dysfunction. Simplified scoring systems designed to recognize and quantify MODS in this patient population may provide opportunities for improved resource allocation and timely, organ-specific treatment.
INTRODUCTION
Malaria is a debilitating disease that remains a significant public health challenge worldwide. An estimated 608,000 deaths occurred in 2022, with a disproportionate number of these fatalities occurring in sub-Saharan Africa.1 Mortality rates have remained relatively stagnant since 2015 despite the implementation of diverse preventive measures and the widespread use of artesunate. African children under 5 years of age continue to account for 76% of all malaria-related deaths.1,2 Cerebral malaria (CM), the most severe manifestation of the Plasmodium falciparum infection, has a mortality rate as high as 40% in African children.3–8
Multiple organ dysfunction syndrome (MODS), defined as the failure of two or more organ systems, is a complication of many critical illnesses including malaria. Multiple organ dysfunction syndrome has been identified in up to 85% of adult patients presenting with severe malaria.9 In adults with CM, the mortality rate increases from 8% in those with “pure CM” to 50% when MODS is also present.9,10 Despite the frequency and significant effects of MODS in adults with malarial infection, the presence of MODS and its impact on outcomes remain poorly understood in children with CM.
Historically, P. falciparum infection in African children was thought to have a “monosyndromic presentation” with minimal complications apart from the presenting coma, anemia, or acidosis.11 With the ongoing improvement of laboratory availability and monitoring, there is a growing recognition of the overlap in disease complications, such as a child admitted to the hospital with both CM and severe malarial anemia.11,12 In addition, it is plausible that multiple organs are simultaneously affected owing to the presence of parasitized red blood cells throughout the host’s microvasculature.10–14 Postmortem histopathological examinations of Malawian children with CM revealed sequestration of P. falciparum parasites within the brain, heart, lungs, spleen, stomach, intestines, and skin.13,14 Namazzi et al.15 evaluated 600 Ugandan children with malaria and reported increased mortality rates in patients diagnosed with both CM and acute kidney injury (AKI). These children with coma and AKI would meet the criteria for MODS. Within the same cohort, increased mortality was observed in patients with AKI and signs of cardiovascular compromise, another presentation of MODS. Improved understanding of the prevalence, severity, and overall impact of MODS in children with CM could have profound implications for clinical management and lead to better outcomes for affected children.
Multiple organ dysfunction syndrome can be diagnosed and quantified in pediatric patients using various scoring systems, including Pediatric Logistic Organ Dysfunction-2 (PELOD-2), Pediatric Sequential Organ Failure Assessment (pSOFA), Signs of Inflammation in Children that Can Kill (SICK), and Lambaréné Organ Dysfunction Score (LODS).16–25 The PELOD-2 and pSOFA scores are commonly used in pediatric intensive care units worldwide and have been validated to quantify MODS and severity of illness.18–21 Our group previously used the PELOD-2 scoring system to elucidate the frequency of MODS in 145 children with CM and identified MODS in 94% of cases.26 In adults with malaria, the SOFA score is used most frequently for diagnosing and quantifying MODS.27–29 To our knowledge, the pSOFA score has not been applied to pediatric patients with malaria. Both the PELOD-2 and pSOFA scoring systems rely heavily on laboratory-based values that are oftentimes not present in resource-limited settings. Contrarily, the SICK score was developed to predict mortality in children with infections in low-resource settings and is based on physical examination and vital sign abnormalities only.22,23 The LODS is a malaria-specific scoring system that aims to rapidly predict mortality in African children with this disease.24,25 No evaluation of the SICK or LODS scoring system to identify MODS has been undertaken. Our study aimed to evaluate the ability of these four scoring systems to recognize MODS and predict mortality in children with CM.
MATERIALS AND METHODS
This was a retrospective review of prospectively collected data at Queen Elizabeth Central Hospital in Blantyre, Malawi, from January 2019 to May 2023. Our work was an ancillary study to Treating Brain Swelling in Pediatric Cerebral Malaria (5U01AI126610-02), an interventional, randomized clinical trial. Ethics approval was received through the Institutional Review Board at Michigan State University and locally through the University of Malawi College of Medicine Research Ethics Committee. Children between 6 months and 12 years of age who met the WHO’s case definition of CM (Blantyre coma score [BCS] of ≤2, peripheral parasitemia with P. falciparum, and no other detectable cause of encephalopathy) were eligible. Exclusion criteria included advanced HIV, gross malnutrition, and recent head trauma.
