Abstract
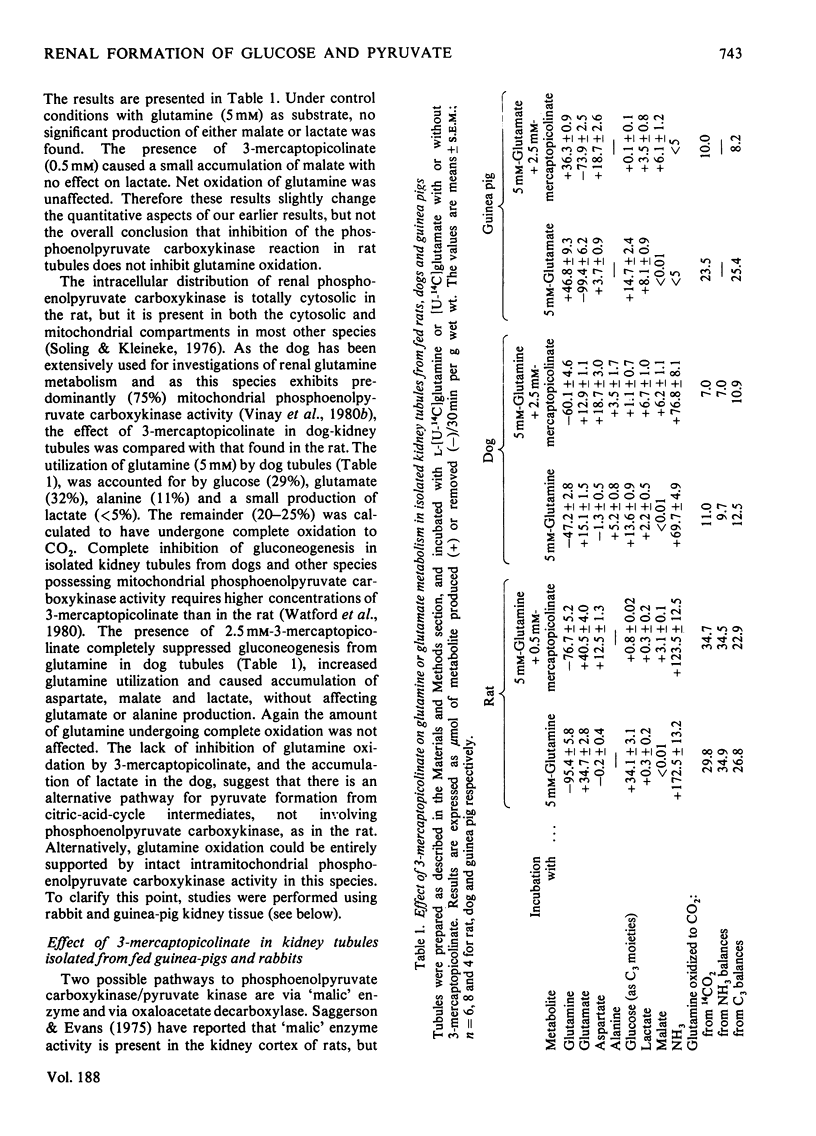
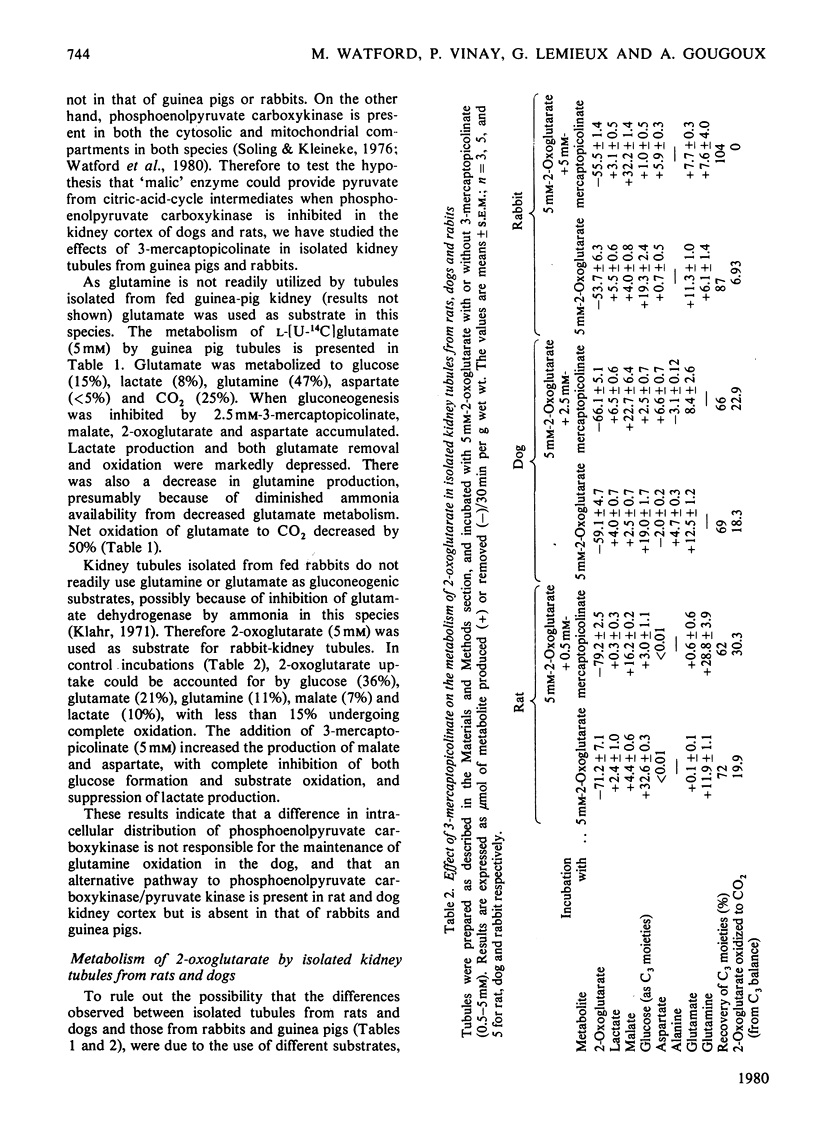
The suppression by 3-mercaptopicolinate of gluconeogenesis from glutamine or 2-oxoglutarate in rat or dog kidney tubules did not affect the amount of these substrates undergoing complete oxidation. Furthermore, 3-mercaptopicolinate caused an accumulation of lactate in dog tubules. 3-Mercaptopicolinate abolished both gluconeogenesis and substrate oxidation in tubules from rabbit and guinea-pig kidney. These results imply the presence of an alternative pathway to phosphoenolpyruvate carboxykinase/pyruvate kinase for the production of pyruvate from citric-acid-cycle intermediates in the kidney cortex of rats and dogs but not in that of rabbits or guinea pigs. Oxaloacetate decarboxylase (present in the kidney cortex of all four species) or 'malic' enzyme (present in rat and dog but absent in rabbit and guinea-pig kidney cortex) could function in this role. Our observations indicate that 'malic' enzyme is probably implicated in this phenomenon. The lactate production observed in dog tubules in the presence of 3-mercaptopicolinate can be suppressed when aspartate formation is inhibited by 2-amino-4-methoxy-trans-but-3-enoic acid. This suggests that the provision of cytosolic NADH from citric-acid-cycle intermediates is facilitated by accumulation of aspartate acting as a 'sink' for cytosolic oxaloacetate.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brdiczka D., Pette D. Intra- and extramitochondrial isozymes of (NADP) malate dehydrogenase. Eur J Biochem. 1971 Apr 30;19(4):546–551. doi: 10.1111/j.1432-1033.1971.tb01347.x. [DOI] [PubMed] [Google Scholar]
- Colombo G., Carlson G. M., Lardy H. A. Phosphoenolpyruvate carboxykinase (guanosine triphosphate) from rat liver cytosol. Separation of homogeneous forms of the enzyme with high and low activity by chromatography on agarose-hexane-guanosine triphosphate. Biochemistry. 1978 Dec 12;17(25):5321–5329. doi: 10.1021/bi00618a001. [DOI] [PubMed] [Google Scholar]
- Creighton D. J., Rose I. A. Oxalacetate decarboxylase activity in muscle is due to pyruvate kinase. J Biol Chem. 1976 Jan 10;251(1):69–72. [PubMed] [Google Scholar]
- Creighton D. J., Rose I. A. Studies on the mechanism and stereochemical properties of the oxalacetate decarboxylase activity of pyruvate kinase. J Biol Chem. 1976 Jan 10;251(1):61–68. [PubMed] [Google Scholar]
