ABSTRACT.
Deer tick virus (DTV), also known as Powassan virus lineage II, is a rising health concern due to increased recognition as a cause of human encephalitis. Since European tick-borne encephalitis virus persists in nature in enzootic foci (i.e., higher prevalence rates in small pockets in nature), we sought to determine whether DTV is also focally maintained in relation to habitat type, to better understand factors leading to human risk of exposure. From 2018 to 2021, questing Ixodes scapularis ticks were collected from five habitats at the Wells National Estuarine Research Reserve (WNERR) in Wells, ME: forest with invasive vegetation in the understory, edge, shrub, forest with native vegetation in the understory, and open field. Deer tick virus prevalence was greater in adult ticks (2.0%) than in nymphs (0.5%). Deer tick virus prevalence in adult ticks collected from forest with invasive vegetation was 3.2% compared to 0 to 1.7% in other habitat types. A hot spot analysis revealed a higher number of infected adults collected per hour on one of the transects located in forest with invasive vegetation. Phylogenetic analysis of 37 full-length DTV genomes sequenced in this study revealed four major clades from the WNERR, and there was high genetic diversity within each transect, suggesting frequent, short-range dispersal between habitats. Analysis of DTV sequences from other New England counties and states also indicated long-distance dispersal to and/or from the WNERR. This study provides preliminary evidence that DTV is focal and that the risk of encountering DTV-infected ticks in forest with invasive vegetation may be greater than in other habitat types.
INTRODUCTION
The tick-borne encephalitis virus (TBEV) (Flaviviridae, Flavivirus) complex was first recognized as a severe central nervous system disease in humans in the late 1930s by Soviet scientists investigating a presumed outbreak of Japanese encephalitis virus among Soviet troops situated in the taiga of the far-eastern border.1,2 The complex includes TBEV, which is widespread across much of Europe and parts of Asia, and Powassan virus (POWV), which circulates in the upper midwestern and northeastern United States, parts of Canada, and the Primorsky krai region of Russia. In North America, POWV is composed of two genetic lineages that share 94% amino acid identity and are indistinguishable serologically.3,4 Lineage I (POW), also known as prototype POWV, circulates in nature among Ixodes cookei Packard and Ixodes marxi Banks ticks and their hosts, including squirrels, woodchucks, and mustelids.5–7 Lineage II, or deer tick virus (DTV), is transmitted by the deer tick (also known as the black-legged tick), Ixodes scapularis Say,8 and is hypothesized to be maintained in nature in the white-footed mouse (Peromyscus leucopus Rafinesque), although recent data suggest that shrews may be an important reservoir host.9
The concept of natural nidality/focality (originally coined “landscape epidemiology” by Pavlovsky in 1966) is often used to explain the long-term persistence of certain vector-borne diseases in nature, including tularemia,10–12 plague,13 and TBEV.14 Natural nidality describes how certain disease pathogens can be maintained in small pockets in nature, also referred to as nidi, or foci, and are dependent on abiotic and biotic factors such as temperature, humidity, vegetation type, and reservoir hosts.10,15 These factors come together to form the “pathobiocenose” that enables the pathogen to persist long-term. Foci can range in size from as small as a nest in a treehole (“microfocus”) to larger areas such as along the border of a forest or where water meets land (“macrofocus”).10,14,16 If the foci persist over time, they are referred to as permanent or “elementary” foci and can be an “environmental reservoir” for dispersal by vectors and hosts to surrounding areas.10,12,16 In Martha’s Vineyard, MA, Francisella tularensis was found to exist in a microfocus on the island, and this also was the source of genetic diversity.12
The focal transmission of TBEV in Europe has been well described in the literature, but little is known whether similar ecological transmission patterns exist for POWV in the United States. In a 2016 and 2017 survey, POWV prevalence rates ranged from 0% to 3.5% in questing adult I. scapularis ticks collected in Maine, depending on location.17 To date, all positives have been of lineage II, or DTV. During the course of this study and subsequent years, the authors noted that DTV virus prevalence rates remained constant in ticks collected from certain areas, whereas other areas continued to be negative for the virus. This led to the current study to determine whether the theory of natural nidality holds true for DTV circulating in nature in Maine. Prior phylogenetic studies support the focality of DTV populations, with distinct virus lineages present in different sites (generally at the town level).18–21 The Wells National Estuarine Research Reserve (WNERR) was chosen as a site for this study because it is a protected reserve with diverse habitat types, and there is a longstanding data set that includes recorded flora, fauna, and environmental conditions. It is also where we previously documented high DTV infection rates in I. scapularis ticks.17 In this study, we aimed to determine if DTV prevalence in questing I. scapularis ticks differed on a small geographic scale and whether higher prevalence rates were associated with a certain habitat type. In addition, we examined the genetic diversity of the DTV genomes from our study site through phylogenetic analysis. This research may provide insight into patterns of transmission in different habitat types and potentially lead to strategies to lower human risk of exposure.
MATERIALS AND METHODS
Study site.
