Abstract
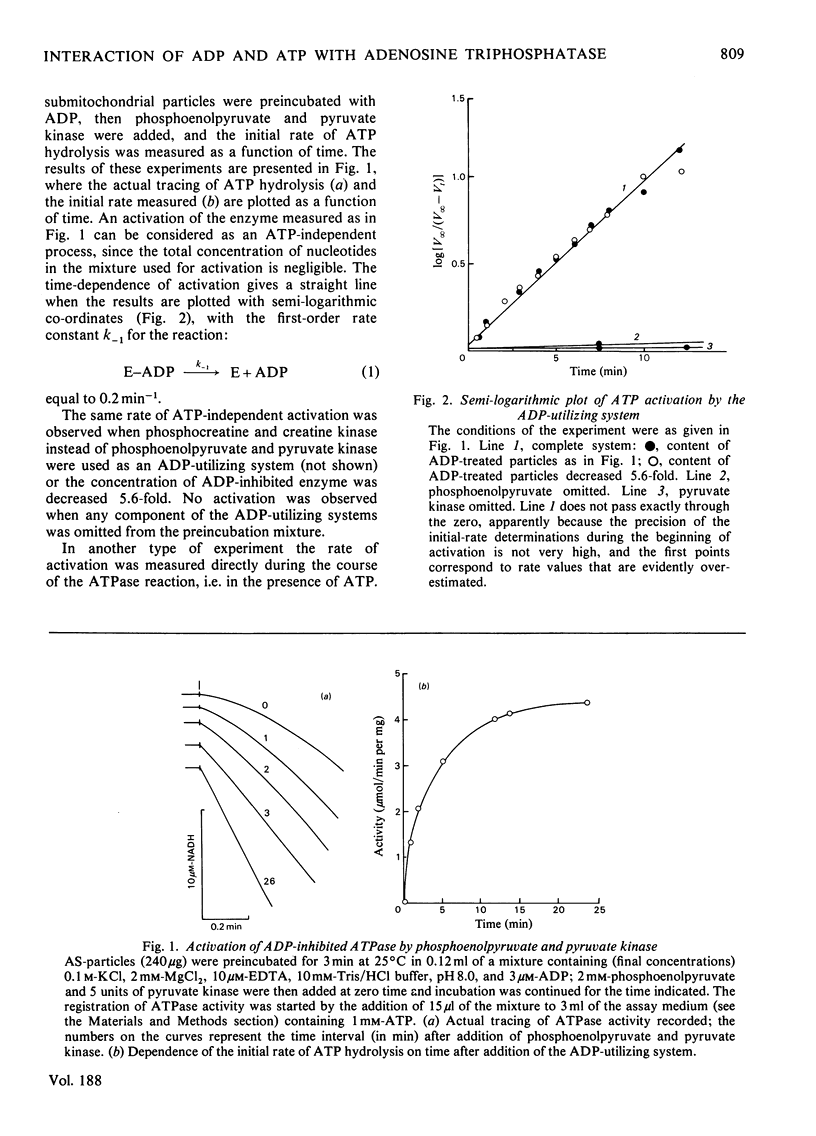
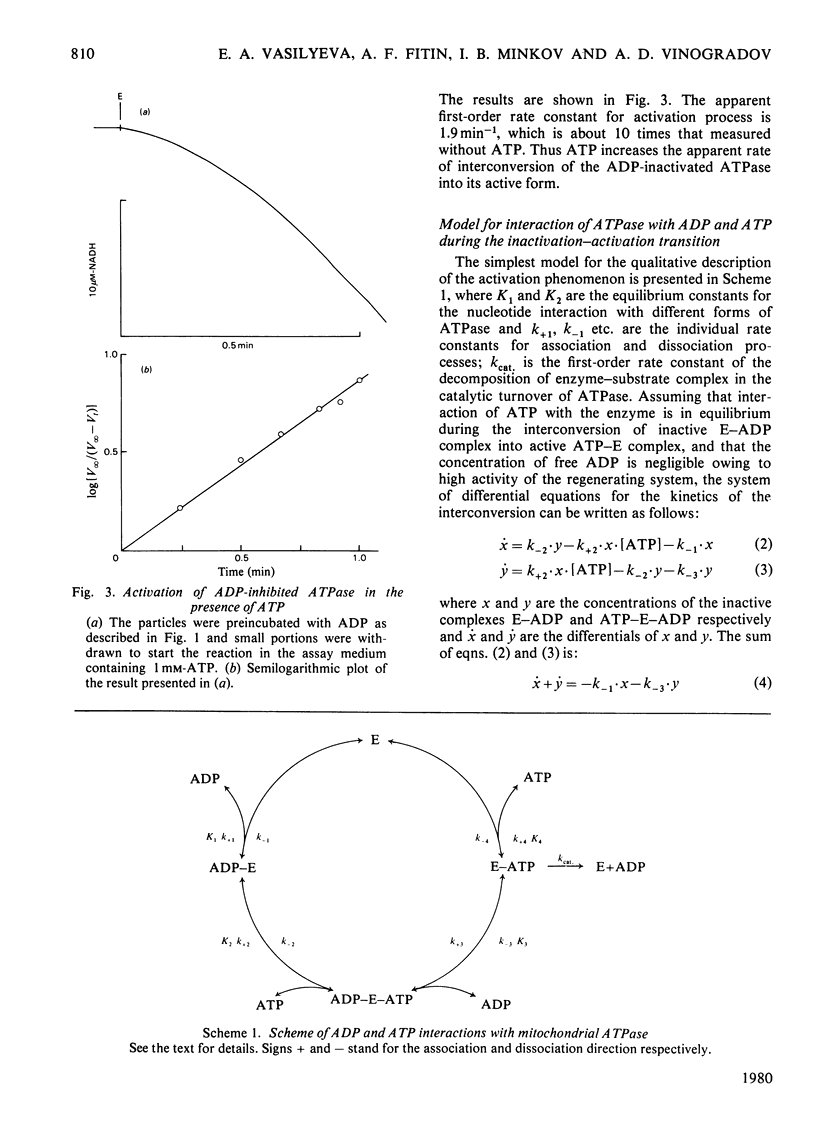
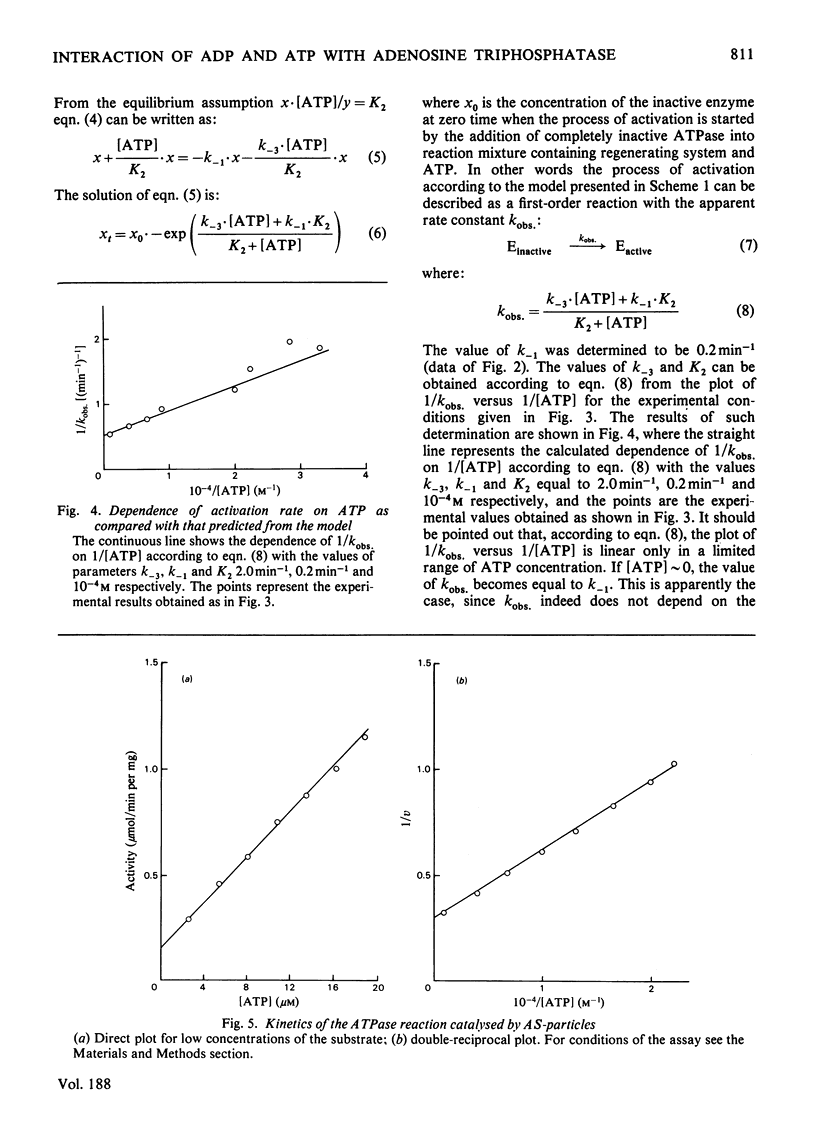
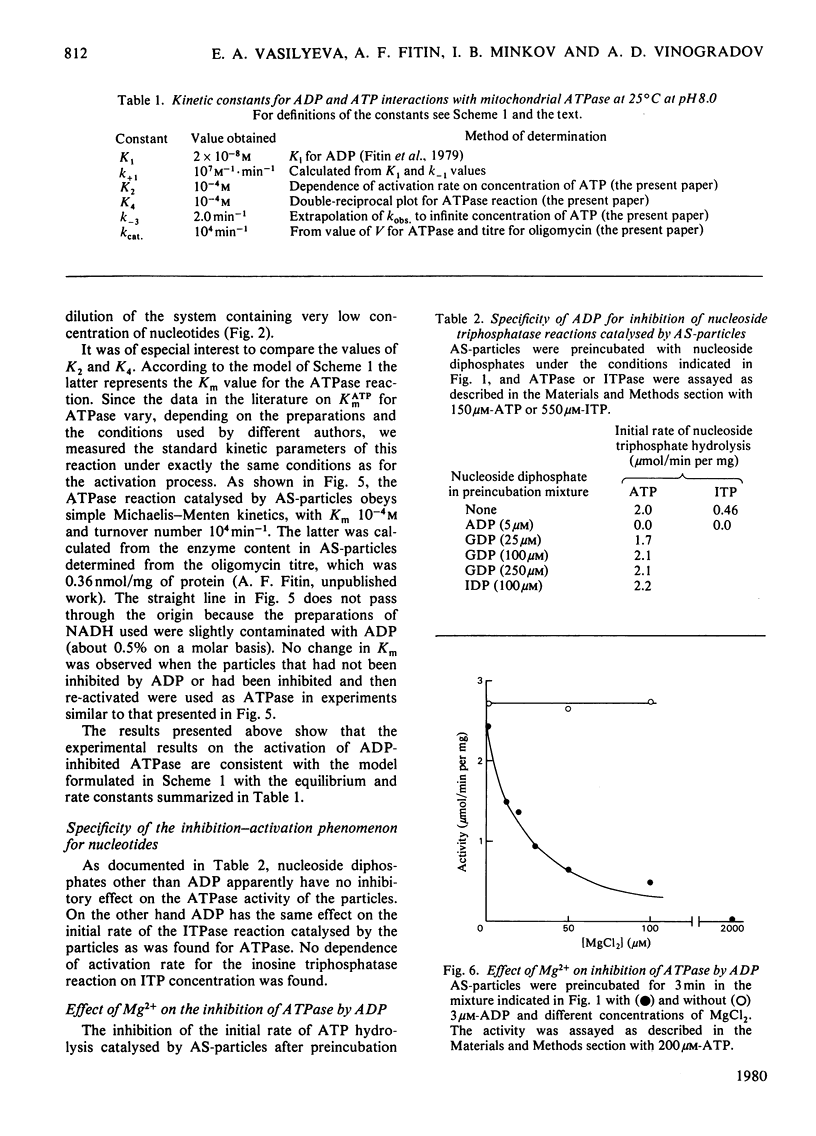
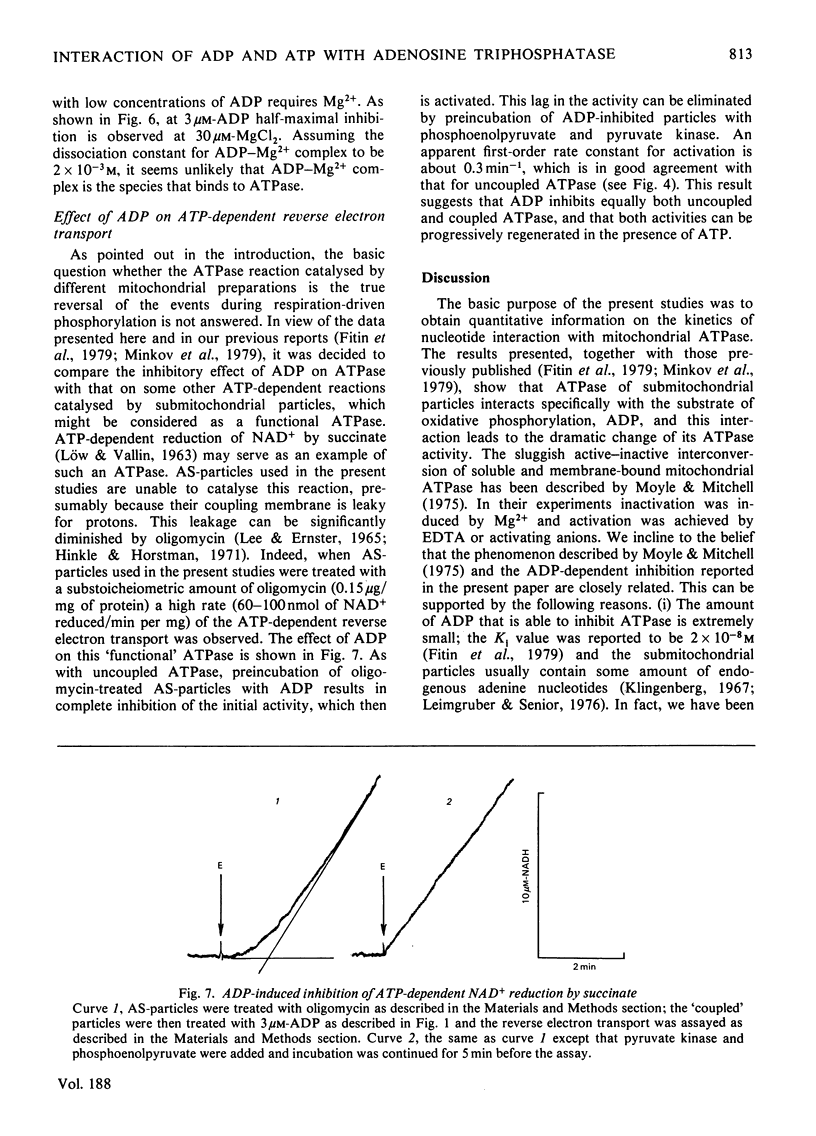
The short preincubation of submitochondrial particles with low concentrations of ADP in the presence of Mg2+ results in a complete loss of their ATPase and inosine triphosphatase activities. Other nucleoside diphosphates (IDP and GDP) do not affect the ATPase activity. The ADP-inhibited ATPase can be activated in a time-dependent manner by treatment of submitochondrial particles with the enzyme converting ADP into ATP (phosphoenolpyruvate plus pyruvate kinase). The activaton is a first-order reaction with rate constant 0.2 min-1 at 25 degrees C. The rate constant of activation is increased in the presence of ATP up to 2 min-1, and this increase shows saturation kinetics with Km value equal to that for ATPase reaction itself (10(-4) M at 25 degrees C at pH 8.0). The experimental results obtained are consistent with the model where two alternative pathways of ADP dissociation from the inhibitory site of ATPase exist; one is spontaneous dissociation and the second is ATP-dependent dissociation through the formation of the ternary complex between ADP, the enzyme and ATP. ADP-induced inactivation