Patient data obtained on admission were extracted from paper charts, including vital signs, physical exam findings, and laboratory values. Laboratory studies included finger prick samples to ascertain the parasite species, packed cell volume, blood glucose concentration, lactate levels (Arkray Lactate Pro 2, Minneapolis, MN), creatinine (StatSensor Creatinine Meter, Nova Biomedical, Waltham, MA), total bilirubin (UNISTAT Bilirubinometer, Reichert Technologies, Depew, NY), and blood gas analysis (Abbott iSTAT, Princeton, NJ). Venous blood samples were collected for a full blood count and electrolyte analysis (Beckman Coulter Life Sciences Coulter Counter, Indianapolis, IN).
All patients in this study received treatment of severe malaria according to the Malawi Standard Treatment Guidelines. Management included the administration of intravenous artesunate; treatment of fever, seizures, and hypoglycemia; and the use of antibiotics as clinically indicated.30 None of the patients were receiving inotropic agents or mechanical ventilation upon admission.
To diagnose and quantify MODS, the data from each patient were scored using the following scoring systems: PELOD-2, pSOFA, SICK, and LODS. Minor adaptations were necessary, and the adapted scoring systems are included in Supplemental Tables 1–4. Notably, the Glasgow coma score (GCS) was not used in any scoring system because it was not recorded. Per standard of care in this patient population, the BCS was recorded and therefore used instead.31 For the neurologic assessment in PELOD-2 and pSOFA, we converted each score’s validated GCS cutoffs to clinically comparable BCS values. Similarly for the SICK score, the BCS was changed to the appropriate Alert, Voice, Pain, Unresponsive (AVPU) grade. To calculate the PELOD-2 score, creatinine values were adjusted from µmmol/L to mg/dL, and the SpO2/FiO2 ratio was used instead of PaO2/FiO2 owing to the complete absence of arterial blood gas values. All missing values were scored as normal for all four scoring systems.
For each patient, the number of affected organs was noted along with the specific organ systems involved. The SICK score and LODS lack predefined, organ-specific categories, necessitating the grouping of measured parameters by organ system (Supplemental Tables 3 and 4). For the SICK score, variables were organized into three organ-specific categories: neurologic (AVPU assessment), cardiovascular (capillary refill time, blood pressure, heart rate measurements), and respiratory (respiratory rate, oxygen saturation evaluations). Temperature, although a parameter in the SICK score, was not included in the number of affected organs as it does not correspond to a specific organ group. For LODS, parameters were grouped into two organ-specific categories: neurologic (coma, prostration) and respiratory (deep breathing).
Values are reported as median (interquartile range), mean (SD), or n (%) as appropriate. Receiver operator characteristic (ROC) curve analysis was used to assess the performance of each score to predict mortality. The sensitivity, specificity, positive predictive value, and negative predictive value of each scoring system were determined using a leave-one-out cross-validation analysis. For this cross-validation, we created training data removing one subject from the dataset. Next, we fit the model using the training dataset and subsequently used the fitted model to compute the optimal cutoff probability, maximizing sensitivity and specificity together. Finally, we used the data of the left-out subject as test data and estimated the outcome using the test data and the optimal cutoff probability from the model using the training data. We repeated this process for all the subjects in the dataset. Comparisons between survivors and nonsurvivors were analyzed using Fisher’s exact test, Mann–Whitney test, or Wilcoxon rank-sum test where appropriate. P-values <0.05 were considered statistically significant throughout. Analyses were conducted using GraphPad Prism v. 9.00 for Windows (GraphPad Software, La Jolla, CA).
RESULTS
One hundred ninety-nine consecutive patients admitted between January 2019 and May 2023 were reviewed. Table 1 provides an overview of the cohort’s basic demographics, vital signs, and admission laboratory values. The mortality rate was 16% (n = 32).
Table 1.
Demographics, laboratory investigations, and outcomes for the cohort
| Variable | All (N = 199) |
|---|---|
| Age, Months, Median [IQR] | 51 [33–75] |
| Male Sex, n (%) | 96 (48) |
| Hours Since Fever Began, Median [IQR] | 60 [48–72] |
| Hours of Unconscious State Prior to Admission, Median [IQR] | 18 [9–24] |
| Had Seizures Prior to Admission, n (%) | 124 (62) |
| Temperature, °C, Median [IQR] | 38.5 [37.9–39.3] |
| Respiratory Rate, Breaths/Minute, Median [IQR] | 36 [28–42] |
| Oxygen Saturation, %, Median [IQR] | 97 [95–98] |
| Heart Rate, Beats/Minute, Median [IQR] | 142 [127–158] |
| Mean Arterial Pressure, mm Hg, Median [IQR] | 77 [70–87] |
| Pallor Present, n (%) | 78 (39) |
| Jaundice Present, n (%) | 10 (5) |
| On Mechanical Ventilation, n (%) | 0 (0) |
| Retinopathy Positive, n (%) | 119 (60) |
| Blantyre Coma Score, n (%) | |
| 0 | 23 (12) |
| 1 | 69 (35) |
| 2 | 107 (54) |
| White Blood Cells, ×103/µL, Median [IQR] | 9.4 [7.0–13.8] |
| Packed Cell Volume, %, Median [IQR] | 26 [21–30] |
| Platelets, ×103/µL, Median [IQR] | 73 [38–127] |
| Creatinine, mg/dL, Median [IQR] | 0.7 [0.54–0.99] |
| Total Bilirubin, mg/dL, Median [IQR] | 1 [0.3–1.8] |
| Lactate, mmol/L, Median [IQR] | 4.1 [2.6–7.4] |
| Glucose, mg/dL, Median [IQR] | 99 [81–121] |
| Mortality, n (%) | 32 (16) |
IQR = interquartile range.