The WNERR in Wells, ME (43°20′18.7″N 70°33′12.2″W), comprises 9.1 km2 (2,250 acres) of protected land along Maine’s southern coast. The reserve contains diverse habitats, including a beach-dune system, freshwater and estuarine wetlands, mowed and unmown shrubby upland fields, and second-growth oak-pine forest.22 Common overstory trees are red oak (Quercus rubra Linnaeus), eastern white pine (Pinus strobus Linnaeus), red maple (Acer rubrum Linnaeus), black cherry (Prunus serotina Ehrhart), apple (Malus spp.), and red spruce (Picea rubens Sargent). Native understory shrub species include bayberry (Morella pensylvanica Mirbel) and high-bush blueberry (Vaccinium corymbosum Linnaeus). Some fields and forest stands are invaded by the nonnative shrub Japanese barberry (Berberis thunbergii de Candolle), which inhibits natural forest succession and canopy regeneration, as well as the nonnative shrub Eurasian honeysuckle (Lonicera spp.) and vine Asiatic bittersweet (Celastrus orbiculata Thunberg). At the WNERR, species documented as hosts for I. scapularis include numerous migratory songbirds, white-tailed deer (Odocoileus virginianus Zimmerman), white-footed mice (P. leucopus), northern red-backed voles (Myodes gapperi Vigors), eastern chipmunks (Tamias stiratus Linnaeus), red squirrels (Tamisciurus hudsonicus Erxleben), southern flying squirrels (Glaucomys volans Linnaeus), short-tailed shrews (Blarina brevicauda Say), and masked shrews (Sorex cinereus Kerr).23
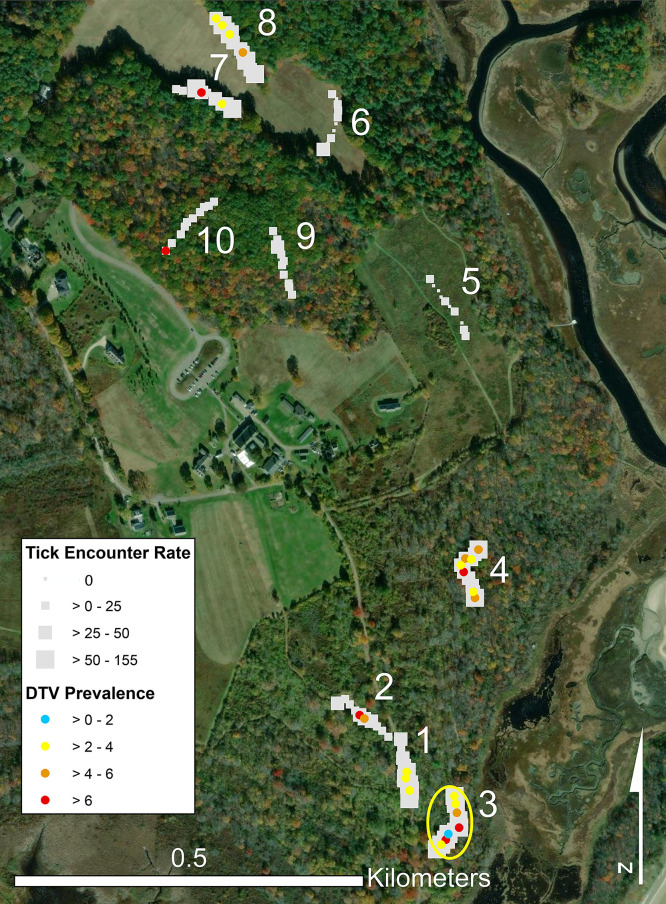
In 2018, we defined the following five different habitat types in upland forest and field portions of the reserve to describe conditions ostensibly ranging from most to least ideal for I. scapularis24–26: 1) forest with invasive vegetation: mostly closed canopy with understory dominated by dense thickets of Japanese barberry with European honeysuckle and Asiatic bittersweet sometimes present; 2) edge: where open field meets the tree line of forest stands; 3) shrub: unmown old fields dominated by grasses and forbs, with scattered cherry, apple, and hawthorn (Crataegus spp. L.) trees and native shrubs, as well as scattered nonnative Japanese barberry, Eurasian honeysuckle, and Asiatic bittersweet shrubs; 4) forest with native vegetation: mostly closed canopy with a sparse understory including native vegetation species; and 5) field: open grasslands mowed once annually. We established ten 100-m transects, two transects per habitat type (Figure 1). Each transect consisted of 10 consecutive 10-m × 1-m plots. The plot was the basic sampling unit (N = 100 plots total, with n = 20 plots per habitat type) and the basis for the hot spot analysis. We recorded latitude and longitude coordinates at plot centers using a handheld GPSMAP® 60CS× unit (Garmin, Olathe, KS).
Figure 1.
Map showing the location of transects sampled at the Wells National Estuarine Research Reserve, Wells, ME, April to November, 2018 to 2021. Squares represent the tick encounter rate on each 10-m × 10-m plot within 100-m transects: shrub (transects 1 and 2), forest with invasive vegetation (3 and 4), field (5 and 6), edge (7 and 8), and forest with native vegetation (9 and 10). DTV prevalence rates are indicated by blue, yellow, orange, and red dots. The yellow circle represents a hot spot analysis showing a focus of the elevated entomological risk index. DTV = deer tick virus.
Tick collections.
Host-seeking I. scapularis ticks were collected by the flagging technique from April through November, 2018–2021, during the active questing seasons of adults (April/May and October/November) and nymphs (June/July). Each of the 10 transects was flagged weekly, when possible, although weather (e.g., rain or drought), COVID-related staffing constraints (2020), and trail maintenance (in 2021) restricted equal sampling efforts. Transects were not flagged during the larval season because of the low numbers of adult and nymphal ticks questing at this time. Each 10-m × 1-m plot was flagged for 30 s. Ticks were transported back to the laboratory in 2-mL cryogenic vials with 0.5 mL of plaster of Paris to maintain humidity and stored at 4°C until sorted as previously described.17 Only live ticks were tested for the presence of DTV RNA.
RNA isolation and reverse transcription polymerase chain reaction.
RNA was isolated using the QIAmp® viral RNA mini kit (Qiagen, Germantown, MD) as previously described17 with one modification. Ticks were homogenized in 300 µL of 1× minimum essential medium (Gibco, Grand Island, NY) with 1× fish gelatin blocking agent (Biotium, Fremont, CA). Homogenates were immediately stored at −80°C after RNA isolation. Reverse transcription polymerase chain reaction (RT-PCR) was used to test I. scapularis ticks for the presence of DTV infection using the POW-bluef (5′AATCCTGTGTGACATCGGGG3′) and POW-bluer (5′CCAGAGCTGCGTTGGATCTC3′) primers, as previously described.17 All positive PCRs from individual ticks were confirmed by purification with the High Pure PCR product purification kit (Roche Molecular Biochemicals, Indianapolis, IN) and sequenced at the University of Maine DNA Sequencing Facility in Orono, ME.
STATISTICAL ANALYSES
We summarized the entomological data in two ways: an annual summary (pooling habitats) and a habitat summary (pooling years). For the annual summary (across habitats, N = 100 plots per year), we summarized the number of ticks collected, effort (time spent collecting), number of ticks tested, and number of ticks positive. From this, we calculated the “tick encounter rate” as the number of ticks collected per hour per plot, which adjusted for variation in sampling time. The tick encounter rate reflects the fact that the true abundance or density of ticks is not known, since flagging collects a small proportion (2–9%) of nymphal and adult ticks.27 We then calculated the annual mean tick encounter rate (i.e., number of ticks per hour). We calculated the annual DTV prevalence as the total number of ticks positive for DTV RNA by RT-PCR divided by the total tested. The “entomological risk index” (ERI) was calculated as the number of infected ticks collected per hour per plot. From this, we then calculated the mean ERI. The ERI is considered a more robust measure of human infection risk than either abundance or infection prevalence as a stand-alone measure.28 Similarly, in the ecological context of this study, the combination of tick abundance and high DTV prevalence was the more robust measure of focality rather than DTV prevalence alone, because prevalence was biased by small denominators where ticks were scarce.