and ATP-dependent activation of ATPase activity of submitochondrial particles is accompanied by the same directed change of their ability to catalyse the ATP-dependent reverse electron transport from succinate to NAD+. The possible implication of the model suggested is discussed in terms of functional role of the inhibitory high-affinity binding site for ADP in the mitochondrial ATPase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. Formation constants for the complexes of adenosine di- or tri-phosphate with magnesium or calcium ions. Biochem J. 1959 Feb;71(2):388–395. doi: 10.1042/bj0710388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Pedersen P. L. Adenosine triphosphatase from rat liver mitochondria. II. Interaction with adenosine diphosphate. J Biol Chem. 1972 Dec 25;247(24):7969–7976. [PubMed] [Google Scholar]
- Fitin A. F., Vasilyeva E. A., Vinogradov A. D. An inhibitory high affinity binding site for ADP in the oligomycin-sensitive ATPase of beef heart submitochondrial particles. Biochem Biophys Res Commun. 1979 Jan 30;86(2):434–439. doi: 10.1016/0006-291x(79)90884-2. [DOI] [PubMed] [Google Scholar]
- Garrett N. E., Penefsky H. S. Interaction of adenine nucleotides with multiple binding sites on beef heart mitochondrial adenosine triphosphatase. J Biol Chem. 1975 Sep 10;250(17):6640–6647. [PubMed] [Google Scholar]
