Abstract
Osteoarthrosis was induced in one knee joint of dogs by an established surgical procedure. Changes in the articular cartilage in the biosynthesis of collagen and other proteins were sought by radiochemical labelling in vivo, with the following findings. (1) Collagen synthesis was stimulated in all cartilage surfaces of the experimental joints at 2, 8 and 24 weeks after surgery. Systemic labelling with [3H]proline showed that over 10 times more collagen was being deposited per dry weight of experimental cartilage compared with control cartilage in the unoperated knee. (2) Type-II collagen was the radiolabelled product in all samples of experimental cartilage ranging in quality from undamaged to overtly fibrillated, and was the only collagen detected chemically in the matrix of osteoarthrotic cartilage from either dog or human joints. (3) Hydroxylysine glycosylation was examined in the newly synthesized cartilage collagen by labelling dog joints in vivo with [3H]lysine. In experimental knees the new collagen was less glycosylated than in controls. However, no difference in glycosylation of the total collagen in the tissues was observed by chemical analysis. (4) Over half the protein-bound tritium was extracted by 4 M-guanidinium chloride from control cartilage labelled with [3H]proline, compared with one-quarter or less from experimental cartilage. Two-thirds of the extracted tritium separated in the upper fraction on density-gradient centrifugation in CsCl under associative conditions. Much of this ran with a single protein band on sodium dodecyl sulphate/polyacrylamide-gel electrophoresis under reducing conditions. The identity of this protein was unknown, although it resembled serum albumin in mobility afte disulphide-bond cleavage.
Full text
PDF




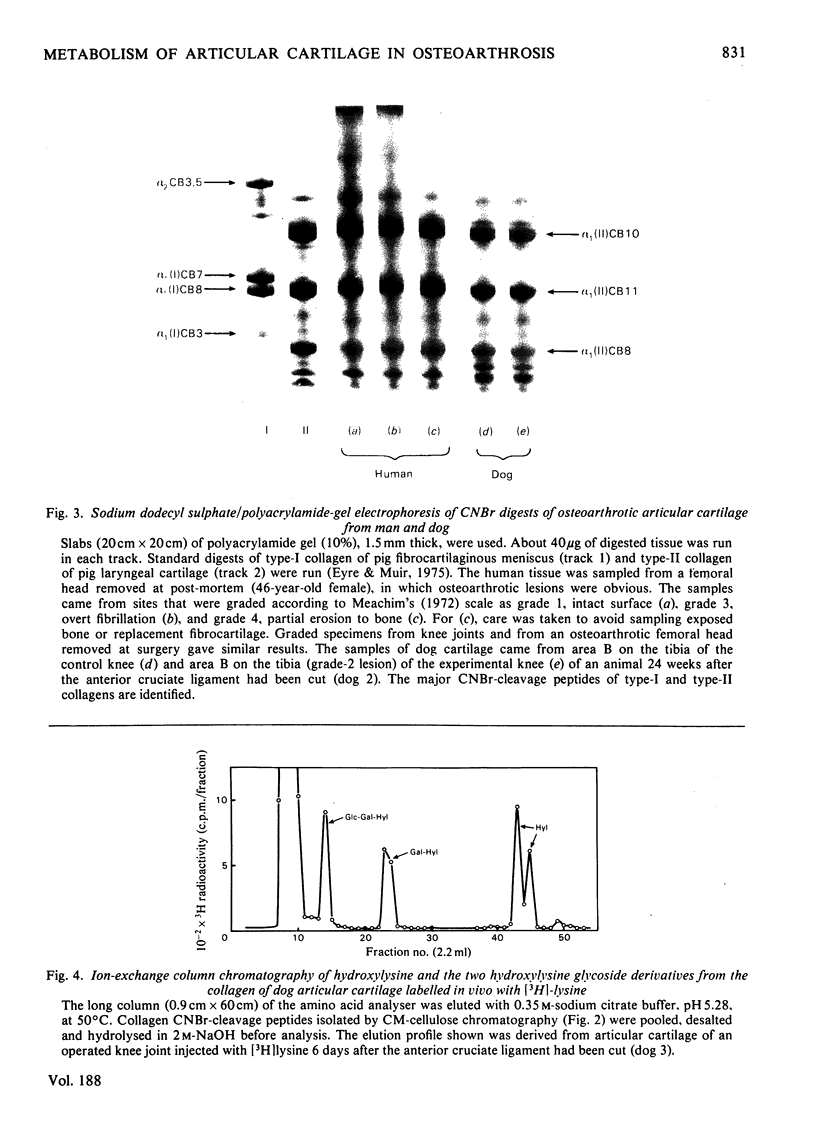
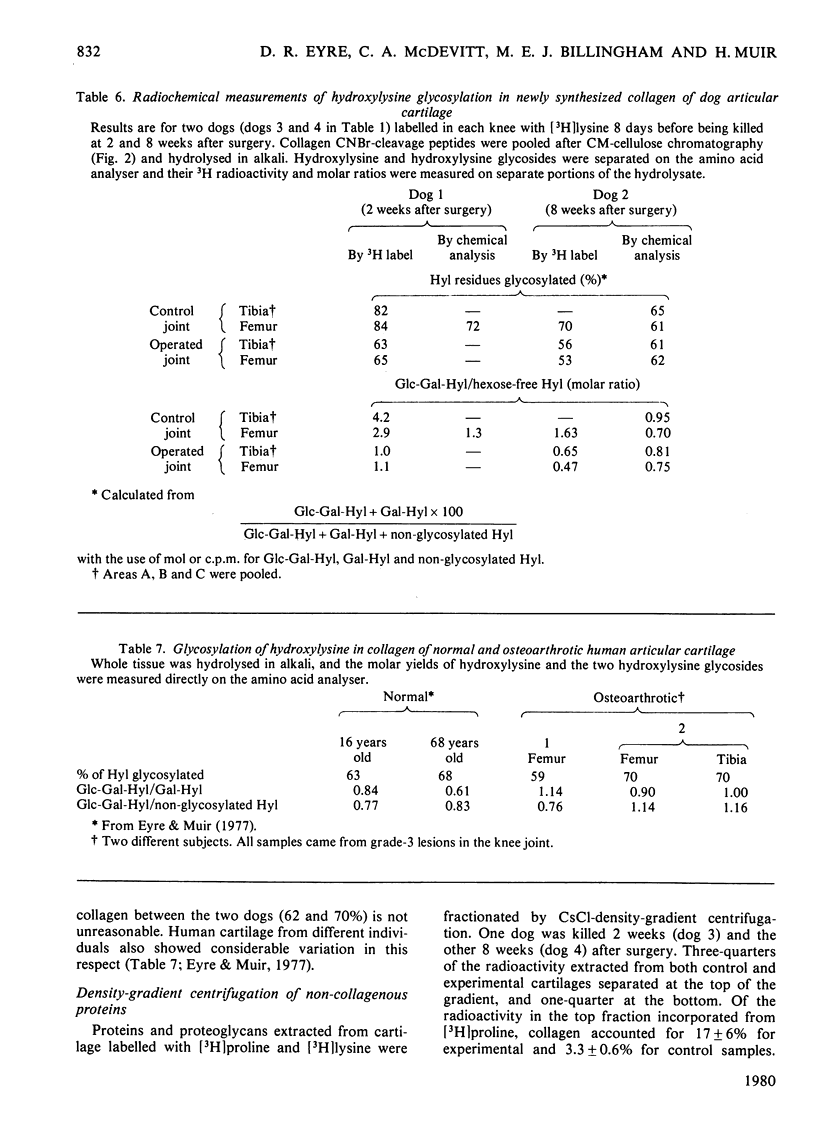
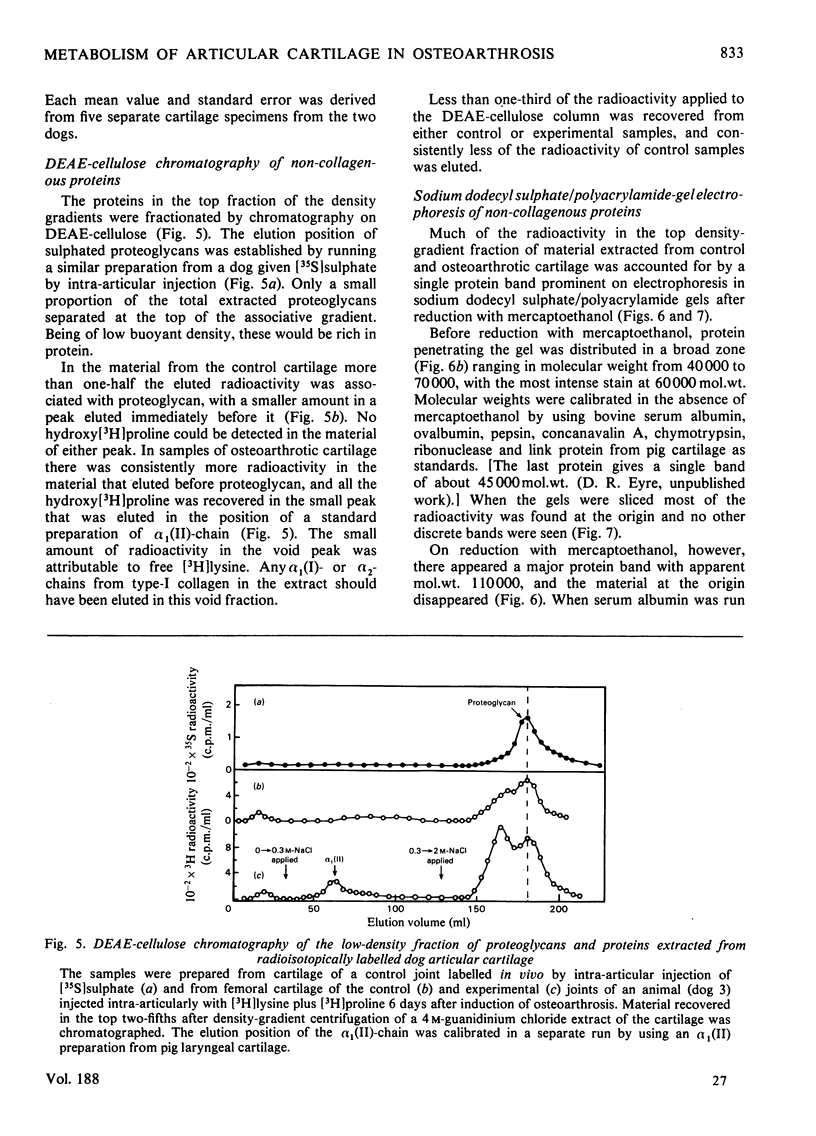