For the habitat summary (across years, n = 20 plots per habitat), we summarized annual counts of adults and nymphs and collection times across sample dates for each plot by habitat type. We then calculated the mean tick encounter rate, DTV prevalence, and mean ERI among habitats. For both the annual and habitat summaries, statistical differences in mean tick encounter rate and mean ERI were tested using pairwise Wilcoxon rank sum tests and DTV prevalence using pairwise Fisher’s exact tests (tests significant at P ≤0.05 and marginally significant at 0.05 < P ≤0.10). Although transect-level comparisons were not germane to the study, we note that there were no differences in DTV prevalence or ERI between transects within any habitat type for adults and nymphs. Also, we did not focus on seasonal peaks for each life stage but rather assessed encounters with adults and nymphs over the course of the entire deer tick questing season, April through November. We used SAS 9.4 for these analyses.29
The spatial hot spot analysis assessed whether there were clusters of plots representing hot spots of risk on the basis of ERI (i.e., number of infected ticks per hour). For the hot spot analysis, we summarized across years and adults by plot and then calculated the ERI for each plot (N = 100 plots). We excluded nymphs from the analysis, since only two were DTV positive, and thus the ERI was 0 for 98 plots. At the spatial level of the 10-m × 1-m plot, ERI overcomes the influence of small denominators that inflate the DTV infection prevalence (e.g., 1 infected tick/1 collected = 100%). We used ArcGIS Desktop 10.8.230 to perform the hot spot analysis, using the Getis-Ord Gi* statistic31 to locate hot (or cool) spots with 90%, 95%, and 99% confidence. To be a statistically significant hot spot, a spatial feature (in this case a plot) with a high response value will be surrounded by other features with lower values.
Whole-genome sequencing and phylogenetic analysis.
Thirty-seven full DTV genomes were sequenced as a component of this study as previously described32 from 25 of the DTV-positive ticks from study transects 1 to 10 plus 12 positive ticks collected from grids established around transects 3 and 4 (i.e., forest with invasive vegetation) as a component of a parallel study conducted in 2020 (L. Baxter, unpublished data). These 12 positive samples were used only for phylogenetic analysis in this study to gain a more in-depth analysis of genetic diversity at the WNERR. Deer tick virus sequences obtained for this study were deposited in GenBank and assigned accession numbers listed in Supplemental Table 2. Briefly, RNA was treated with heat-labile double-stranded deoxyribonuclease (ArcticZymes, TromsØ, Norway) and converted to complementary DNA using random primers and Superscript IIITM (Invitrogen, Carlsbad, CA). Libraries were tagmented and amplified using Nextera® XT (Illumina, San Diego, CA) and were sequenced on an Illumina platform with 150-base pair paired-end reads. Reference-based assembly was performed using viral-ngs v2.0.21 software33 and reference HM440559.1. Our analysis included 51 sequences from the WNERR (37 DTV sequences generated in this study plus 14 sequences available through GenBank). In addition, 25 publicly available reference sequences from other locations in Maine were also used for analysis. These Maine DTV genome sequences were first aligned with all available reference sequences from GenBank (N = 270) to generate a maximum-likelihood phylogenetic tree. Visual inspection of this tree allowed identification of 20 reference DTV sequences from other states that either clustered with WNERR sequences or were important in separating clusters of WNERR sequences. Together with the 76 Maine sequences described above, this final set of 96 sequences was used to produce the figures shown here. All included sequences had 95% or higher coverage of the DTV coding region. Alignment of the coding region was performed using MAFFT as implemented in Geneious, and pairwise nucleotide differences were calculated in Geneious.34 Sequences with <100% coverage were excluded from pairwise calculations. Maximum-likelihood phylogenetic analysis was performed using IQ-TREE (v1.6.12) with ModelFinder and 1,000 ultrafast bootstraps.35,36 The best-fit substitution model, TIM+F+G4, was determined using the Bayesian information criterion in IQ-TREE. A maximum-likelihood tree was constructed using this model, with rate heterogeneity modeled using a gamma distribution with four categories. The tree was visualized using iTOL,37 marking nodes with >95% ultrafast bootstrap support.
RESULTS
A total of 2,576 questing nymphal and adult I. scapularis ticks were collected from the WNERR during the course of this study from 2018 to 2021 (Supplemental Table 1). Of these, 2,388 were tested for the presence of DTV RNA by RT-PCR. Of 1,964 adult deer ticks tested, 40 (2.0%) were DTV positive, with 2.1% of females (21/987) and 1.9% of males (19/977) positive (prevalence not different, χ2 test for differences in proportions, P = 0.77). Only two nymphs tested positive over the course of this study (N = 424, 0.5%).
Tick encounter rate, DTV prevalence, and ERI among years.
From 2018 through 2021, the adult deer tick encounter rate showed minor variation, ranging from 22.2 ticks collected per hour in 2018 to 35.3/hour in 2020 (Table 1). Nymphal deer tick encounter rates varied substantially, with the highest in 2019 (17.0/hour) and the lowest in 2020 (1.0/hour). There was no statistical significance in adult and nymphal DTV prevalence or ERI among years. Adult DTV prevalence was 2.0% overall (n = 40/1,964) and ranged from 1.1% in 2020 (n = 4/353) to 3.6% in 2018 (n = 15/416). Only 2 of 424 nymphs tested were DTV positive over the course of this study, both collected in 2019 (DTV prevalence, 0.9%). The ERI for adult deer ticks ranged from 0.6 (2019 and 2020) to 1.3 (2018) and was 0.2 for nymphs in 2019.
Table 1.