- Gomez-Fernandez J. C., Harris D. A. A thermodynamic analysis of the interaction between the mitochondrial coupling adenosine triphosphatase and its naturally occurring inhibitor protein. Biochem J. 1978 Dec 15;176(3):967–975. doi: 10.1042/bj1760967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes G. G., Hilborn D. A. Steady state kinetics of soluble and membrane-bound mitochondrial ATPase. Biochim Biophys Acta. 1971 Jun 1;233(3):580–590. doi: 10.1016/0005-2736(71)90156-8. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Gomez-Fernandez J. C., Klungsøyr L., Radda G. K. Specificity of nucleotide binding and coupled reactions utilising the mitochondrial ATPase. Biochim Biophys Acta. 1978 Dec 7;504(3):364–383. doi: 10.1016/0005-2728(78)90060-9. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Radda G. K., Slater E. C. Tightly bound nucleotides of the energy-transducing ATPase, and their role in oxidative phosphorylation. II. The beef heart mitochondrial system. Biochim Biophys Acta. 1977 Mar 11;459(3):560–572. doi: 10.1016/0005-2728(77)90054-8. [DOI] [PubMed] [Google Scholar]
- Harris D. A., Rosing J., van de Stadt R. J., Slater E. C. Tight binding of adenine nucleotides to beef-heart mitochondrial ATPase. Biochim Biophys Acta. 1973 Aug 31;314(2):149–153. doi: 10.1016/0005-2728(73)90130-8. [DOI] [PubMed] [Google Scholar]
