Abstract
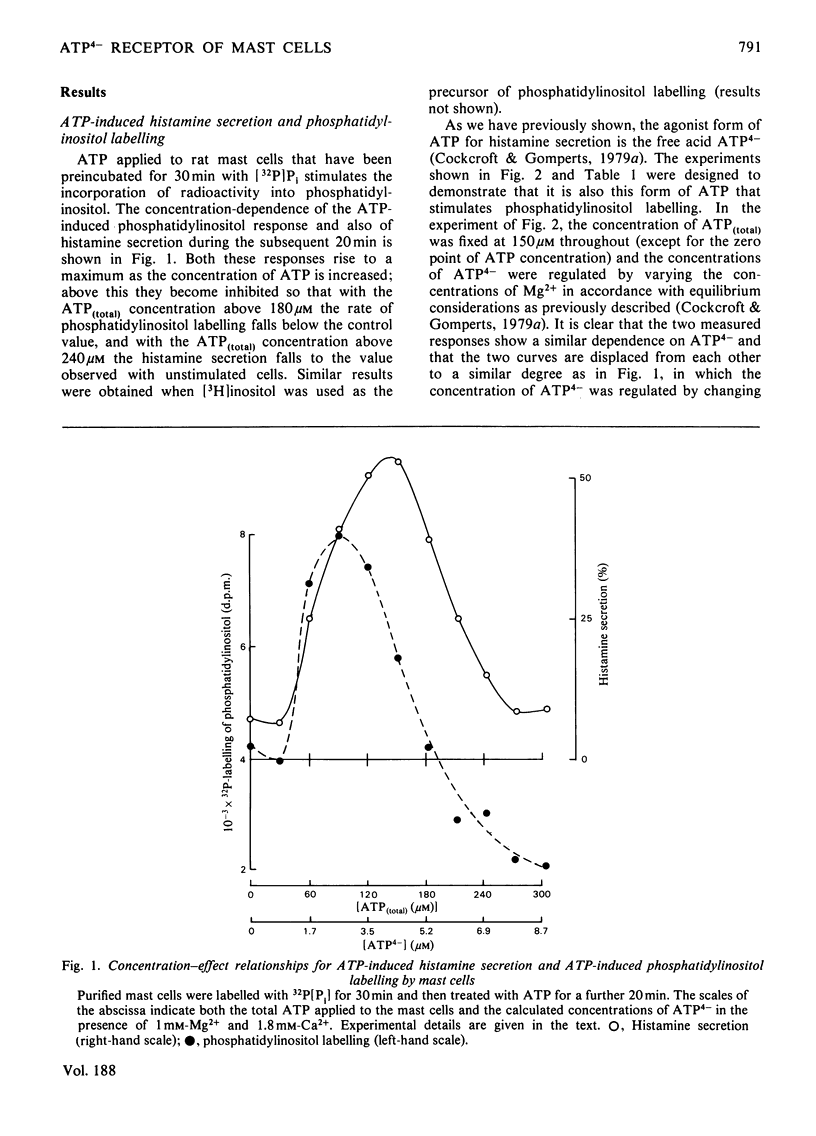
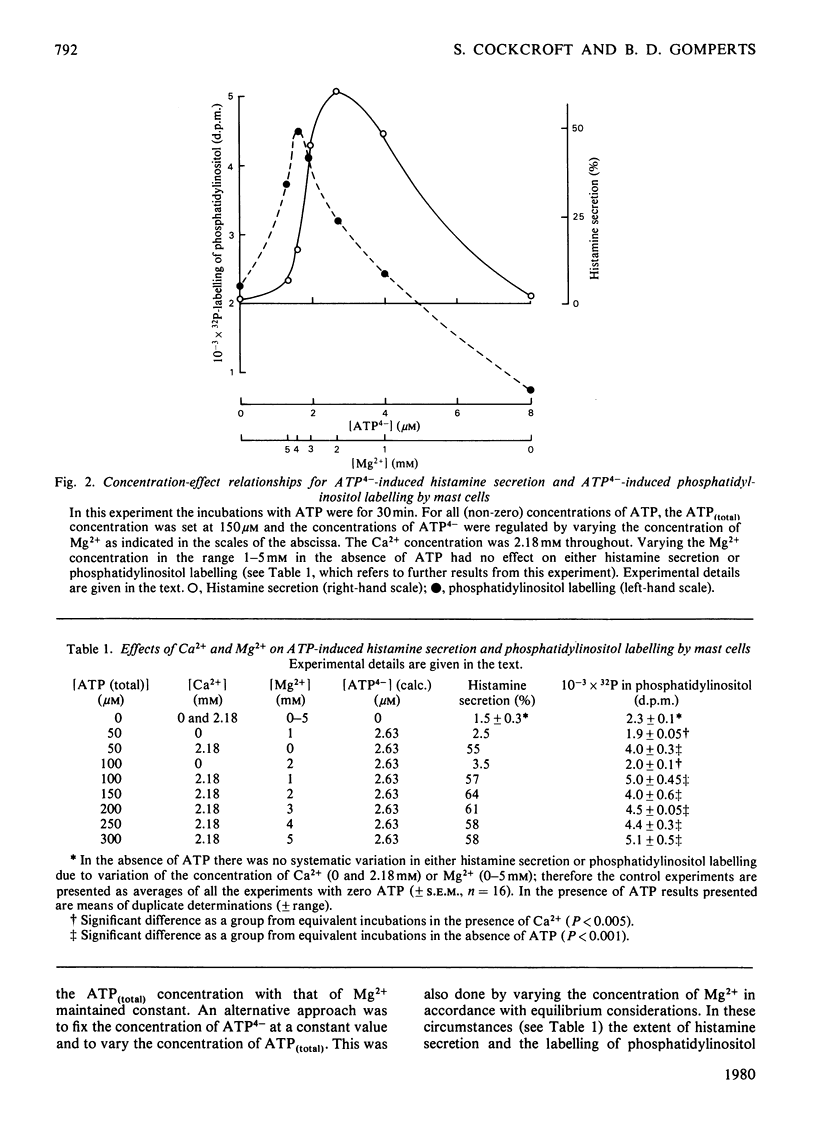
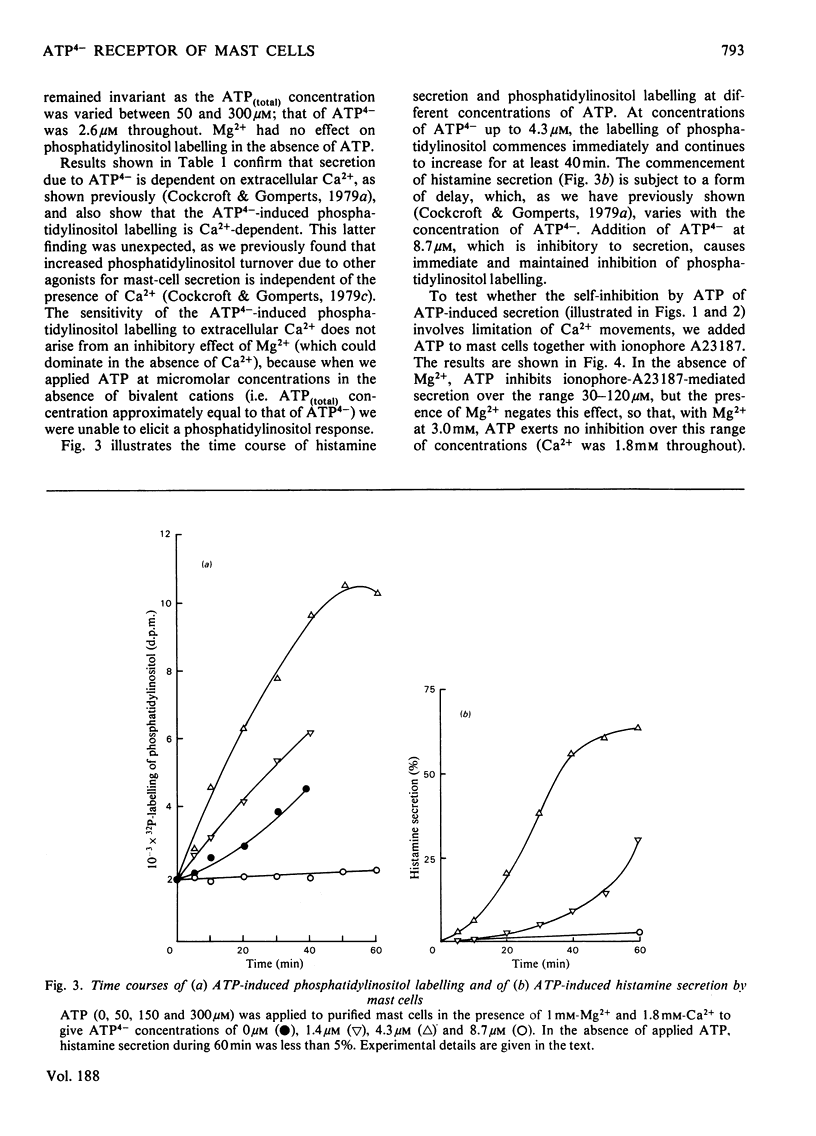
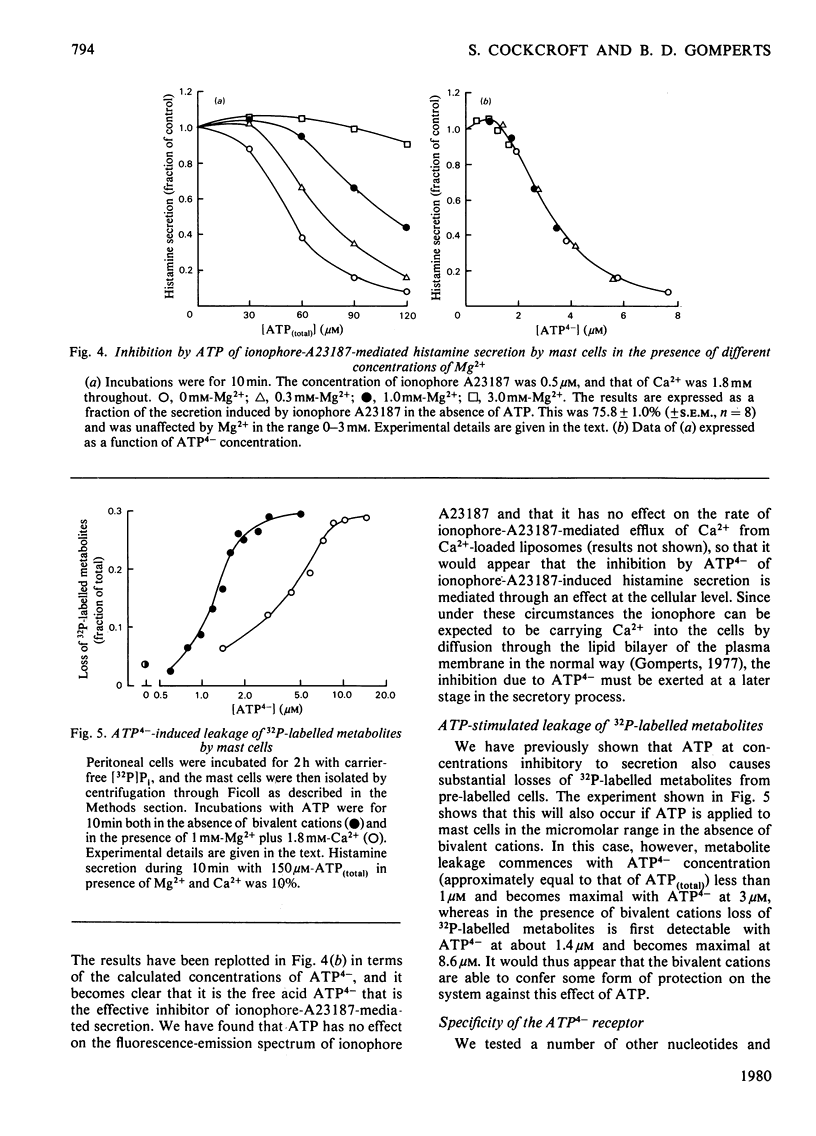
The concentration-dependence on exogenous ATP of activation and inhibition of mast-cell histamine secretion, phosphatidylinositol labelling and leakage of metabolites shows that all these functions are regulated by the free acid ATP4-. Maximal histamine secretion and phosphatidylinositol labelling occur with ATP4- at approx. 2 microM, but higher concentrations, which cause inhibition of secretion and phosphatidylinositol labelling, are required to maximize leakage of 32P-labelled metabolites. Both enhancement and inhibition of phosphatidylinositol labelling (due to low and high concentrations of ATP4- respectively) are rapid in onset; histamine secretion is characterized by a delay, especially at low concentrations of ATP4- (approx. 1 microM). Phosphatidylinositol labelling and histamine secretion are dependent on extracellular Ca2+. Metabolite leakage due to the presence of exogenous ATP4- is slow and does not require Ca2+. Of 18 analogues of ATP that were tested, only four were agonists for secretion, and only these four permitted leakage of 32P-labelled metabolites. It is argued that activation and inhibition of histamine