Tick encounter rate, DTV prevalence, and ERI for adult and nymphal Ixodes scapularis ticks collected at the Wells National Estuarine Research Reserve, Wells, ME, 2018–2021
| Stage | Year | Tick Encounter Rate*† | Prevalence‡§ | ERI†‖ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Mean (95% CI) | Significance | Total | + | % (95% CI) | Significance | Mean (95% CI) | Significance | ||
| Adult | 2018 | 443 | 22.2 (16.1–28.3) | B | 416 | 15 | 3.6 (1.8–5.4) | A¶ | 1.3 (0.4–2.1) | A |
| 2019 | 691 | 34.2 (26.1–42.3) | A | 653 | 10 | 1.5 (0.6–2.5) | A | 0.6 (0.2–1.1) | AB | |
| 2020 | 353 | 35.3 (27.4–43.2) | A | 353 | 4 | 1.1 (0.0–2.2) | A | 0.6 (0.0–1.1) | B | |
| 2021 | 551 | 34.7 (26.8–42.7) | A | 542 | 11 | 2.0 (0.8–3.2) | A | 0.9 (0.3–1.5) | AB | |
| All years | 2,038 | – | – | 1,964 | 40 | – | – | – | – | |
| Nymph | 2018 | 156 | 8.3 (5.8–10.7) | B | 138 | 0 | 0.0 | A | 0.0 | A |
| 2019 | 322 | 17.0 (11.7–22.2) | A | 226 | 2 | 0.9 (0.0–2.1) | A | 0.2 (0.0–0.5) | A | |
| 2020 | 10 | 1.0 (0.3–1.7) | D | 10 | 0 | 0.0 | A | 0.0 | A | |
| 2021 | 50 | 3.0 (2.0–4.0) | C | 50 | 0 | 0.0 | A | 0.0 | A | |
| All years | 538 | – | – | 424 | 2 | – | – | – | – | |
DTV = deer tick virus; ERI = entomological risk index.
Mean number of ticks collected/hour; N = 100 plots/year.
Same letters indicate that values are not statistically different (pairwise Wilcoxon rank sum tests, P <0.05).
Number of DTV-positive ticks/total tested.
Same letters indicate that values are not statistically different (Fisher’s exact test, P <0.05).
Mean number of infected ticks collected/hour; N = 100 plots/year.4
DTV prevalence was marginally higher in 2018 than in 2019 and 2020, both P = 0.09.
Tick encounter rate by habitat type.
The tick encounter rate (number of I. scapularis collected per hour) varied substantially among habitat types (Table 2). The adult tick encounter rate (54.6/hour) was marginally higher in forest with invasive vegetation in the understory than in edge habitat and significantly higher than in all other habitat types. Adult tick encounter rates in edge (44.1/hour) and shrub (42.6/hour) habitats were similar to that in forest with invasive vegetation but higher than those in uninvaded forest (11.8/hour) and field habitats (3.8/hour). Nymphal tick encounter rates were highest in forest with invasive vegetation (20.4/hour) and lowest in field habitat (0.4/hour) but otherwise did not align with the pattern of adult tick encounter rates (Table 2). The nymphal tick encounter rate was second highest in forest with native vegetation (12.1/hour) and third highest in edge (7.7/hour) (Table 2).
Table 2.
Tick encounter rate, DTV prevalence, and ERI for adult and nymphal Ixodes scapularis ticks collected in five habitat types at the Wells National Estuarine Research Reserve, Wells, ME, 2018–2021
| Stage | Habitat Type | Transect(s) | Tick Encounter Rate*† | Prevalence‡§ | ERI†‖ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Mean (95% CI) | Significance | Total | + | % (95% CI) | Significance | Mean (95% CI) | Significance | |||
| Adult | Forest w/invasive | 3, 4 | 764 | 54.6 (45.9, 63.2) | A¶ | 742 | 24 | 3.2 (2.0, 4.5) | A¶ | 1.7 (0.9, 2.5) | A |
| Edge | 7, 8 | 544 | 44.1 (34.4, 53.8) | A | 529 | 9 | 1.7 (0.6, 2.8) | AB | 0.7 (0.2, 1.3) | AB | |
| Shrub | 1, 2 | 546 | 42.6 (25.4, 59.8) | B | 524 | 6 | 1.1 (0.2, 2.1) | B | 0.5 (0.0, 0.9) | BC | |
| Forest w/native | 9, 10 | 137 | 11.8 (8.7, 15.0) | C | 127 | 1 | 0.8 (0.0, 2.3) | AB | 0.1 (0.0, 0.3) | C | |
| Field | 5, 6 | 47 | 3.8 (0, 8.8) | D | 42 | 0 | 0 | AB | 0 | C | |
| All habitats | – | 2,038 | – | 1,964 | 40 | – | – | – | – | ||
| Nymph | Forest w/invasive | 3, 4 | 286 | 20.4 (16.0, 24.9) | A | 230 | 1 | 0.4 (0.0, 1.3) | A | 0.1 (0.0, 0.2) | A |
| Edge | 7, 8 | 95 | 7.7 (2.9, 12.5) | C | 78 | 0 | 0 | A | 0 | A | |
| Shrub | 1, 2 | 12 | 1.0 (0.3, 1.6) | D | 8 | 0 | 0 | A | 0 | A | |
| Forest w/native | 9, 10 | 140 | 12.1 (7.7, 16.5) | B | 103 | 1 | 1.0 (0.0, 2.9) | A | 0.1 (0.0, 0.3) | A | |
| Field | 5, 6 | 5 | 0.4 (0, 0.9) | D | 5 | 0 | 0 | A | 0 | A | |
| All habitats | – | 538 | – | – | 424 | 2 | – | – | – | – | |
DTV = deer tick virus; ERI = entomological risk index; Forest w/invasive = forest with invasive vegetation in the understory; Forest w/native = forest with native vegetation in the understory.
Mean number of ticks collected/hour; n = 20 plots/habitat.
Same letters indicate that values are not statistically different (pairwise Wilcoxon rank sum tests, P <0.05).
Number of DTV-positive ticks/total tested.
Same letters indicate that values are not statistically different (Fisher’s exact test, P <0.05).
Mean number of infected ticks collected/hour; n = 20 plots/habitat.
Mean ticks/hour and DTV prevalence in “forest w/invasive” were marginally greater than those in edge, P = 0.06 and 0.09, respectively.
DTV prevalence by habitat type.
In forest with invasive vegetation, adult DTV prevalence (3.2%, n = 24/742) was marginally higher than that of adults in edge (1.7% (n = 9/529) and significantly higher than in shrub (1.1% (n = 6/524)) (Table 2). Adult DTV prevalences in forest with native vegetation and field appeared lower, at 0.8% (n = 1/127) and 0% (n = 0/42), respectively, but were not statistically differentiated from those in the other habitats, because sample sizes were low by virtue of the presence of relatively few ticks in forest with native vegetation and field. This highlights the difficulty of obtaining adequate power to detect statistical differences when low tick abundance is combined with low pathogen prevalence.38 Only two nymphs tested positive by RT-PCR over the course of this study—one was from forest with invasive vegetation (0.4%, n = 1/230) and the other was from forest with native vegetation (1.0%, n = 1/103).
