Abstract
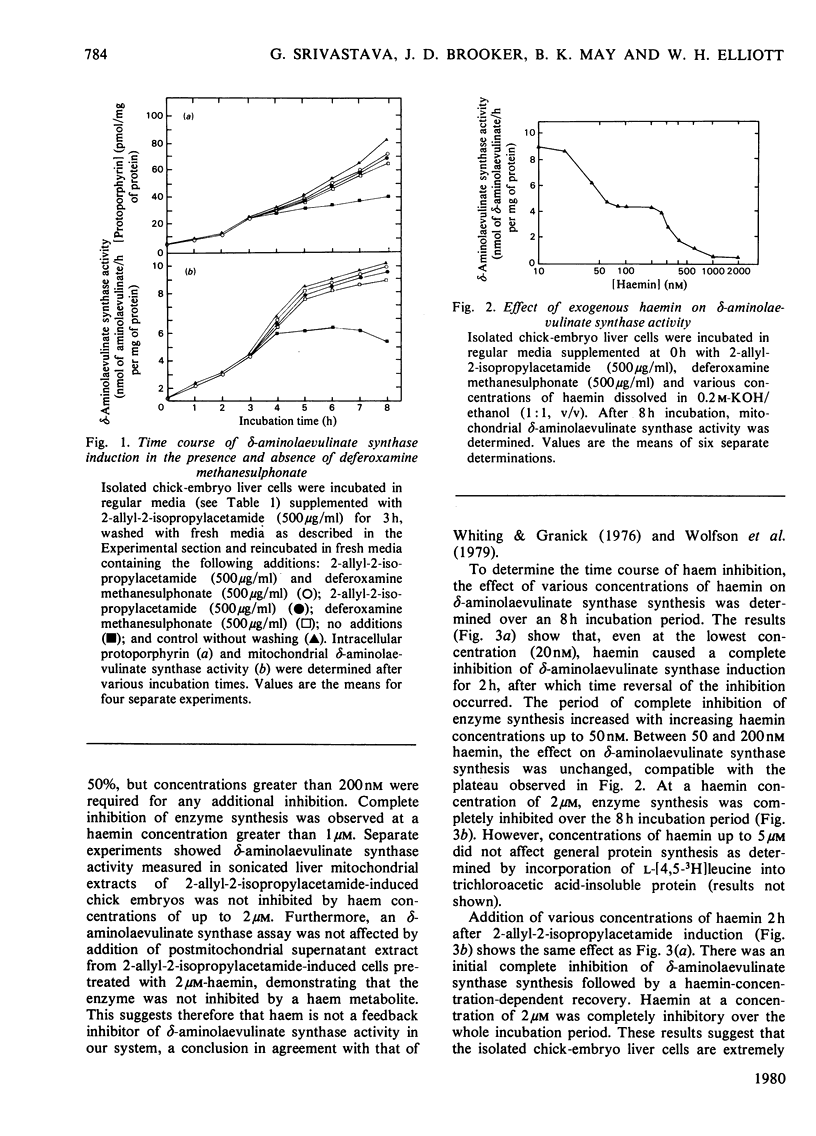
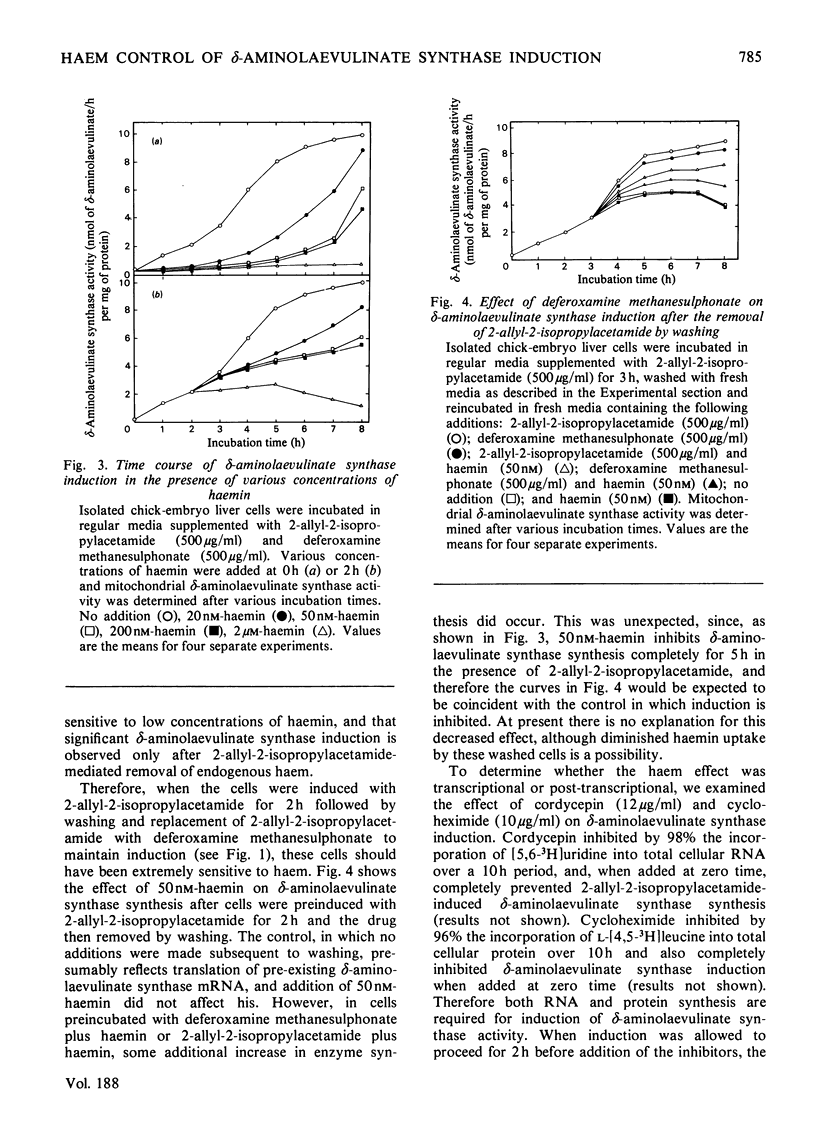
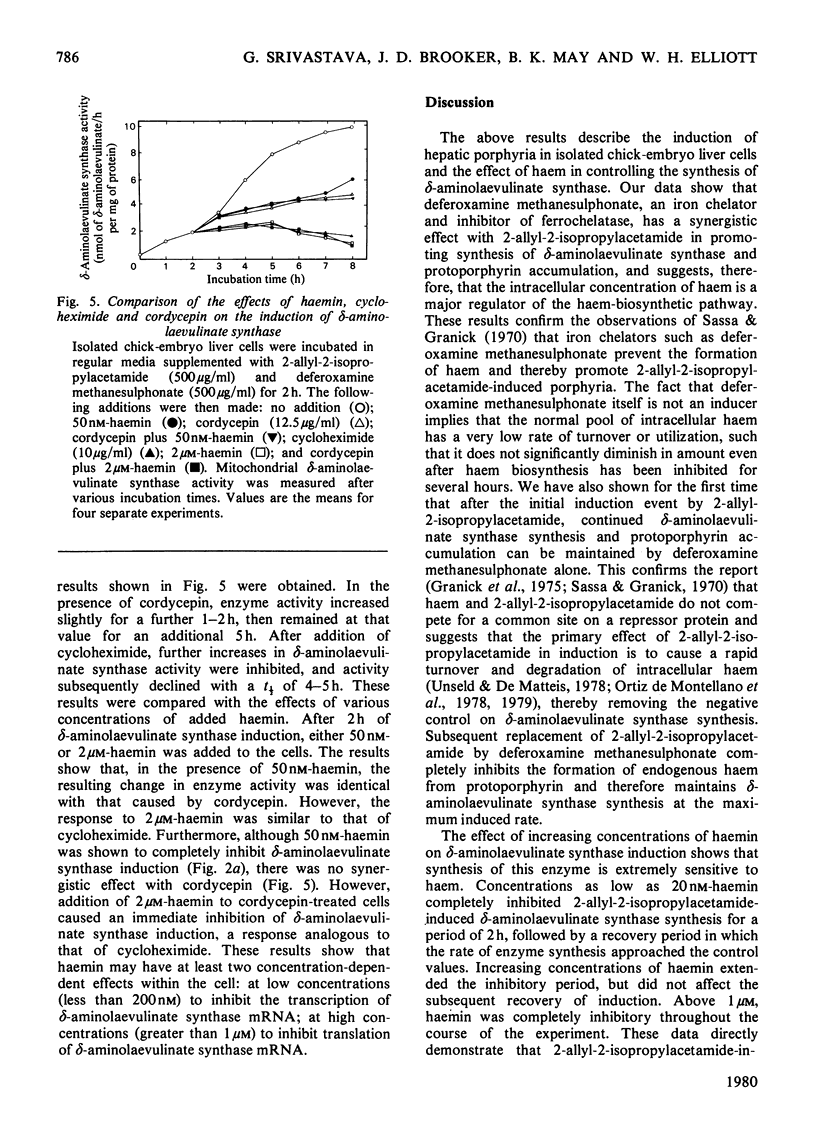
2-Allyl-2-isopropylacetamide-mediated induction of hepatic porphyria was studied in isolated chick-embryo liver cells. Increased δ-aminolaevulinate synthase activity occurred within 1h of induction and continued to increase for 8h. Protoporphyrins synthesized during this time accumulated to a concentration 10-fold greater than that in the control. Removal of 2-allyl-2-isopropylacetamide from the cells by washing at 3h immediately inhibited further increases in δ-aminolaevulinate synthase synthesis. However substitution of 2-allyl-2-isopropylacetamide at 3h by deferoxamine methane-sulphonate, an inhibitor of haem synthesis, allowed continued δ-aminolaevulinate synthase induction at an unaltered rate, even though this agent did not, by itself, induce enzyme synthesis. Exogenously added haemin was shown completely to inhibit 2-allyl-2-isopropylacetamide-mediated δ-aminolaevulinate synthase induction at concentrations as low as 20nm, a value that is less than the reported physiological one. The duration of inhibition was dependent on the concentration of added haemin and was followed by a period of δ-aminolaevulinate synthase synthesis at a rate similar to that of the control. These data are consistent with the hypothesis that δ-aminolaevulinate synthase synthesis is regulated by the concentration of intracellular haem and that induction is initiated by 2-allyl-2-isopropylacetamide-mediated destruction of haem. Induction of δ-aminolaevulinate synthase was shown to be dependent on both RNA and protein synthesis, and a study of the comparative