Abstract
1. A method was developed for the isolation of essentially pure myosin light chains from perfused rat heart. The phosphorylation of the P-light chains was estimated by hydrolysis and measurement of phosphate released, by electrophoresis in 8 M-urea and by 32P incorporation in perfusion with [32P]Pi. 2. In control perfusions there was 0.5-0.6 mol of phosphate/mol of P-light chain. This was not changed by perfusion with 5 microM-adrenaline for 10-40s. Perfusion for 1 min with medium containing 7.5 mM-CaCl2, or for 30s with medium containing 118 mM-KCl, also did not change the phosphorylation of P-light chains. 3. It is concluded that phosphorylation of P-light chains is not important in mediating the action of inotropic agents in the heart.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIANCHI C. P., SHANES A. M. Calcium influx in skeletal muscle at rest, during activity, and during potassium contracture. J Gen Physiol. 1959 Mar 20;42(4):803–815. doi: 10.1085/jgp.42.4.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány K., Bárány M., Gillis J. M., Kushmerick M. J. Phosphorylation-dephosphorylation of the 18,000-dalton light chain of myosin during the contraction-relaxation cycle of frog muscle. J Biol Chem. 1979 May 10;254(9):3617–3623. [PubMed] [Google Scholar]
- Dabrowska R., Aromatorio D., Sherry J. M., Hartshorne D. J. Composition of the myosin light chain kinase from chicken gizzard. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1263–1272. doi: 10.1016/0006-291x(77)91429-2. [DOI] [PubMed] [Google Scholar]
- England P. J. A simple modification to an enzymic method for the preparation of [gamma-32P]ATP free of salt and buffer. Anal Biochem. 1979 Mar;93(2):272–274. doi: 10.1016/s0003-2697(79)80151-7. [DOI] [PubMed] [Google Scholar]
- England P. J. Correlation between contraction and phosphorylation of the inhibitory subunit of troponin in perfused rat heart. FEBS Lett. 1975 Jan 15;50(1):57–60. doi: 10.1016/0014-5793(75)81040-4. [DOI] [PubMed] [Google Scholar]
- England P. J. Studies on the phosphorylation of the inhibitory subunit of troponin during modification of contraction in perfused rat heart. Biochem J. 1976 Nov 15;160(2):295–304. doi: 10.1042/bj1600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England P. J., Walsh D. A. A rapid method for the measurement of [gamma-32P]ATP specific radioactivity in tissue extracts and its application to the study of 32Pi uptake in perfused rat heart. Anal Biochem. 1976 Oct;75(2):429–435. doi: 10.1016/0003-2697(76)90096-8. [DOI] [PubMed] [Google Scholar]
- Fenner C., Traut R. R., Mason D. T., Wikman-Coffelt J. Quantification of Coomassie Blue stained proteins in polyacrylamide gels based on analyses of eluted dye. Anal Biochem. 1975 Feb;63(2):595–602. doi: 10.1016/0003-2697(75)90386-3. [DOI] [PubMed] [Google Scholar]
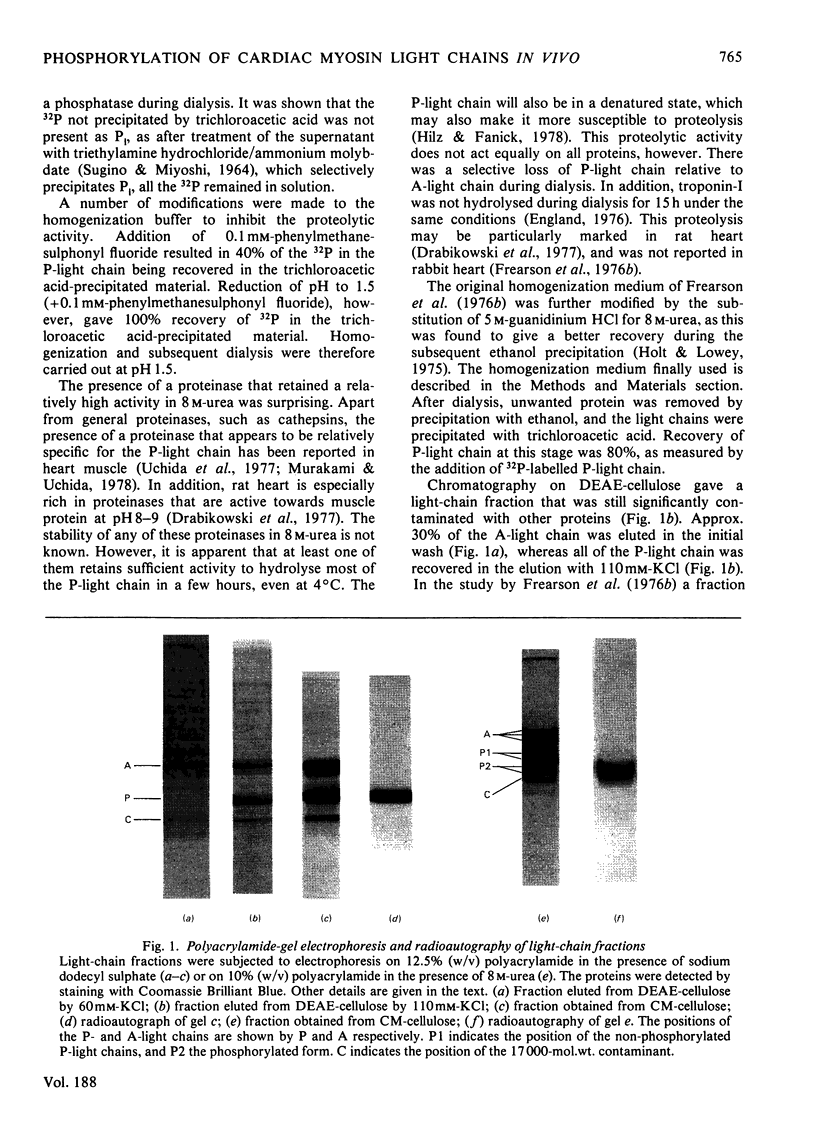
- Frearson N., Focant B. W., Perry S. V. Phosphorylation of a light chain component of myosin from smooth muscle. FEBS Lett. 1976 Mar 15;63(1):27–32. doi: 10.1016/0014-5793(76)80187-1. [DOI] [PubMed] [Google Scholar]
- Frearson N., Perry S. V. Phosphorylation of the light-chain components of myosin from cardiac and red skeletal muscles. Biochem J. 1975 Oct;151(1):99–107. doi: 10.1042/bj1510099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frearson N., Solaro R. J., Perry S. V. Changes in phosphorylation of P light chain of myosin in perfused rabbit heart. Nature. 1976 Dec 23;264(5588):801–802. doi: 10.1038/264801a0. [DOI] [PubMed] [Google Scholar]