Entomological risk index by habitat type.
The adult ERI was marginally higher in forest with invasive vegetation than in edge habitat (1.7 versus 0.7 DTV-infected ticks collected per hour, P = 0.07) but significantly higher than that in all other habitats: shrub (0.5/hour), forest with native vegetation (0.1/hour), and field (0/hour) (Table 2). Because of the very low nymphal infection prevalence among habitats (n = 2, 0–1%), there were no differences in ERI among habitats for nymphs.
Hot spot analysis using the ERI.
The hot spot analysis searched for foci of potentially elevated human exposure to tick bites across the I. scapularis questing season from April to November. Using the ERI (infected ticks collected per hour), the hot spot analysis revealed one hot spot (95–99% confidence) on transect 3, which ran through forest with invasive vegetation in the understory (Figure 1). Within the transect 3 focus, the ERI ranged from 0 to 5.5 DTV-infected ticks per hour across plots 1–8. This showed that a plot could have had zero infected ticks but still be part of a focus if enough neighbors had high ERIs. All plots along transect 3 were infested by Japanese barberry, and five of the eight plots in transect 3 had Asiatic bittersweet.
Phylogenetic analysis of DTV at the WNERR.
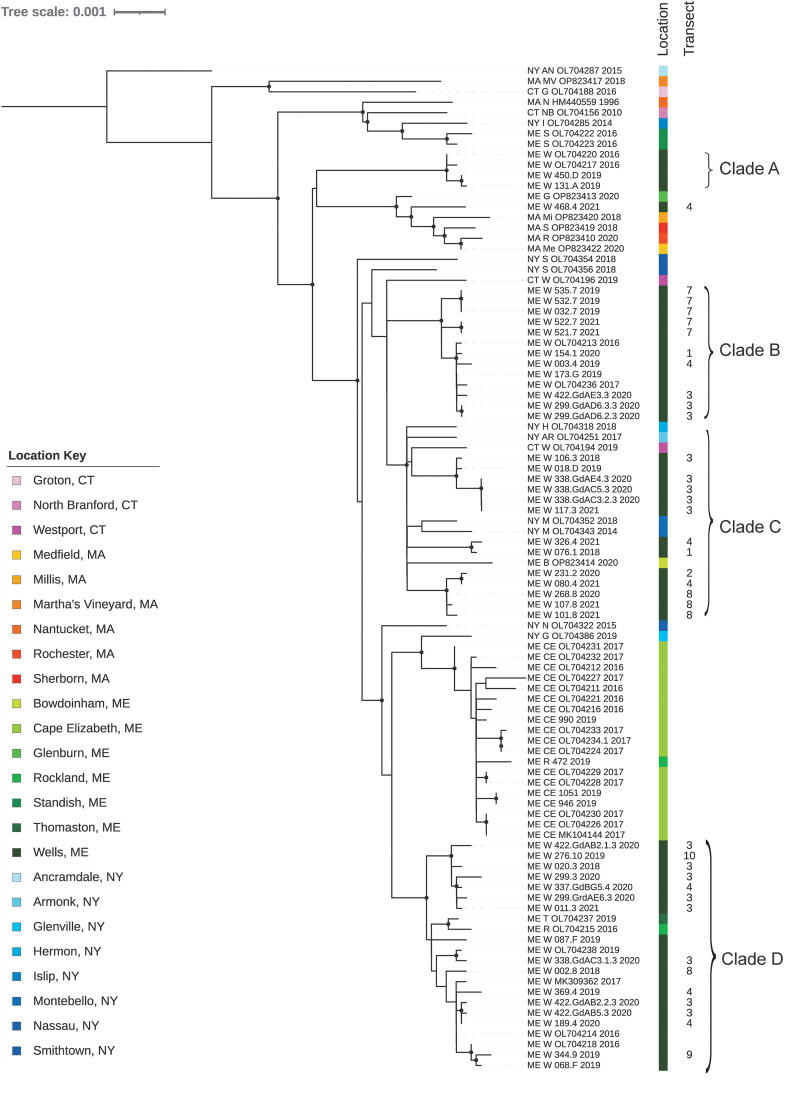
We analyzed 25 full-length DTV genome sequences from ticks collected in study transects between 2018 and 2021, 12 ticks collected from grids surrounding transects 3 and 4, 14 previously published sequences from other areas at the WNERR, 25 ticks collected at other locations in Maine, and 20 publicly available reference sequences (Supplemental Table 2). Phylogenetic analysis demonstrated that most sequences from the WNERR belonged to one of four clades, each of which was supported by >95% ultrafast bootstrap support and contained at least two WNERR sequences; these clades were separated from one another by DTV sequences from other cities and states (Figure 2). Clade A was composed of four samples collected from the WNERR between 2016 and 2019; these sequences differed from one another by an average of only 3.5 nucleotides (0.03%), and the clade was most closely related to sequences from Massachusetts. Clade B was composed of 12 samples collected from the WNERR between 2016 and 2021 whose sequences differed from one another by an average of 6.3 nucleotides (0.06%). Clade C was composed of 15 WNERR samples collected between 2018 and 2021, 4 samples from New York (2014–2018), 1 sample from Bowdoinham, ME (2020), and 1 sample from Connecticut (2019). Sequences within this clade differed from one another by an average of 32.4 nucleotides (0.29%), and notably there were three subclades of WNERR-only samples within this clade, each of which spanned multiple years. Clade D was composed of 21 samples from the WNERR collected between 2016 and 2021, one sample from Rockland, ME (2016), and one sample from Thomaston, ME (2019). Sequences within clade D differed from one another by an average of 15.3 nucleotides (0.14%), and clade D was most closely related to sequences from Cape Elizabeth, ME. Thus, overall, we identified four genetically distinct DTV clades at the WNERR, each of which persisted across multiple years. Two of the clades contained sequences from different cities and states, suggesting the dispersal of closely related viruses to and/or from the WNERR.
Figure 2.
Maximum-likelihood phylogenetic analysis demonstrating four primary deer tick virus clades at the Wells National Estuarine Research Reserve, Wells, ME. Tips are labeled by state, city, unique identifier, and year. Transect numbers are indicated as shown in Figure 1. City and state are color coded as shown in the location key. Major clades are labeled. Nodes with at least 95% ultrafast bootstrap support are marked with black circles.