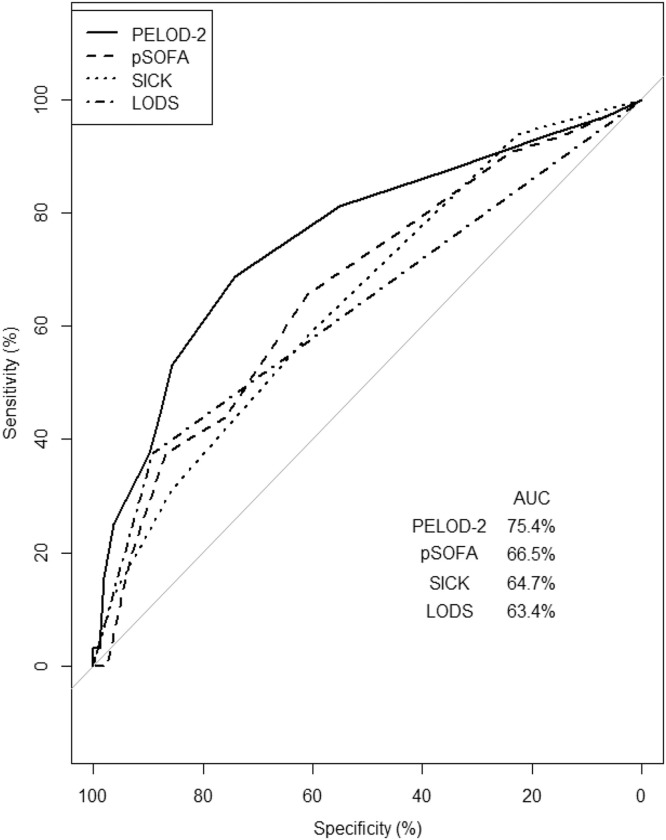
When calculated based on admission data, a higher score on each of the four scoring systems was positively correlated with the risk of death. For every unit increase in the PELOD-2 score, the estimated odds of death increased by 36%. The odds of death increased by 29% for every unit increase in the pSOFA score and 64% for every point scored in the SICK score. All patients with CM had a minimum LODS of 2 based on their CM diagnosis. When the LODS increased to 3, the odds of death increased by 397%. When model performance to predict mortality was further evaluated, the PELOD-2 score outperformed all other models with an area under the curve value of 0.75. The ROC curves for the four scoring systems are illustrated in Figure 1. Further information regarding the sensitivity, specificity, positive predictive value, and negative predictive value of each scoring system can be found in Table 2.
Figure 1.
Receiver operator characteristic curve illustrating the ability of four different scoring systems to predict mortality. LODS = Lambaréné Organ Dysfunction Score; PELOD-2 = Pediatric Logistic Organ Dysfunction-2; pSOFA = Pediatric Sequential Organ Failure Assessment; SICK = Signs of Inflammation in Children that Can Kill.
Table 2.
Results of leave-one-out cross-validation using the test datasets
| Characteristic | PELOD-2 (N = 199)* | pSOFA (N = 199)* | SICK (N = 199)* | LODS (N = 199)* |
|---|---|---|---|---|
| Sensitivity | 0.69 (0.5–0.84) | 0.66 (0.47–0.81) | 0.59 (0.41–0.76) | 0.38 (0.21–0.56) |
| Specificity | 0.74 (0.67–0.81) | 0.61 (0.53–0.69) | 0.60 (0.52–0.67) | 0.89 (0.84–0.93) |
| Positive Predictive Value | 0.34 (0.23–0.47) | 0.24 (0.16–0.35) | 0.22 (0.14–0.32) | 0.40 (0.23–0.59) |
| Negative Predictive Value | 0.93 (0.87–0.96) | 0.90 (0.83–0.95) | 0.88 (0.81–0.94) | 0.88 (0.82–0.93) |
LODS = Lambaréné Organ Dysfunction Score; PELOD-2 = Pediatric Logistic Organ Dysfunction-2; pSOFA = Pediatric Sequential Organ Failure Assessment; SICK = Signs of Inflammation in Children that Can Kill.