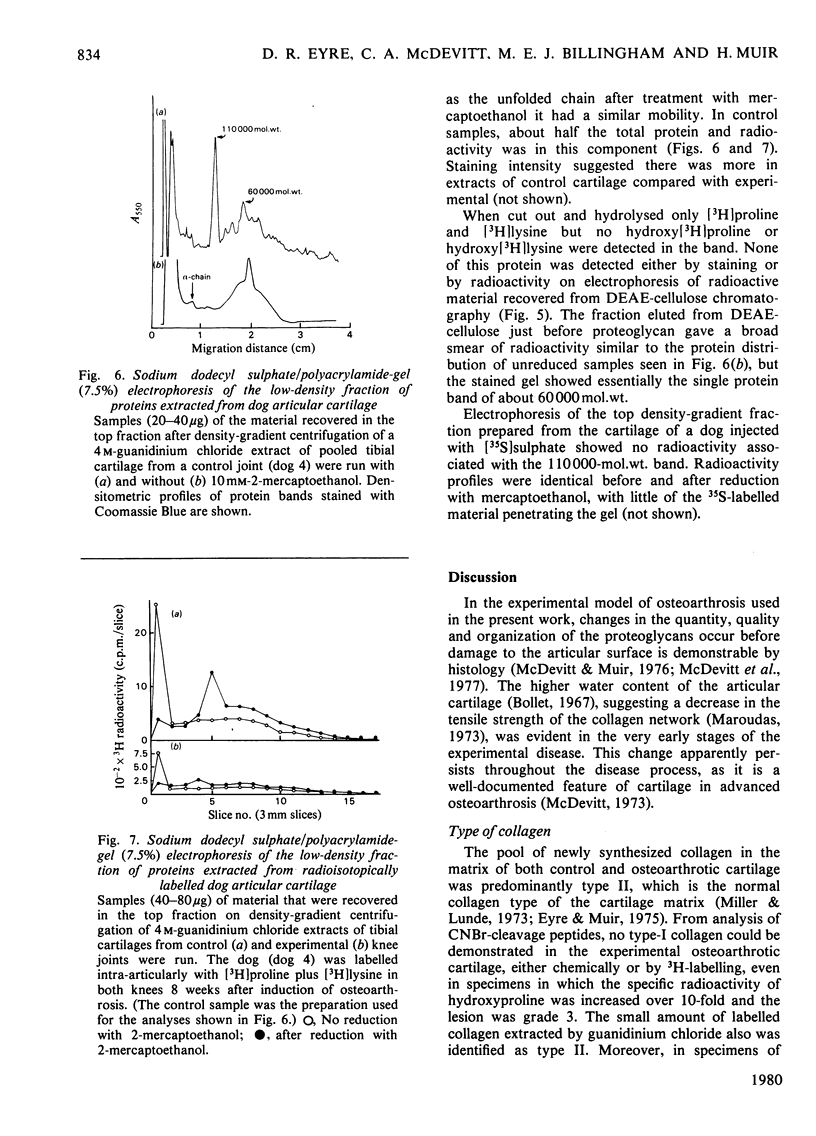



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss M. T., Ali S. Y. Isolation of proteoglycans from human articular cartilage. Biochem J. 1978 Jan 1;169(1):123–132. doi: 10.1042/bj1690123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya P. D., Padilla S. R., Nimni M. E. The progeny of rabbit articular chondrocytes synthesize collagen types I and III and type I trimer, but not type II. Verifications by cyanogen bromide peptide analysis. Biochemistry. 1977 Mar 8;16(5):865–872. doi: 10.1021/bi00624a009. [DOI] [PubMed] [Google Scholar]
- Bollet A. J. Connective tissue polysaccharide metabolism and the pathogenesis of osteoarthritis. Adv Intern Med. 1967;13:33–60. [PubMed] [Google Scholar]
- Burgeson R. E., El Adli F. A., Kaitila I. I., Hollister D. W. Fetal membrane collagens: identification of two new collagen alpha chains. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2579–2583. doi: 10.1073/pnas.73.8.2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgeson R. E., Hollister D. W. Collagen heterogeneity in human cartilage: identification of several new collagen chains. Biochem Biophys Res Commun. 1979 Apr 27;87(4):1124–1131. doi: 10.1016/s0006-291x(79)80024-8. [DOI] [PubMed] [Google Scholar]
- Chung E., Rhodes K., Miller E. J. Isolation of three collagenous components of probable basement membrane origin from several tissues. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1167–1174. doi: 10.1016/0006-291x(76)90776-2. [DOI] [PubMed] [Google Scholar]
- Deshmukh K., Hemrick S. Metabolic changes in rabbit articular cartilage due to inflammation. Arthritis Rheum. 1976 Mar-Apr;19(2):199–208. doi: 10.1002/art.1780190212. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Brickley-Parsons D. M., Glimcher M. J. Predominance of type I collagen at the surface of avian articular cartilage. FEBS Lett. 1978 Jan 15;85(2):259–263. doi: 10.1016/0014-5793(78)80468-2. [DOI] [PubMed] [Google Scholar]