Notably, the identified DTV hot spot (transect 3) within the WNERR contained genetically diverse viruses; the 16 samples from transect 3 differed from one another by an average of 52 nucleotides (0.45%) and were distributed across phylogenetic clades B, C, and D. In addition, the seven samples from neighboring transect 4 (also forest with invasive vegetation) differed from one another by an average of 42 nucleotides (0.41%), were distributed across clades B, C, and D, and also contained a unique sequence that phylogenetically clustered with samples from Massachusetts (ME_W_468.4_2021). More broadly, sequences from the same transect did not cluster together on the phylogenetic tree, with the exception of a few sets of identical or near-identical sequences from the same transect.
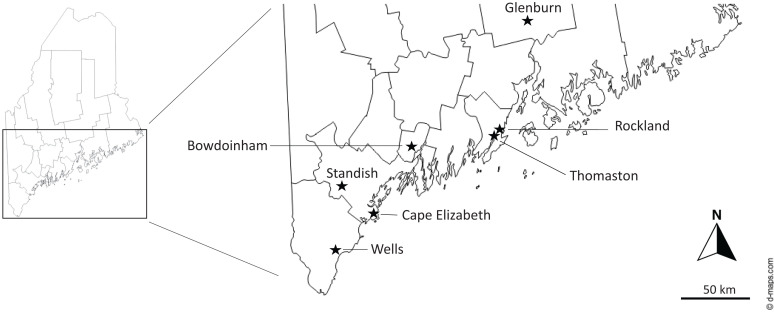
On a broader geographic scale, we observed several patterns of DTV diversity within Maine. Sequences from the coastal cities of Rockland and Thomaston clustered within the WNERR clade D, despite a geographic distance of ∼100 miles north of the WNERR (Figure 3).39 By contrast, DTV sequences from nearby Cape Elizabeth (∼30 miles north) formed an independent cluster that was more closely related to sequences from Massachusetts and New York than to the WNERR sequences. Deer tick virus sequences from the inland city of Standish, ME (northwest), were distantly related to other Maine sequences and clustered with New York, Connecticut, and Massachusetts sequences.
Figure 3.
Location of tick collection sites (★) in Maine.
DISCUSSION
Although natural foci for DTV have been referred to in the literature,40,41 the results presented here provide additional clues characterizing the multifactorial pathobiocenose (i.e., the associations of pathogen, flora, and fauna) of DTV in nature. Taken together, the DTV prevalence, ERI, and hot spot analysis provide preliminary support for the idea that DTV is focal and habitat associated and that the risk of encountering DTV-infected ticks in forest invaded by Japanese barberry and Eurasian honeysuckle may be approximately twice that of natural habitats. Research has shown that Japanese barberry is an ideal habitat type for I. scapularis,25,26 as it provides protection to small mammals from predators as well as higher humidity in the leaf litter for optimal tick survival. A higher tick density coupled with protection for small rodents may create an ideal habitat type for a DTV to propagate, which can then spill over to other environments.
Further research is needed to determine if this hot spot demonstrates a well-defined focus at the macro or microlevel at the WNERR. Low DTV prevalence rates in combination with transect sampling design (as opposed to a grid design) limited our ability to determine if this focus was specific to a single transect or contiguous with bordering similar or different habitats. Although the use of mean ERI (infected ticks per hour) across the entire collection season for hot spot analysis allowed adjustment for sampling effort, it may underestimate the risk for each life stage during the ticks’ peak questing season (e.g., one might expect a higher adult ERI in spring and fall than in summer). In addition, the mean ERI measures the risk of a human being bitten by an infected tick and does not take into account other mechanisms of DTV persistence in nature. Therefore, other measures of DTV activity [e.g., small mammal seroprevalence, DTV persistence within a reservoir host(s), etc.] would help better define this focus, as would future studies looking at seasonal differences in DTV prevalence in questing ticks. It is likely, however, that forest with invasive vegetation is an ideal habitat for a DTV focus. In Central Europe, TBEV foci were also associated with a high population density of ticks and mammals, as well as a relatively humid environment with a well-developed layer of forest litter.42 Further research is needed to better delineate the extent of the DTV focus at the WNERR, as well as whether other areas in Maine show similar patterns of high DTV prevalence rates associated with forested areas with invasive vegetation in the understory.
Although the structure of TBEV foci has been well documented in the literature,14,42 little is known about the ecology of DTV and how it is perpetuated in nature. Because I. scapularis larval and nymphal stages readily feed on P. leucopus, the primary reservoir host for Borrelia burgdorferi in nature, it has been predicted that the white-footed mouse is also a reservoir host for DTV.40,43 However, unlike with B. burgdorferi, this study as well as previous research suggests that DTV is focally maintained in nature,18,19 thus suggesting that a different host(s) may act as a primary reservoir(s). Studies attempting to detect DTV from I. scapularis ticks collected from wild-caught P. leucopus mice have thus far resulted in negative tests for DTV,40,44 including for ticks collected from 297 wild-caught P. leucopus mice from our DTV hot spot in Wells, ME (L. Baxter, unpublished data). In a recent study using retrotransposon blood meal analysis, I. scapularis nymphs that were positive for DTV RNA showed evidence for having fed on a shrew (likely Blarina or Sorex spp.) during the larval stage.9 Although the implication for shrews as a potential reservoir host for DTV needs further investigation, small burrowing rodents (including several species of vole) have also been implicated as the primary reservoir host for TBEV in Europe because of their high and often prolonged viremia.45–48 There is a need to determine whether perpetuation of DTV in North America follows transmission patterns similar to those of TBEV in Europe, i.e., existing in natural foci in nature with small, burrowing rodents as the primary reservoir host.
The two transects with high DTV prevalence rates at the WNERR (i.e., in forest with invasive vegetation) contained a genetically diverse set of viruses, all belonging to the Northeast sublineage within DTV (lineage II). Viruses from four distinct phylogenetic clades (A–D) were distributed between the two transects and the other transects in this study site, suggesting frequent dispersal between nearby locations. This differs from focal genetic homogeneity that has been described on a larger geographic scale in prior studies.18–21 Here, analyzing sequences from well-characterized nearby transects allowed us to capture dispersal across short distances (<1 mile), likely involving larval and nymphal ticks and small mammal hosts. Dispersal of female ticks feeding on deer, the main reproductive host, provides another mechanism for local dispersal of DTV, as vertical transmission has been demonstrated.49 Our results also indicate long-distance dispersal of DTV, with clustering of sequences from other Maine cities and other states (New York and Connecticut) within and between WNERR clades. This has been reported to a lesser extent in other studies,20,21 and further work is needed to investigate the frequency and mechanisms of long-distance dispersal, e.g., by avian hosts or vertical transmission as described above.