Mean (95% CI).
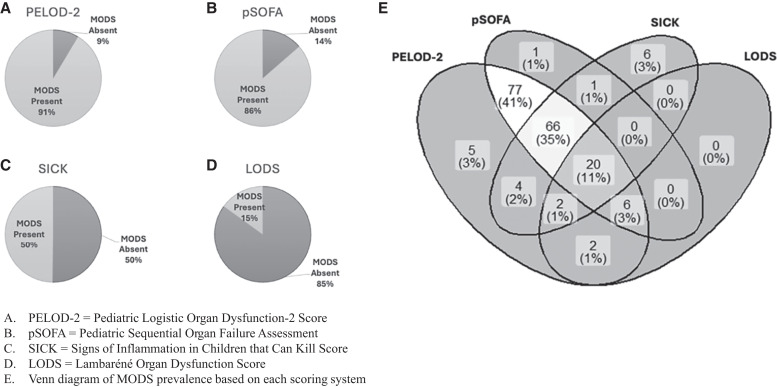
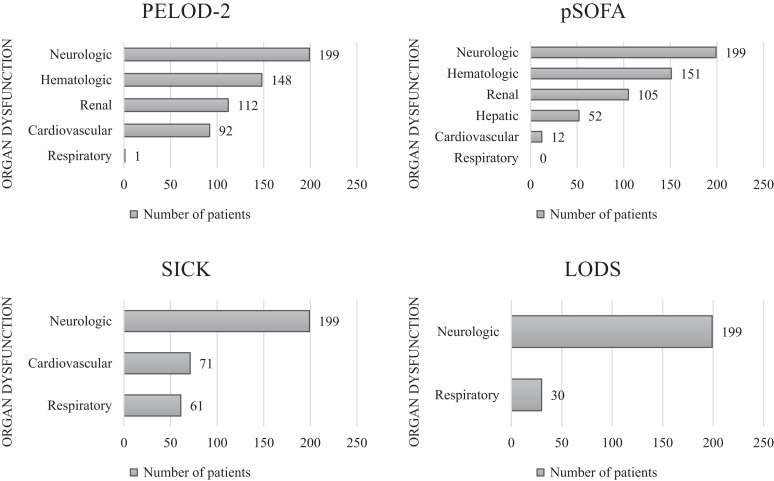
Multiple organ dysfunction syndrome was identified in 190 of 199 patients (95%) in this cohort. The PELOD-2 score and pSOFA score revealed the presence of MODS in 91% and 86% of patients, respectively (Figure 2). Both scores indicated neurologic dysfunction in all 199 patients. The hematologic and renal systems were the second- and third-most affected organ systems based on the PELOD-2 and pSOFA scores (Figure 3). Moreover, children with more organs involved had higher rates of mortality (Table 3). When evaluating for MODS using the SICK score and LODS, 50% of patients and 15% of patients, respectively, met our predefined criteria.
Figure 2.
MODS prevalence using four different scoring systems in 199 children with CM. CM = cerebral malaria; MODS = multiple organ dysfunction syndrome.
Figure 3.
Individual organ dysfunctions by scoring system for the cohort. LODS = Lambaréné Organ Dysfunction Score; PELOD-2 = Pediatric Logistic Organ Dysfunction-2; pSOFA = Pediatric Sequential Organ Failure Assessment; SICK = Signs of Inflammation in Children that Can Kill. N = 199 patients.
Table 3.
Organ dysfunction and mortality
| Evaluation of Organ Dysfunction | All (N = 199) | Survivors (n = 167) | Nonsurvivors (n = 32) | P-Value |
|---|---|---|---|---|
| Organ Dysfunction Scores | ||||
| PELOD-2, median [IQR] | 5.00 [3.00–6.00] | 4.00 [3.00–6.00] | 7.00 [5.00–9.25] | <0.001 |
| pSOFA, median [IQR] | 6.00 [5.00–8.00] | 6.00 [5.00–7.00] | 7.00 [6.00–9.00] | 0.003 |
| SICK, median [IQR] | 2.00 [2.00–3.00] | 2.00 [2.00–3.00] | 3.00 [2.00–4.00] | 0.006 |
| LODS, n (%) | ||||
| 2 | 169 (85) | 149 (89) | 20 (63) | <0.001 |
| 3 | 30 (15) | 18 (11) | 12 (38) | |
| Number of organ dysfunctions using PELOD-2, median [IQR] | 3.00 [2.00–3.00] | 3.00 [2.00–3.00] | 3.00 [2.00–4.00] | 0.059 |
| Number of organ dysfunctions using pSOFA, median [IQR] | 3.00 [2.00–3.00] | 3.00 [2.00–3.00] | 3.00 [2.00–3.00] | 0.328 |
IQR = interquartile range; LODS = Lambaréné Organ Dysfunction Score; PELOD-2 = Pediatric Logistic Organ Dysfunction-2; pSOFA = Pediatric Sequential Organ Failure Assessment; SICK = Signs of Inflammation in Children that Can Kill.