- Harris D. A. The interactions of coupling ATPases with nucleotides. Biochim Biophys Acta. 1978 Mar 10;463(3-4):245–273. doi: 10.1016/0304-4173(78)90002-2. [DOI] [PubMed] [Google Scholar]
- Hilborn D. A., Hammes G. G. Equilibrium binding of nucleotides to beef heart mitochondrial adenosine triphosphatase. Biochemistry. 1973 Feb 27;12(5):983–990. doi: 10.1021/bi00729a030. [DOI] [PubMed] [Google Scholar]
- Hinkle P. C., Horstman L. L. Respiration-driven proton transport in submitochondrial particles. J Biol Chem. 1971 Oct 10;246(19):6024–6028. [PubMed] [Google Scholar]
- LEE C. P., ERNSTER L. RESTORATION OF OXIDATIVE PHOSPHORYLATION IN NON-PHOSPHORYLATING SUBMITOCHONDRIAL PARTICLES BY OLIGOMYCIN. Biochem Biophys Res Commun. 1965 Feb 17;18:523–529. doi: 10.1016/0006-291x(65)90785-0. [DOI] [PubMed] [Google Scholar]
- Leimgruber R. M., Senior A. E. Removal of "tightly bound" nucleotides from phosphorylating submitochondrial particles. J Biol Chem. 1976 Nov 25;251(22):7110–7113. [PubMed] [Google Scholar]
- Leimgruber R. M., Senior A. E. Tightly-bound ATP and ADP in reconstituted submitochondrial particles. Biochem Biophys Res Commun. 1978 Aug 14;83(3):837–842. doi: 10.1016/0006-291x(78)91470-5. [DOI] [PubMed] [Google Scholar]