The identification of higher DTV prevalence rates (and a possible DTV focus) in a forested area with dense invasive vegetation (e.g., Japanese barberry and honeysuckle) in the understory at the WNERR will allow us to probe deeper into environmental factors (i.e., the pathobiocenose) that enable the virus to persist long term in the environment. Prior research has described how invasive vegetation provides an ideal habitat for ticks due to high humidity and an ample population of small mammals for feeding.25,26 These three factors (high humidity and dense populations of ticks and mammals) have been shown to be important in maintaining TBEV microfoci in Europe42 and appear to be important in maintaining DTV foci at the WNERR in Wells, ME, as well. There is a current need to better characterize the reservoir mammalian hosts that are important in maintaining the DTV transmission cycle in nature. The recent development of blood meal analysis assays50 will provide a useful tool in taking this next step, as well as more field and controlled laboratory studies looking at vertical transmission and seroprevalence rates in small mammals. Additional genetics studies are also warranted to determine the role small mammals and avian hosts have in the short- and long-distance dispersal of the virus, how this impacts the genetic diversity of the virus, and how these factors form the pathobiocenose.
Supplemental Materials
ACKNOWLEDGMENTS
We thank the following seasonal staff for assistance with field collections on this project: Jake Angelico, Henry Becker, Ella Buchanan, Ben Clark, Elizabeth Copeland, Mer Feero, Ina Guzja, Hannah Richelieu, Amanda Waugh, Keenan Ernste, and Kyle Timmer. We also thank Gregory Ebel (Colorado State University) and Heidi Goethert (Tufts University) for providing us with feedback on the manuscript.
Note: Supplemental material appears at www.ajtmh.org.
REFERENCES
- 1.Zilber LA, 1939. Spring epidemic tick-borne encephalitis. Arch Biol Sci 56: 9–37. [Google Scholar]
- 2.Zoblin VI, Pogodina VV, Kahl O, 2017. A brief history of the discovery of tick-borne encephalitis virus in the late 1930s (based on reminiscences of members of the expeditions, their colleagues, and relatives). Ticks Tick Borne Dis 8: 813–820. [DOI] [PubMed] [Google Scholar]
- 3.Beasley DW, Suderman MT, Holbrook MR, Barrett AD, 2001. Nucleotide sequencing and serological evidence that the recently recognized deer tick virus is a genotype of Powassan virus. Virus Res 79: 81–89. [DOI] [PubMed] [Google Scholar]
- 4.Kuno G, Artsob H, Karabatsos N, Tsuchiya KR, Chang GJ, 2001. Genomic sequencing of deer tick virus and phylogeny of Powassan-related viruses of North America. Am J Trop Med Hyg 65: 671–676. [DOI] [PubMed] [Google Scholar]
- 5.McLean DM, Larke RP, 1963. Powassan and Silverwater viruses: Ecology of two Ontario arboviruses. Can Med Assoc J 88: 182–185. [PMC free article] [PubMed] [Google Scholar]
- 6.McLean DM, Cobb C, Gooderham SE, Smart CA, Wilson AG, Wilson WE, 1967. Powassan virus: Persistence of virus activity during 1966. Can Med Assoc J 96: 660–664. [PMC free article] [PubMed] [Google Scholar]
- 7.Main AJ, Carey AB, Downs WG, 1979. Powassan virus in Ixodes cookei and Mustelidae in New England. J Wildl Dis 15: 585–591. [DOI] [PubMed] [Google Scholar]
- 8.Telford SR, 3rd, Armstrong PM, Katavolos P, Foppa I, Garcia AS, Wilson ML, Spielman A, 1997. A new tick-borne encephalitis-like virus infecting New England deer ticks, Ixodes dammini. Emerg Infect Dis 3: 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goethert HK, Mather TN, Johnson RW, Telford SR, 3rd, 2021. Incrimination of shrews as a reservoir for Powassan virus. Commun Biol 4: 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pavlovsky EN, 1966. Natural Nidality of Transmissible Diseases, with Special Reference to the Landscape Epidemiology of Zooanthroponse. Urbana, IL: University of Illinois Press. [Google Scholar]
- 11.Pollitzer R, 1967. History and Incidence of Tularemia in the Soviet Union. Bronx, NY: Fordham University, Institute of Contemporary Russian Studies. [Google Scholar]
- 12.Goethert HK, Telford SR, 3rd, 2009. Nonrandom distribution of vector ticks (Dermacentor variabilis) infected by Francisella tularensis. PLoS Pathog 5: e1000319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson BC, 1980. Plague Studies in California—The Roles of Various Species of Sylvatic Rodents in Plague Ecology in California. Proceedings of the Vertebrate Pest Conference March 4–6, 1980: Hilton Hotel, Fresno, CA. [Google Scholar]
- 14.Blaskovic D, Nosek J, 1972. The ecological approach to the study of tick-borne encephalitis. Prog Med Virol 14: 275–320. [PubMed] [Google Scholar]
- 15.Emmanuel NN, Loha N, Ojogba OM, Ikenna OK, 2011. Landscape epidemiology: An emerging perspective in the mapping and modeling of disease and disease risk factors. Asian Pac J Trop Dis 1: 247–250. [Google Scholar]
- 16.Audy JR, 1958. The localization of disease with special reference to the zoonoses. Trans R Soc Trop Med Hyg 52: 308–328. [DOI] [PubMed] [Google Scholar]
- 17.Robich RM, Cosenza DS, Elias SP, Henderson EF, Lubelczyk CB, Welch M, Smith RP, 2019. Prevalence and genetic characterization of deer tick virus (Powassan virus, lineage II) in Ixodes scapularis ticks collected in Maine. Am J Trop Med Hyg 101: 467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pesko KN, Torres-Perez F, Hjelle BL, Ebel GD, 2010. Molecular epidemiology of Powassan virus in North America. J Gen Virol 91: 2698–2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson JF, Armstrong PM, 2012. Prevalence and genetic characterization of Powassan virus strains infecting Ixodes scapularis in Connecticut. Am J Trop Med Hyg 87: 754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMinn RJ, et al. , 2023. Phylodynamics of deer tick virus in North America. Virus Evol 9: vead008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogels CBF, et al. , 2023. Phylogeographic reconstruction of the emergence and spread of Powassan virus in the northeastern United States. Proc Natl Acad Sci USA 120: e2218012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells Reserve at Laudholm These 2,250 Acres. Available at: https://www.wellsreserve.org/conservation/these-2250-acres. Accessed May 17, 2023.