DISCUSSION
Multiple organ dysfunction syndrome is a major cause of death in critically ill children.32–38 Furthermore, mortality continues to escalate as the severity of organ dysfunction increases and as the number of organ systems involved increases.33–40 Despite the belief that MODS is a complication only seen in adults with severe malaria, our group has previously demonstrated both the presence of MODS in pediatric patients with CM and its association with increased mortality rates.11,26,41 This current study augments our previous findings of MODS prevalence in children with CM by applying a second validated organ dysfunction scoring system as well as two scoring systems specifically developed for use in resource-limited areas. In addition, our findings align with rates of observed MODS in adults with severe malaria.27,28
The PELOD-2 scores and the pSOFA scores revealed comparable rates of MODS within our cohort upon admission. One notable difference in the results of these two scoring systems was the presence of cardiovascular dysfunction, which was identified in more patients when using the PELOD-2 score (92 patients) compared with the pSOFA score (12 patients). This variance may be attributable to the use of lactate as one of the two markers of cardiovascular dysfunction in the PELOD-2 score. Although elevated lactate can indicate cardiovascular dysfunction and shock, multiple other factors are more likely causative in children with CM. For example, tissue hypoxia often results from profound anemia and microvascular occlusion that is classically associated with P. falciparum infection. Parasite metabolism also results in lactate production and release, further increasing a patient’s lactatemia. In addition, impaired clearance of lactate may occur owing to concurrent liver dysfunction.42,43 It is also possible that we underestimated cardiovascular dysfunction when using the pSOFA score if children with profound hypotension did not survive long enough to be admitted to the hospital.
The SICK score and LODS identified lower rates of MODS in pediatric CM patients. These scores offer the advantage of being designed for low-resource settings, are easily applicable, and perform well when predicting mortality. However, our attempt to organize these scores into system-based categories for the diagnosis of MODS proved unsuccessful. The SICK score is heavily reliant on vital sign abnormalities, which may not indicate dysfunction in a specific organ system. For example, tachycardia could represent cardiovascular dysfunction but may also be secondary to fever, anemia, respiratory distress, dehydration, or pain. The use of LODS as a MODS screening tool is limited as there are only three variables, two of which are distinctly neurologic in origin. Future work should focus on scoring systems that are easy to apply in resource-limited settings and target recognition of specific organ dysfunction in malaria patients.
Our study does have several limitations. This was a retrospective study conducted in a resource-limited setting. Despite having access to more laboratory capabilities than is typical in these settings, we had multiple missing values, most notably creatinine and bilirubin (Supplemental Table 5). Several missing data points are due to the rapid clinical decline and death of patients, but most of our missing data were due to lack of testing strips or reagents. Consequently, there is a strong possibility that we underestimated the prevalence of MODS or the severity of MODS in our cohort. This is especially true when utilizing the pSOFA score, which requires bilirubin, a laboratory value we did not obtain in 80 patients (40%) included in this study.
In addition, both respiratory dysfunction and renal dysfunction were potentially underrecognized in our cohort. Scoring systems such as PELOD-2 and pSOFA were developed and validated in high-resource settings. Owing to the absence of emergency airway management skills and resources in many malaria-endemic areas, a child with profound hypercapnia or hypoxemia would likely die prior to arrival or admission to the hospital. The use of laboratory values such as an arterial blood gas or the use of ventilator requirements is not feasible or applicable in this environment. Further work is needed to establish a more practical approach to evaluate the contribution of respiratory failure in this population. Similarly, these scoring systems use creatinine cutoffs based on data obtained from children in high-resource settings, an important limitation as serum creatinine levels are affected by nutritional status and muscle mass.44,45 Using the provided cutoffs, we likely underestimated AKI because of the risk of undernutrition in our cohort of Malawian children. Notably, PELOD-2 and pSOFA do not incorporate urine output criteria, the alternate recommended way to evaluate renal dysfunction. Monitoring the change in creatinine values when available or including urine output measurements as recommended by the Kidney Disease Improving Global Guidelines are promising potentials for evaluation of renal dysfunction in resource-limited settings.46
Lastly, it is possible that the prevalence of MODS was overestimated in our study. The presence of coma is part of the diagnostic criteria for CM. Therefore, all children had neurologic involvement. Multiple organ dysfunction syndrome could also be overestimated in this cohort owing to specific abnormalities related to the P. falciparum infection itself. Beyond elevated lactate, thrombocytopenia is regularly identified in children with malaria because of several mechanisms including immune-mediated destruction, splenic sequestration, bone marrow suppression, and consumption in the microvasculature.47,48 Unconjugated hyperbilirubinemia is also common in P. falciparum infections owing to hemolysis of both parasitized and unparasitized erythrocytes.49–51 In these patients, thrombocytopenia may not be the best marker of hematologic organ dysfunction, and hyperbilirubinemia may be a poor marker of liver dysfunction. Point-of-care tests are increasingly used in resource-challenged settings. Establishing which laboratory parameters are of most use in identifying children with severe malaria and concomitant MODS is imperative and will help to focus development efforts on affordable, feasible, and robust assays.