secretion, phosphatidylinositol labelling and metabolite leakage are all initiated by ATP4- acting at the same receptor. For mast cells stimulated with ATP4- enhancement of phosphatidylinositol metabolism is not sufficient by itself to cause Ca2+-dependent secretion.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. P., Cockcroft S., Gomperts B. D. Ionomycin stimulates mast cell histamine secretion by forming a lipid-soluble calcium complex. Nature. 1979 Dec 20;282(5741):851–853. doi: 10.1038/282851a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Bennett J. P., Gomperts B. D. f-MetLeuPhe-induced phosphatidylinositol turnover in rabbit neutrophils is dependent on extracellular calcium. FEBS Lett. 1980 Jan 28;110(1):115–118. doi: 10.1016/0014-5793(80)80036-6. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. ATP induces nucleotide permeability in rat mast cells. Nature. 1979 Jun 7;279(5713):541–542. doi: 10.1038/279541a0. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Activation and inhibition of calcium-dependent histamine secretion by ATP ions applied to rat mast cells. J Physiol. 1979 Nov;296:229–243. doi: 10.1113/jphysiol.1979.sp013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Evidence for a role of phosphatidylinositol turnover in stimulus-secretion coupling. Studies with rat peritoneal mast cells. Biochem J. 1979 Mar 15;178(3):681–687. doi: 10.1042/bj1780681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlquist R., Diamant B. Interaction of ATP and calcium on the rat mast cell: effect on histamine release. Acta Pharmacol Toxicol (Copenh) 1974 May;34(5):368–384. doi: 10.1111/j.1600-0773.1974.tb03533.x. [DOI] [PubMed] [Google Scholar]
- Day H. J., Holmsen H. Concepts of the blood platelet release reaction. Ser Haematol. 1971;4(1):3–27. [PubMed] [Google Scholar]
- Diamant B., Patkar S. A. Stimulation and inhibition of histamine release from isolated rat mast cells. Dual effects of the ionophore A23187. Int Arch Allergy Appl Immunol. 1975;49(1-2):183–207. doi: 10.1159/000231394. [DOI] [PubMed] [Google Scholar]
- Diamant B. The influence of adenosine triphosphate on isolated rat peritoneal mast cells. Int Arch Allergy Appl Immunol. 1969;36(1):3–21. doi: 10.1159/000230715. [DOI] [PubMed] [Google Scholar]
- Grosman N., Diamant B. Effects of adenosine-5'-triphosphate (ATP) on rat mast cells: influence on anaphylactic and compound 48/80-induced histamine release. Agents Actions. 1975 May;5(2):108–114. doi: 10.1007/BF02027349. [DOI] [PubMed] [Google Scholar]
- HILLARP N. A., THIEME G. Nucleotides in the catechol amine granules of the adrenal medulla. Acta Physiol Scand. 1959 Apr 22;45(4):328–338. doi: 10.1111/j.1748-1716.1959.tb01705.x. [DOI] [PubMed] [Google Scholar]