- Crow K. E., Cornell N. W., Veech R. L. Lactate-stimulated ethanol oxidation in isolated rat hepatocytes. Biochem J. 1978 Apr 15;172(1):29–36. doi: 10.1042/bj1720029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean B., Bartley W. Oxaloacetate decarboxylases of rat liver. Biochem J. 1973 Dec;135(4):667–672. doi: 10.1042/bj1350667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dies F., Lotspeich W. D. Hexose monophosphate shunt in the kidney during acid-base and electrolyte imbalance. Am J Physiol. 1967 Jan;212(1):61–71. doi: 10.1152/ajplegacy.1967.212.1.61. [DOI] [PubMed] [Google Scholar]
- Elliott K. R., Pogson C. I. The effects of starvation and experimental diabetes on phosphoenol-pyruvate carboxykinase in the guinea pig. Biochem J. 1977 May 15;164(2):357–361. doi: 10.1042/bj1640357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Brosnan J. T. Effects of metabolic acidosis and starvation on the content of intermediary metabolites in rat kidney. Biochem J. 1971 Jul;123(3):391–397. doi: 10.1042/bj1230391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomain-Baum M., Schramm V. L., Hanson R. W. Mechanism of 3-mercaptopicolinic acid inhibition of hepatic phosphoenolpyruvate carboxykinase (GTP). J Biol Chem. 1976 Jan 10;251(1):37–44. [PubMed] [Google Scholar]
- Jomain-Baum M., Schramm V. L. Kinetic mechanism of phosphoenolpyruvate carboxykinase (GTP) from rat liver cytosol. Product inhibition, isotope exchange at equilibrium, and partial reactions. J Biol Chem. 1978 May 25;253(10):3648–3659. [PubMed] [Google Scholar]
- Jursinic S. B., Robinson J. L. The active site of rabbit muscle pyruvate kinase. Evidence for a site common to the oxalacetate decarboxylase and pyruvate kinase reactions. Biochim Biophys Acta. 1978 Apr 12;523(2):358–367. doi: 10.1016/0005-2744(78)90038-4. [DOI] [PubMed] [Google Scholar]
- Klahr S. Relation of renal gluconeogenesis to ammonia production in the rabbit. Am J Physiol. 1971 Jul;221(1):69–74. doi: 10.1152/ajplegacy.1971.221.1.69. [DOI] [PubMed] [Google Scholar]
- Kostos V., DiTullio N. W., Rush J., Cieslinski L., Saunders H. L. The effect of 3-mercaptopicolinic acid on phosphoenolpyruvate carboxykinase (GTP) in the rat and guinea pig. Arch Biochem Biophys. 1975 Dec;171(2):459–465. doi: 10.1016/0003-9861(75)90054-5. [DOI] [PubMed] [Google Scholar]
- Lin R. C., Davis E. J. Malic enzymes of rabbit heart mitochondria. Separation and comparison of some characteristics of a nicotinamide adenine dinucleotide-preferring and a nicotinamide adenine dinucleotide phosphate-specific enzyme. J Biol Chem. 1974 Jun 25;249(12):3867–3875. [PubMed] [Google Scholar]