- Minkov I. B., Fitin A. F., Vasilyeva E. A., Vinogradov A. D. Mg2+-induced ADP-dependent inhibition of the ATPase activity of beef heart mitochondrial coupling factor F1. Biochem Biophys Res Commun. 1979 Aug 28;89(4):1300–1306. doi: 10.1016/0006-291x(79)92150-8. [DOI] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. Active/inactive state transitions of mitochondrial ATPase molecules influenced by Mg2+, anions and aurovertin. FEBS Lett. 1975 Aug 1;56(1):55–61. doi: 10.1016/0014-5793(75)80110-4. [DOI] [PubMed] [Google Scholar]
- PENEFSKY H. S., PULLMAN M. E., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. II. Participation of a soluble adenosine tolphosphatase in oxidative phosphorylation. J Biol Chem. 1960 Nov;235:3330–3336. [PubMed] [Google Scholar]
- PULLMAN M. E., PENEFSKY H. S., DATTA A., RACKER E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. I. Purification and properties of soluble dinitrophenol-stimulated adenosine triphosphatase. J Biol Chem. 1960 Nov;235:3322–3329. [PubMed] [Google Scholar]
- Racker E., Horstman L. L. Partial resolution of the enzymes catalyzing oxidative phosphorylation. 13. Structure and function of submitochondrial particles completely resolved with respect to coupling factor. J Biol Chem. 1967 May 25;242(10):2547–2551. [PubMed] [Google Scholar]