- Eyre D. R. Collagen: molecular diversity in the body's protein scaffold. Science. 1980 Mar 21;207(4437):1315–1322. doi: 10.1126/science.7355290. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Glimcher M. J. Collagen cross-linking. Isolation of cross-linked peptides from collagen of chicken bone. Biochem J. 1973 Nov;135(3):393–403. doi: 10.1042/bj1350393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre D. R., Muir H. Collagen polymorphism: two molecular species in pig intervertebral disc. FEBS Lett. 1974 Jun 1;42(2):192–196. doi: 10.1016/0014-5793(74)80783-0. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Muir H. Quantitative analysis of types I and II collagens in human intervertebral discs at various ages. Biochim Biophys Acta. 1977 May 27;492(1):29–42. doi: 10.1016/0005-2795(77)90211-2. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Muir H. The distribution of different molecular species of collagen in fibrous, elastic and hyaline cartilages of the pig. Biochem J. 1975 Dec;151(3):595–602. doi: 10.1042/bj1510595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furthmayr H., Timpl R. Characterization of collagen peptides by sodium dodecylsulfate-polyacrylamide electrophoresis. Anal Biochem. 1971 Jun;41(2):510–516. doi: 10.1016/0003-2697(71)90173-4. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Eyre D. R., Koide S., Glimcher M. J. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg Am. 1980 Jan;62(1):79–89. [PubMed] [Google Scholar]
- Gay S. Immunohistological studies on collagen type distribution in tissues. Suppl Thromb Haemost. 1978;63:171–179. [PubMed] [Google Scholar]