- Holmsen H., Day H. J., Storm E. Adenine nucleotide metabolism of blood platelets. VI. Subcellular localization of nucleotide pools with different functions in the platelet release reaction. Biochim Biophys Acta. 1969 Aug 20;186(2):254–266. doi: 10.1016/0005-2787(69)90003-3. [DOI] [PubMed] [Google Scholar]
- Khan M. M., Martell A. E. Thermodynamic quantities associated with the interaction of adenosine triphosphate with metal ions. J Am Chem Soc. 1966 Feb 20;88(4):668–671. doi: 10.1021/ja00956a008. [DOI] [PubMed] [Google Scholar]
- Kiernan J. A. Action of adenosine triphosphate on mast cells in normal and denervated skin. Arch Dermatol Forsch. 1974;251(1):83–86. doi: 10.1007/BF00561714. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Jafferji S. S., Jones L. M. The possible involvement of phosphatidylinositol breakdown in the mechanism of stimulus-response coupling at receptors which control cell-surface calcium gates. Adv Exp Med Biol. 1977;83:447–464. doi: 10.1007/978-1-4684-3276-3_41. [DOI] [PubMed] [Google Scholar]
- Sugiyama K. Calcium-dependent histamine release with degranulation from isolated rat mast cells by adenosine 5'-triphosphate. Jpn J Pharmacol. 1971 Apr;21(2):209–226. doi: 10.1254/jjp.21.209. [DOI] [PubMed] [Google Scholar]