- Lopes-Cardozo M., van den Bergh S. G. Ketogenesis in isolated rat-liver mitochondria. IV. Oxaloacetate decarboxylation: consequences for metabolic calculations. Biochim Biophys Acta. 1974 Aug 23;357(2):193–203. doi: 10.1016/0005-2728(74)90060-7. [DOI] [PubMed] [Google Scholar]
- Mandella R. D., Sauer L. A. The mitochondrial malic enzymes. I. Submitochondrial localization and purification and properties of the NAD(P)+-dependent enzyme from adrenal cortex. J Biol Chem. 1975 Aug 10;250(15):5877–5884. [PubMed] [Google Scholar]
- Meijer A. J., Gimpel J. A., Deleeuw G., Tischler M. E., Tager J. M., Williamson J. R. Interrelationships between gluconeogenesis and ureogenesis in isolated hepatocytes. J Biol Chem. 1978 Apr 10;253(7):2308–2320. [PubMed] [Google Scholar]
- Meijer A. J., Van Dam K. The metabolic significance of anion transport in mitochondria. Biochim Biophys Acta. 1974 Dec 30;346(3-4):213–244. doi: 10.1016/0304-4173(74)90001-9. [DOI] [PubMed] [Google Scholar]
- Pogson C. I., Wolfe R. G. Oxaloacetic acid. Tautomeric and hydrated forms in solution. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1048–1054. doi: 10.1016/s0006-291x(72)80078-0. [DOI] [PubMed] [Google Scholar]
- Rando R. R., Relyea N., Cheng L. Mechanism of the irreversible inhibition of aspartate aminotransferase by the bacterial toxin L-2-amino-4-methoxy-trans-3-butenoic acid. J Biol Chem. 1976 Jun 10;251(11):3306–3312. [PubMed] [Google Scholar]
- Richards T. C., Knox W. E. The distribution of malate NADP dehydrogenase in adult, fetal and neoplastic tissues of the rat. Enzyme. 1972;13(5-6):320–323. doi: 10.1159/000459680. [DOI] [PubMed] [Google Scholar]
- Robinson B. H., Oei J. 3-Mercaptopicolinic acid, a preferential inhibitor of the cytosolic phosphoenolpyruvate carboxykinase. FEBS Lett. 1975 Oct 15;58(1):12–15. doi: 10.1016/0014-5793(75)80214-6. [DOI] [PubMed] [Google Scholar]
- Rognstad R. Pyruvate cycling involving possible oxaloacetate decarboxylase activity. Biochim Biophys Acta. 1979 Aug 22;586(2):242–249. doi: 10.1016/0304-4165(79)90096-5. [DOI] [PubMed] [Google Scholar]
- Ross B. D. Effect of inhibition of gluconeogenesis on ammonia production in the perfused rat kidney. Clin Sci Mol Med. 1976 Jun;50(6):493–498. doi: 10.1042/cs0500493. [DOI] [PubMed] [Google Scholar]