Multiple organ dysfunction syndrome was identified in nearly all children with CM. Although these results may not be generalizable to children with other types of severe malaria without further investigations, it is important to recognize the association of MODS and increased mortality in pediatric CM. Limitations in the currently available scoring systems may lead to over- as well as under-recognition of MODS in these patients. The development of a novel scoring system that rapidly and accurately identifies organ dysfunction in children across the spectrum of severe malaria manifestations would enhance clinical decision-making and facilitate effective resource allocation.
Supplemental Materials
ACKNOWLEDGMENT
We thank the patients, their families, and the staff of the Blantyre Malaria Project.
Note: Supplemental materials appear at www.ajtmh.org.
REFERENCES
- 1.World Health Organization, 2023. World Malaria Report 2023. Geneva, Switzerland: WHO. [Google Scholar]
- 2.Rosenthal PJ, 2022. Malaria in 2022: Challenges and progress. Am J Trop Med Hyg 106: 1565–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John CC, Bangirana P, Byarugaba JS, Opoka RO, Idro R, Jurek AM, Wu B, Boivin MJ, 2008. Cerebral malaria in children is associated with long-term cognitive impairment. Pediatrics 122: e92–e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien NF, Mutatshi Taty T, Moore-Clingenpeel M, Bodi Mabiala J, Mbaka Pongo J, Ambitapio Musungufu D, Uchama M, Yotebieng M, 2018. Transcranial Doppler ultrasonography provides insights into neurovascular changes in children with cerebral malaria. J Pediatr 203: 116–124.e3. [DOI] [PubMed] [Google Scholar]
- 5.Idro R, Jenkins NE, Newton CRJC, 2005. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol 4: 827–840. [DOI] [PubMed] [Google Scholar]
- 6.Idro R, Marsh K, John CC, Newton CR, 2010. Cerebral malaria: Mechanisms of brain injury and strategies for improved neurocognitive outcome. Pediatr Res 68: 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiess N, Villabona-Rueda A, Cottier KE, Huether K, Chipeta J, Stins MF, 2020. Pathophysiology and neurologic sequelae of cerebral malaria. Malar J 19: 266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langfitt JT, McDermott MP, Brim R, Mboma S, Potchen MJ, Kampondeni SD, Seydel KB, Semrud-Clikeman M, Taylor T, 2019. Neurodevelopmental impairments 1 year after cerebral malaria. Pediatrics 143: e20181026. [DOI] [PubMed] [Google Scholar]
- 9.Krishnan A, Karnad DR, 2003. Severe falciparum malaria: An important cause of multiple organ failure in Indian intensive care unit patients. Crit Care Med 31: 2278–2284. [DOI] [PubMed] [Google Scholar]
- 10.Newton CR, Hien TT, White N, 2000. Cerebral malaria. J Neurol Neurosurg Psychiatry 69: 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milner DA, Jr, 2018. Malaria pathogenesis. Cold Spring Harb Perspect Med 8: a025569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsh K, et al. , 1995. Indicators of life-threatening malaria in African children. N Engl J Med 332: 1399–1404. [DOI] [PubMed] [Google Scholar]
- 13.Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, Lewallen S, Liomba NG, Molyneux ME, 2004. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med 10: 143–145. [DOI] [PubMed] [Google Scholar]
- 14.Milner DA, et al. , 2015. Quantitative assessment of multiorgan sequestration of parasites in fatal pediatric cerebral malaria. J Infect Dis 212: 1317–1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Namazzi R, Opoka R, Datta D, Bangirana P, Batte A, Berrens Z, Goings MJ, Schwaderer AL, Conroy AL, John CC, 2022. Acute kidney injury interacts with coma, acidosis, and impaired perfusion to significantly increase risk of death in children with severe malaria. Clin Infect Dis 75: 1511–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferreira AM, Sakr Y, 2011. Organ dysfunction: General approach, epidemiology, and organ failure scores. Semin Respir Crit Care Med 32: 543–551. [DOI] [PubMed] [Google Scholar]