- 23.Dionne M, Dalton C, Wilhelm H. (ed), 2006. Site Profile of the Wells National Estuarine Research Reserve. Wells, ME: Wells National Estuarine Research Reserve. [Google Scholar]
- 24.Lubelczyk CB, Elias SL, Rand PW, Holman MS, LaCombe EH, Smith RP, Jr., 2004. Habitat associations of Ixodes scapularis (Acari: Ixodidae) in Maine. Environ Entomol 33: 900–906. [Google Scholar]
- 25.Elias SP, Lubelczyk CB, Rand PW, Lacombe EH, Holman MS, Smith RP, Jr., 2006. Deer browse resistant exotic-invasive understory: An indicator of elevated human risk of exposure to Ixodes scapularis (Acari: Ixodidae) in southern coastal Maine woodlands. J Med Entomol 43: 1142–1152. [DOI] [PubMed] [Google Scholar]
- 26.Williams SC, Ward JS, 2010. Effects of Japanese barberry (Ranunculales: Berberidaceae) removal and resulting microclimatic changes on Ixodes scapularis (Acari: Ixodidae) abundances in Connecticut, USA. Environ Entomol 39: 1911–1921. [DOI] [PubMed] [Google Scholar]
- 27.Daniels TJ, Falco RC, Fish D, 2000. Estimating population size and drag sampling efficiency for the blacklegged tick (Acari: Ixodidae). J Med Entomol 37: 357–363. [DOI] [PubMed] [Google Scholar]
- 28.Eisen L, Eisen RJ, 2016. Critical evaluation of the linkage between tick-based risk measures and the occurrence of Lyme disease cases. J Med Entomol 53: 1050–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SAS Institute, Inc, 2023. SAS OnlineDoc 9.4. Available at: https://support.sas.com/en/documentation.html. Accessed August 4, 2022.
- 30.ESRI, 2023. ArcGIS Desktop. Available at: https://desktop.arcgis.com/. Accessed February 9, 2024.
- 31.Getis A, Ord JK, 1992. The analysis of spatial association by use of distance statistics. Geogr Anal 24: 189–206. [Google Scholar]
- 32.Normandin E, Solomon IH, Zamirpour S, Lemieux J, Freije CA, Mukerji SS, Tomkins-Tinch C, Park D, Sabeti PC, Piantadosi A, 2020. Powassan virus neuropathology and genomic diversity in patients with fatal encephalitis. Open Forum Infect Dis 7: ofaa392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Broadinstitute/Viral-PipelinesAvailable at: https://github.com/broadinstitute/viral-pipelines. Accessed May 17, 2017.
- 34.Geneious by Dotmatics, 2023. MAFFT. Available at: https://www.geneious.com/. Accessed September 20, 2023.
- 35.Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ, 2015. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol 32: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS, 2017. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat Methods 14: 587–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Letunic I, Bork P, 2007. Interactive Tree Of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 23: 127–128. [DOI] [PubMed] [Google Scholar]
- 38.Kilpatrick AM, et al. , 2017. Lyme disease ecology in a changing world: Consensus, uncertainty and critical gaps for improving control. Philos Trans R Soc Lond B Biol Sci 372: 20160117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.d-mapsAvailable at: https://d-maps.com/m/america/usa/maine/maine/maine52.gif. Accessed September 8, 2023.
- 40.Hassett EM, Thangamani S, 2021. Ecology of Powassan virus in the United States. Microorganisms 9: 2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brackney DE, Nofchissey RA, Fitzpatrick KA, Brown IK, Ebel GD, 2008. Stable prevalence of Powassan virus in Ixodes scapularis in a northern Wisconsin focus. Am J Trop Med Hyg 79: 971–973. [PMC free article] [PubMed] [Google Scholar]
- 42.Nosek J, Kožuch O, Grulich I, 1970. The structure of tick-borne encephalitis (TBE) foci in Central Europe. Oecologia 5: 61–73. [DOI] [PubMed] [Google Scholar]
- 43.Ebel GD, Campbell E, Goethert HK, Spielman A, Telford SR, 2000. Enzootic transmission of deer tick virus in New England and Wisconsin sites. Am J Trop Med Hyg 63: 36–42. [DOI] [PubMed] [Google Scholar]
- 44.Dupuis AP, 2nd, Peters RJ, Prusinski MA, Falco RC, Ostfeld RS, Kramer LD, 2013. Isolation of deer tick virus (Powassan virus, lineage II) from Ixodes scapularis and detection of antibody in vertebrate hosts sampled in the Hudson Valley, New York State. Parasit Vectors 6: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakhvalova VN, Dobrotvorsky AK, Panov VV, Matveeva VA, Tkachev SE, Morozova OV, 2006. Natural tick-borne encephalitis virus infection among wild small mammals in the southeastern part of western Siberia, Russia. Vector Borne Zoonotic Dis 6: 32–41. [DOI] [PubMed] [Google Scholar]
- 46.Bakhvalova VN, Potapova OF, Panov VV, Morozova OV, 2009. Vertical transmission of tick-borne encephalitis virus between generations of adapted reservoir small rodents. Virus Res 140: 172–178. [DOI] [PubMed] [Google Scholar]
- 47.Tonteri E, Kipar A, Voutilainen L, Vene S, Vaheri A, Vapalahti O, Lundkvist A, 2013. The three subtypes of tick-borne encephalitis virus induce encephalitis in a natural host, the bank vole (Myodes glareolus). PLoS One 8: e81214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valarcher JF, Hägglund S, Juremalm M, Blomqvist G, Renström L, Zohari S, Leijon M, Chirico J, 2015. Tick-borne encephalitis. Rev Sci Tech Off Int Epiz 34: 453–466. [DOI] [PubMed] [Google Scholar]
- 49.Lange RE, Prusinski MA, Dupuis AP, Ciota AT, 2024. Direct evidence of Powassan virus vertical transmission in Ixodes scapularis in nature. Viruses 16: 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goethert HK, Mather TN, Buchthal J, Telford SR, 2021. Retrotransposon-based blood meal analysis of nymphal deer ticks demonstrates spatiotemporal diversity of Borrelia burgdorferi and Babesia microti reservoirs. Appl Environ Microbiol 87: e02370-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.