- Hilz H., Fanick W. Divergent denaturation of proteases by urea and dodecylsulfate in the absence of substrate. Hoppe Seylers Z Physiol Chem. 1978 Oct;359(10):1447–1450. [PubMed] [Google Scholar]
- Holt J. C., Lowey S. An immunological approach to the role of the low molecular weight subunits in myosin. I. Physical--chemical and immunological characterization of the light chains. Biochemistry. 1975 Oct 21;14(21):4600–4609. doi: 10.1021/bi00692a007. [DOI] [PubMed] [Google Scholar]
- Itaya K., Ui M. A new micromethod for the colorimetric determination of inorganic phosphate. Clin Chim Acta. 1966 Sep;14(3):361–366. doi: 10.1016/0009-8981(66)90114-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine F. H., Gold H. K., Leinbach R. C., Daggett W. M., Austen W. G., Buckley M. J. Safe early revascularization for continuing ischemia after acute myocardial infarction. Circulation. 1979 Aug;60(2 Pt 2):5–9. doi: 10.1161/01.cir.60.2.5. [DOI] [PubMed] [Google Scholar]
- Manning D. R., Stull J. T. Myosin light chain phosphorylation and phosphorylase A activity in rat extensor digitorum longus muscle. Biochem Biophys Res Commun. 1979 Sep 12;90(1):164–170. doi: 10.1016/0006-291x(79)91604-8. [DOI] [PubMed] [Google Scholar]
- Meisler M. H., Langan T. A. Characterization of a phosphatase specific for phosphorylated histones and protamine. J Biol Chem. 1969 Sep 25;244(18):4961–4968. [PubMed] [Google Scholar]
- Morgan M., Perry S. V., Ottaway J. Myosin light-chain phosphatase. Biochem J. 1976 Sep 1;157(3):687–697. doi: 10.1042/bj1570687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami U., Uchida K. Purification and characterization of a myosin-cleaving protease from rat heart myofibrils. Biochim Biophys Acta. 1978 Jul 7;525(1):219–229. doi: 10.1016/0005-2744(78)90217-6. [DOI] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrie W. T., Smillie L. B., Perry S. B. A phosphorylated light-chain component of myosin from skeletal muscle. Biochem J. 1973 Sep;135(1):151–164. doi: 10.1042/bj1350151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires E. M., Perry S. V. Purification and properties of myosin light-chain kinase from fast skeletal muscle. Biochem J. 1977 Oct 1;167(1):137–146. doi: 10.1042/bj1670137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pires E., Perry S. V., Thomas M. A. Myosin light-chain kinase, a new enzyme from striated muscle. FEBS Lett. 1974 May 1;41(2):292–296. doi: 10.1016/0014-5793(74)81232-9. [DOI] [PubMed] [Google Scholar]
- SUGINO Y., MIYOSHI Y. THE SPECIFIC PRECIPITATION OF ORTHOPHOSPHATE AND SOME BIOCHEMICAL APPLICATIONS. J Biol Chem. 1964 Jul;239:2360–2364. [PubMed] [Google Scholar]
- Stull J. T., High C. W. Phosphorylation of skeletal muscle contractile proteins in vivo. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1078–1083. doi: 10.1016/s0006-291x(77)80088-0. [DOI] [PubMed] [Google Scholar]
- Uchida K., Murakami U., Hiratsuka T. Purification of cardiac myosin from rat heart Proteolytic cleavage and its inhibition. J Biochem. 1977 Aug;82(2):469–476. [PubMed] [Google Scholar]
- Van Winkle W. B., Schwartz A. Ions and inotropy. Annu Rev Physiol. 1976;38:247–272. doi: 10.1146/annurev.ph.38.030176.001335. [DOI] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Yazawa M., Kuwayama H., Yagi K. Modulator protein as a Ca2+-dependent activator of rabbit skeletal myosin light-chain kinase. Purification and characterization. J Biochem. 1978 Nov;84(5):1253–1258. doi: 10.1093/oxfordjournals.jbchem.a132243. [DOI] [PubMed] [Google Scholar]
- Yazawa M., Yagi K. Calcium-binding subunit of myosin light chain kinase. J Biochem. 1977 Jul;82(1):287–289. doi: 10.1093/oxfordjournals.jbchem.a131681. [DOI] [PubMed] [Google Scholar]