- 17.Bembea MM, et al. , 2022. Pediatric organ dysfunction information update mandate (PODIUM) contemporary organ dysfunction criteria: Executive summary. Pediatrics 149 (Suppl 1): S1–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F, 2013. PELOD-2: An update of the pediatric logistic organ dysfunction score. Crit Care Med 41: 1761–1773. [DOI] [PubMed] [Google Scholar]
- 19.Suari NMR, Latief A, Pudjiadi AH, 2021. New PELOD-2 cut-off score for predicting death in children with sepsis. Paediatr Indones 61: 39–45. [Google Scholar]
- 20.Kumbar S, Chandrashekhara, 2020. Assessment of pSOFA-L score in predicting the clinical outcome of critically ill children. Int J Contemp Pediatr 7: 925. [Google Scholar]
- 21.Mohamed El-Mashad G, Said El-Mekkawy M, Helmy Zayan M, 2020. Paediatric sequential organ failure assessment (pSOFA) score: A new mortality prediction score in the paediatric intensive care unit [in Spanish]. An Pediatr (Engl Ed) 92: 277–285. [DOI] [PubMed] [Google Scholar]
- 22.Bhal S, Tygai V, Kumar N, Sreenivas V, Puliyel JM, 2006. Signs of inflammation in children that can kill (SICK score): Preliminary prospective validation of a new non-invasive measure of severity-of-illness. J Postgrad Med 52: 102–105. [PubMed] [Google Scholar]
- 23.Gupta MA, Chakrabarty A, Halstead R, Sahni M, Rangasami J, Puliyel A, Sreenivas V, Green DA, Puliyel JM, 2010. Validation of “Signs of Inflammation in Children that Kill” (SICK) score for immediate non-invasive assessment of severity of illness. Ital J Pediatr 36: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Helbok R, et al. , 2009. The Lambaréné Organ Dysfunction Score (LODS) is a simple clinical predictor of fatal malaria in African children. J Infect Dis 200: 1834–1841. [DOI] [PubMed] [Google Scholar]
- 25.Conroy AL, Hawkes M, Hayford K, Namasopo S, Opoka RO, John CC, Liles WC, Kain KC, 2015. Prospective validation of pediatric disease severity scores to predict mortality in Ugandan children presenting with malaria and non-malaria febrile illness. Crit Care 19: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson H, Raees M, Urbina E, Muszynski J, Seydel K, Taylor T, O’Brien N, 2022. Multiple organ dysfunction syndrome and pediatric logistic organ dysfunction-2 score in pediatric cerebral malaria. Am J Trop Med Hyg 107: 820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhutani A, Kaushik RM, Kaushik R, 2020. A study on multi-organ dysfunction syndrome in malaria using sequential organ failure assessment score. Trop Parasitol 10: 86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makowiecki M, et al. , 2018. Usefulness of SOFA score and artesunate – based treatment in severe malaria – a single center study. Przegl Epidemiol 72: 215–221. [PubMed] [Google Scholar]
- 29.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S, 1998. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Crit Care Med 26: 1793–1800. [DOI] [PubMed] [Google Scholar]
- 30.Ministry of Health, 2015. Malawi Standard Treatment Guidelines. 5th ed. Lilongwe, Malawi: Ministry of Health. [Google Scholar]
- 31.Taylor TE, 2009. Caring for children with cerebral malaria: Insights gleaned from 20 years on a research ward in Malawi. Trans R Soc Trop Med Hyg 103 (Suppl 1): S6–S10. [DOI] [PubMed] [Google Scholar]
- 32.Matics TJ, Pinto NP, Sanchez-Pinto LN, 2019. Association of organ dysfunction scores and functional outcomes following pediatric critical illness. Pediatr Crit Care Med 20: 722–727. [DOI] [PubMed] [Google Scholar]
- 33.Typpo KV, Petersen NJ, Hallman DM, Markovitz BP, Mariscalco MM, 2009. Day 1 multiple organ dysfunction syndrome is associated with poor functional outcome and mortality in the pediatric intensive care unit. Pediatr Crit Care Med 10: 562–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kutko MC, Calarco MP, Flaherty MB, Helmrich RF, Ushay HM, Pon S, Greenwald BM, 2003. Mortality rates in pediatric septic shock with and without multiple organ system failure. Pediatr Crit Care Med 4: 333–337. [DOI] [PubMed] [Google Scholar]