- Saggerson D., Evans C. J. The activities and intracellular distribution of nicotinamide-adenine dinucleotide phosphate-malate dehydrogenase, phosphoenolpyruvate carboxykinase and pyruvate carboxylase in rat, guinea-pig and rabbit tissues. Biochem J. 1975 Feb;146(2):329–332. doi: 10.1042/bj1460329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimerlik M. I., Cleland W. W. Inhibition and alternate-substrate studies on the mechanism of malic enzyme. Biochemistry. 1977 Feb 22;16(4):565–570. doi: 10.1021/bi00623a001. [DOI] [PubMed] [Google Scholar]
- Smith S. B., Briggs S., Triebwasser K. C., Freedland R. A. Re-evaluation of amino-oxyacetate as an inhibitor. Biochem J. 1977 Feb 15;162(2):453–455. doi: 10.1042/bj1620453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. J., Frenkel R. Dietary induction of hepatic malic enzyme activity: differentiation of the induction process. Life Sci. 1974 Apr 16;14(8):1563–1575. doi: 10.1016/0024-3205(74)90167-2. [DOI] [PubMed] [Google Scholar]
- Stark M. J., Thompson B., Frenkel R. Possible alternative functions of rat liver malic enzyme. Arch Biochem Biophys. 1975 Jan;166(1):174–180. doi: 10.1016/0003-9861(75)90377-x. [DOI] [PubMed] [Google Scholar]
- Tsoncheva A. V. O nekotorykh svoistvakh izofermentov NADP-malatdegidrogenazy korkovogo sloia pochek krysy. Biokhimiia. 1974 Nov-Dec;39(6):1172–1178. [PubMed] [Google Scholar]
- WALLACH D. P. The inhibition of gamma aminobutyric-alpha-ketoglutaric acid transaminase in vitro and in vivo by amino-oxyacetic acid. Biochem Pharmacol. 1960 Oct;5:166–167. doi: 10.1016/0006-2952(60)90019-8. [DOI] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- Watford M., Lund P., Krebs H. A. Isolation and metabolic characteristics of rat and chicken enterocytes. Biochem J. 1979 Mar 15;178(3):589–596. doi: 10.1042/bj1780589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watford M., Vinay P., Lemieux G., Gougoux A. The formation of pyruvate from citric acid-cycle intermediates in kidney cortex [proceedings]. Biochem Soc Trans. 1979 Aug;7(4):753–755. doi: 10.1042/bst0070753. [DOI] [PubMed] [Google Scholar]
- Wojtczak A. B., Walajtys E. Mitochondrial oxaloacetate decarboxylase from rat liver. Biochim Biophys Acta. 1974 May 22;347(2):168–182. doi: 10.1016/0005-2728(74)90042-5. [DOI] [PubMed] [Google Scholar]
- YOUNG J. W., SHRAGO E., LARDY H. A. METABOLIC CONTROL OF ENZYMES INVOLVED IN LIPOGENESIS AND GLUCONEOGENESIS. Biochemistry. 1964 Nov;3:1687–1692. doi: 10.1021/bi00899a015. [DOI] [PubMed] [Google Scholar]