- Gilbertson E. M. Development of periarticular osteophytes in experimentally induced osteoarthritis in the dog. A study using microradiographic, microangiographic, and fluorescent bone-labelling techniques. Ann Rheum Dis. 1975 Feb;34(1):12–25. doi: 10.1136/ard.34.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D. Extraction, fractionation and characterization of proteoglycans from bovine tracheal cartilage. Biochim Biophys Acta. 1972 Nov 28;285(1):181–192. doi: 10.1016/0005-2795(72)90190-0. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Howell D. S., Sapolsky A. I., Pita J. C., Woessner J. F. The pathogenesis of osteoarthritis. Semin Arthritis Rheum. 1976 May;4(4):365–383. doi: 10.1016/0049-0172(76)90015-9. [DOI] [PubMed] [Google Scholar]
- Inerot S., Heinegård D., Audell L., Olsson S. E. Articular-cartilage proteoglycans in aging and osteoarthritis. Biochem J. 1978 Jan 1;169(1):143–156. doi: 10.1042/bj1690143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalides N. A. Basement membranes: structural and biosynthetic considerations. J Invest Dermatol. 1975 Jul;65(1):85–92. doi: 10.1111/1523-1747.ep12598062. [DOI] [PubMed] [Google Scholar]
- Kempson G. E., Muir H., Swanson S. A., Freeman M. A. Correlations between stiffness and the chemical constituents of cartilage on the human femoral head. Biochim Biophys Acta. 1970 Jul 21;215(1):70–77. doi: 10.1016/0304-4165(70)90388-0. [DOI] [PubMed] [Google Scholar]
- Kostuik J. P. Recent advances in the treatment of painful adult scoliosis. Clin Orthop Relat Res. 1980 Mar-Apr;(147):238–252. [PubMed] [Google Scholar]
- Layman D. L., Sokoloff L., Miller E. J. Collagen synthesis by articular in monolayer culture. Exp Cell Res. 1972 Jul;73(1):107–112. doi: 10.1016/0014-4827(72)90107-3. [DOI] [PubMed] [Google Scholar]
- Lee P., Rooney P. J., Sturrock R. D., Kennedy A. C., Dick W. C. The etiology and pathogenesis of osteoarthrosis: a review. Semin Arthritis Rheum. 1974 Spring;3(3):189–218. doi: 10.1016/0049-0172(74)90019-5. [DOI] [PubMed] [Google Scholar]