effects of cordycepin, cycloheximide and haem has shown that, at haemin concentrations up to 50nm, the inhibition of δ-aminolaevulinate synthase synthesis followed kinetics similar to the effect of cordycepin, with no synergism between cordycepin and 50nm-haemin. However, at a haemin concentration of 2μm, the inhibition of δ-aminolaevulinate synthase synthesis followed similar kinetics to the effect of cycloheximide. These data demonstrate the control of δ-aminolaevulinate synthase synthesis by low concentrations of haemin and suggests that the primary effect of haemin is at the level of transcription.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissell D. M., Hammaker L. E. Cytochrome p-450 heme and the regulation of delta-aminolevulinic acid synthetase in the liver. Arch Biochem Biophys. 1976 Sep;176(1):103–112. doi: 10.1016/0003-9861(76)90145-4. [DOI] [PubMed] [Google Scholar]
- Delta-Aminolevulinic acid synthase from chick embryo liver mitochondria. II. Immunochemical correlation between synthesis and activity in induction and repression. J Biol Chem. 1976 Mar 10;251(5):1347–1353. [PubMed] [Google Scholar]
- GRANICK S. Induction of the synthesis of delta-aminolevulinic acid synthetase in liver parenchyma cells in culture by chemical that induce acute porphyria. J Biol Chem. 1963 Jun;238:2247–2249. [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Granick S., Sinclair P., Sassa S., Grieninger G. Effects by heme, insulin, and serum albumin on heme and protein synthesis in chick embryo liver cells cultured in a chemically defined medium, and a spectrofluorometric assay for porphyrin composition. J Biol Chem. 1975 Dec 25;250(24):9215–9225. [PubMed] [Google Scholar]