- 35.Leteurtre S, et al. , 2003. Validation of the Paediatric Logistic Organ Dysfunction (PELOD) score: Prospective, observational, multicentre study. Lancet 362: 192–197. [DOI] [PubMed] [Google Scholar]
- 36.Wilkinson JD, Pollack MM, Glass NL, Kanter RK, Katz RW, Steinhart CM, 1987. Mortality associated with multiple organ system failure and sepsis in pediatric intensive care unit. J Pediatr 111: 324–328. [DOI] [PubMed] [Google Scholar]
- 37.Watson RS, Crow SS, Hartman ME, Lacroix J, Odetola FO, 2017. Epidemiology and outcomes of pediatric multiple organ dysfunction syndrome. Pediatr Crit Care Med 18 (Suppl 1): S4–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leclerc F, Leteurtre S, Duhamel A, Grandbastien B, Proulx F, Martinot A, Gauvin F, Hubert P, Lacroix J, 2005. Cumulative influence of organ dysfunctions and septic state on mortality of critically ill children. Am J Respir Crit Care Med 171: 348–353. [DOI] [PubMed] [Google Scholar]
- 39.Badke CM, Mayampurath A, Sanchez-Pinto LN, 2022. Multiple organ dysfunction interactions in critically ill children. Front Pediatr 10: 874282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamburro RF, Jenkins TL, 2017. Multiple organ dysfunction syndrome: A challenge for the pediatric critical care community. Pediatr Crit Care Med 18: S1–S3. [DOI] [PubMed] [Google Scholar]
- 41.Africa Health Organisation, 2020. Protecting Health in Africa. Available at: https://aho.org/fact-sheets/malaria-fact-sheet/. Accessed May 20, 2024.
- 42.2014. Severe malaria. Trop Med Int Health 19 (Suppl 1): 7–131. [DOI] [PubMed] [Google Scholar]
- 43.Krishna S, et al. , 1994. Lactic acidosis and hypoglycaemia in children with severe malaria: Pathophysiological and prognostic significance. Trans R Soc Trop Med Hyg 88: 67–73. [DOI] [PubMed] [Google Scholar]
- 44.Batte A, Starr MC, Schwaderer AL, Opoka RO, Namazzi R, Phelps Nishiguchi ES, Ssenkusu JM, John CC, Conroy AL, 2020. Methods to estimate baseline creatinine and define acute kidney injury in lean Ugandan children with severe malaria: A prospective cohort study. BMC Nephrol 21: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klahr S, Alleyne GA, 1973. Effects of chronic protein-calorie malnutrition on the kidney. Kidney Int 3: 129–141. [DOI] [PubMed] [Google Scholar]
- 46.Khwaja A, 2012. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120: c179–c184. [DOI] [PubMed] [Google Scholar]
- 47.Krishna P, Chalamalasetty MK, 2023. Thrombocytopenia in malaria and its diagnostic significance: A prospective study. J Clin Sci Res 12 (Suppl 1): S1–S4. [Google Scholar]
- 48.Lacerda MV, Mourão MP, Coelho HC, Santos JB, 2011. Thrombocytopenia in malaria: Who cares? Mem Inst Oswaldo Cruz 106 (Suppl 1): 52–63. [DOI] [PubMed] [Google Scholar]
- 49.Al-Salahy M, Shnawa B, Abed G, Mandour A, Al-Ezzi A, 2016. Parasitaemia and its relation to hematological parameters and liver function among patients malaria in Abs, Hajjah, northwest Yemen. Interdiscip Perspect Infect Dis 2016: 5954394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anand AC, Puri P, 2005. Jaundice in malaria. J Gastroenterol Hepatol 20: 1322–1332. [DOI] [PubMed] [Google Scholar]
- 51.Bhattacharjee D, Mukherjee K, Sarkar R, Chakraborti G, Das O, 2021. Abnormalities of liver function tests in acute malaria with hepatic involvement: A case–control study in eastern India. Med J Dr DY Patil Univ 14: 21–25. [Google Scholar]
- 52.Vercesi V, et al. ; Lung Ultrasound Consortium, 2018. External confirmation and exploration of the Kigali modification for diagnosing moderate or severe ARDS. Intensive Care Med 44: 523–524. [DOI] [PubMed] [Google Scholar]
- 53.Khemani RG, Thomas NJ, Venkatachalam V, Scimeme JP, Berutti T, Schneider JB, Ross PA, Willson DF, Hall MW, Newth CJ; Pediatric Acute Lung Injury and Sepsis Network Investigators (PALISI), 2012. Comparison of SpO2 to PaO2 based markers of lung disease severity for children with acute lung injury. Crit Care Med 40: 1309–1316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.