- MEACHIM G., COLLINS D. H. Cell counts of normal and osteoarthritic articular cartilage in relation to the uptake of sulphate (35SO4) in vitro. Ann Rheum Dis. 1962 Mar;21:45–50. doi: 10.1136/ard.21.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Mayne P. M., Miller E. J. Changes in type of collagen synthesized as clones of chick chondrocytes grow and eventually lose division capacity. Proc Natl Acad Sci U S A. 1976 May;73(5):1674–1678. doi: 10.1073/pnas.73.5.1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayne R., Vail M. S., Miller E. J. Analysis of changes in collagen biosynthesis that occur when chick chondrocytes are grown in 5-bromo-2'-deoxyuridine. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4511–4515. doi: 10.1073/pnas.72.11.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt C. A. Biochemistry of articular cartilage. Nature of proteoglycans and collagen of articular cartilage and their role in ageing and in osteoarthrosis. Ann Rheum Dis. 1973 Jul;32(4):364–378. doi: 10.1136/ard.32.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt C. A., Muir H. Biochemical changes in the cartilage of the knee in experimental and natural osteoarthritis in the dog. J Bone Joint Surg Br. 1976 Feb;58(1):94–101. doi: 10.1302/0301-620X.58B1.131804. [DOI] [PubMed] [Google Scholar]
- McDevitt C., Gilbertson E., Muir H. An experimental model of osteoarthritis; early morphological and biochemical changes. J Bone Joint Surg Br. 1977 Feb;59(1):24–35. doi: 10.1302/0301-620X.59B1.576611. [DOI] [PubMed] [Google Scholar]
- Meachim G. Light microscopy of Indian ink preparations of fibrillated cartilage. Ann Rheum Dis. 1972 Nov;31(6):457–464. doi: 10.1136/ard.31.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. R., Lust G. Accumulation of procollagen in the degenerative articular cartilage of dogs with osteoarthritis. Biochim Biophys Acta. 1979 Mar 7;583(2):218–231. doi: 10.1016/0304-4165(79)90429-x. [DOI] [PubMed] [Google Scholar]
- Miller E. J. Biochemical characteristics and biological significance of the genetically-distinct collagens. Mol Cell Biochem. 1976 Dec 10;13(3):165–192. doi: 10.1007/BF01731779. [DOI] [PubMed] [Google Scholar]