- Rosing J., Harris D. A., Kemp A., Jr, Slater E. C. Nucleotide-binding properties of native and cold-treated mitochondrial ATPase. Biochim Biophys Acta. 1975 Jan 31;376(1):13–26. doi: 10.1016/0005-2728(75)90201-7. [DOI] [PubMed] [Google Scholar]
- Schuster S. M., Ebel R. E., Lardy H. A. Kinetic studies on rat liver and beef heart mitochondrial ATPase. Evidence for nucleotide binding at separate regulatory and catalytic sites. J Biol Chem. 1975 Oct 10;250(19):7848–7853. [PubMed] [Google Scholar]
- Selwyn M. J. Preparation and general properties of a soluble adenosine triphosphatase from mitochondria. Biochem J. 1967 Oct;105(1):279–288. doi: 10.1042/bj1050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgato M. C., Ferguson S. J., Kell D. B., John P. The protonmotive force in bovine heart submitochondrial particles. Magnitude, sites of generation and comparison with the phosphorylation potential. Biochem J. 1978 Jul 15;174(1):237–256. doi: 10.1042/bj1740237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalkin H., Pullman M. E., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. IV. Formation of a complex between coupling factor 1 and adenosine diphosphate and its relation to the 14C-adenosine diphosphate-adenosine triphosphate exchange reaction. J Biol Chem. 1965 Oct;240(10):4011–4016. [PubMed] [Google Scholar]