- Granick S. The induction in vitro of the synthesis of delta-aminolevulinic acid synthetase in chemical porphyria: a response to certain drugs, sex hormones, and foreign chemicals. J Biol Chem. 1966 Mar 25;241(6):1359–1375. [PubMed] [Google Scholar]
- Hayashi N., Kurashima Y., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of -aminolevulinate synthetase in liver mitochondria. V. Mechanism of regulation by hemin of the level of -aminolevulinate synthetase in rat liver mitochondria. Arch Biochem Biophys. 1972 Jan;148(1):10–21. doi: 10.1016/0003-9861(72)90109-9. [DOI] [PubMed] [Google Scholar]
- Oashi A., Kikuchi G. Mechanism of allylisopropylacetamide-induced increase of -aminolevulinate synthetase in liver mitochondria. VI. Multiple molecular forms of -aminolevulinate synthetase in the cytosol and mitochondria of induced cock liver. Arch Biochem Biophys. 1972 Nov;153(1):34–46. doi: 10.1016/0003-9861(72)90417-1. [DOI] [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Kunze K. L., Yost G. S., Mico B. A. Self-catalyzed destruction of cytochrome P-450: covalent binding of ethynyl sterols to prosthetic heme. Proc Natl Acad Sci U S A. 1979 Feb;76(2):746–749. doi: 10.1073/pnas.76.2.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz de Montellano P. R., Mico B. A., Yost G. S. Suicidal inactivation of cytochrome P-450. Formation of a heme-substrate covalent adduct. Biochem Biophys Res Commun. 1978 Jul 14;83(1):132–137. doi: 10.1016/0006-291x(78)90407-2. [DOI] [PubMed] [Google Scholar]
- Poland A., Glover E. 2,3,7,8-Tetrachlorodibenzo-p-dioxin: a potent inducer of -aminolevulinic acid synthetase. Science. 1973 Feb 2;179(4072):476–477. doi: 10.1126/science.179.4072.476. [DOI] [PubMed] [Google Scholar]