- Miller E. J., Lunde L. G. Isolation and characterization of the cyanogen bromide peptides from the alpha 1(II) chain of bovine and human cartilage collagen. Biochemistry. 1973 Aug 14;12(17):3153–3159. doi: 10.1021/bi00741a003. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr, Glossmann H. Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol. 1974;32:92–102. doi: 10.1016/0076-6879(74)32012-5. [DOI] [PubMed] [Google Scholar]
- Nimni M., Deshmukh K. Differences in collagen metabolism between normal and osteoarthritic human articular cartilage. Science. 1973 Aug 24;181(4101):751–752. doi: 10.1126/science.181.4101.751. [DOI] [PubMed] [Google Scholar]
- Norby D. P., Malemud C. J., Sokoloff L. Differences in the collagen types synthesized by lapine articular chondrocytes in spinner and monolayer culture. Arthritis Rheum. 1977 Mar;20(2):709–716. doi: 10.1002/art.1780200211. [DOI] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Peyron J. G. Epidemiologic and etiologic approach of osteoarthritis. Semin Arthritis Rheum. 1979 May;8(4):288–306. doi: 10.1016/0049-0172(79)90006-4. [DOI] [PubMed] [Google Scholar]
- Pond M. J., Nuki G. Experimentally-induced osteoarthritis in the dog. Ann Rheum Dis. 1973 Jul;32(4):387–388. doi: 10.1136/ard.32.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repo R. U., Mitchell N. Collagen synthesis in mature articular cartilage of the rabbit. J Bone Joint Surg Br. 1971 Aug;53(3):541–548. [PubMed] [Google Scholar]
- Sajdera S. W., Hascall V. C. Proteinpolysaccharide complex from bovine nasal cartilage. A comparison of low and high shear extraction procedures. J Biol Chem. 1969 Jan 10;244(1):77–87. [PubMed] [Google Scholar]
- Tirgari M., Vaughan L. C. Arthritis of the canine stifle joint. Vet Rec. 1975 May 3;96(18):394–399. doi: 10.1136/vr.96.18.394. [DOI] [PubMed] [Google Scholar]