- Sassa S., Kappas A. Induction of aminolevulinate synthase and porphyrins in cultured liver cells maintained in chemically defined medium. Permissive effects of hormones on induction process. J Biol Chem. 1977 Apr 10;252(7):2428–2436. [PubMed] [Google Scholar]
- Srivastava G., May B. K., Elliott W. H. cAMP-dependent induction of delta-aminolevulinate synthase in isolated embryonic chick liver cells. Biochem Biophys Res Commun. 1979 Sep 12;90(1):42–49. doi: 10.1016/0006-291x(79)91587-0. [DOI] [PubMed] [Google Scholar]
- Tonita Y., Oashi A., Kikuchi G. Induction of delta-aminolevulinate synthetase in organ culture of chick embryo liver by allylisopropylacetamide and 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biochem. 1974 May;75(5):1007–1007. doi: 10.1093/oxfordjournals.jbchem.a130472. [DOI] [PubMed] [Google Scholar]
- Tyrrell D. L., Marks G. S. Drug-induced porphyrin biosynthesis. V. Effect of protohemin on the transcriptional and post-transcriptional phases of -aminolevulinic acid synthetase induction. Biochem Pharmacol. 1972 Aug 1;21(15):2077–2093. doi: 10.1016/0006-2952(72)90161-x. [DOI] [PubMed] [Google Scholar]
- Unseld A., de Matteis F. Destruction of endogenous and exogenous haem by 2-allyl-2-isopropylacetamide: role of the liver cytochrome P-450 which is inducible by phenobarbitone. Int J Biochem. 1978;9(12):865–869. doi: 10.1016/0020-711x(78)90061-7. [DOI] [PubMed] [Google Scholar]
- Wolfson S. J., Bartczak A., Bloomer J. R. Effect of endogenous heme generation on delta-aminolevulinic acid synthase activity in rat liver mitochondria. J Biol Chem. 1979 May 10;254(9):3543–3546. [PubMed] [Google Scholar]


