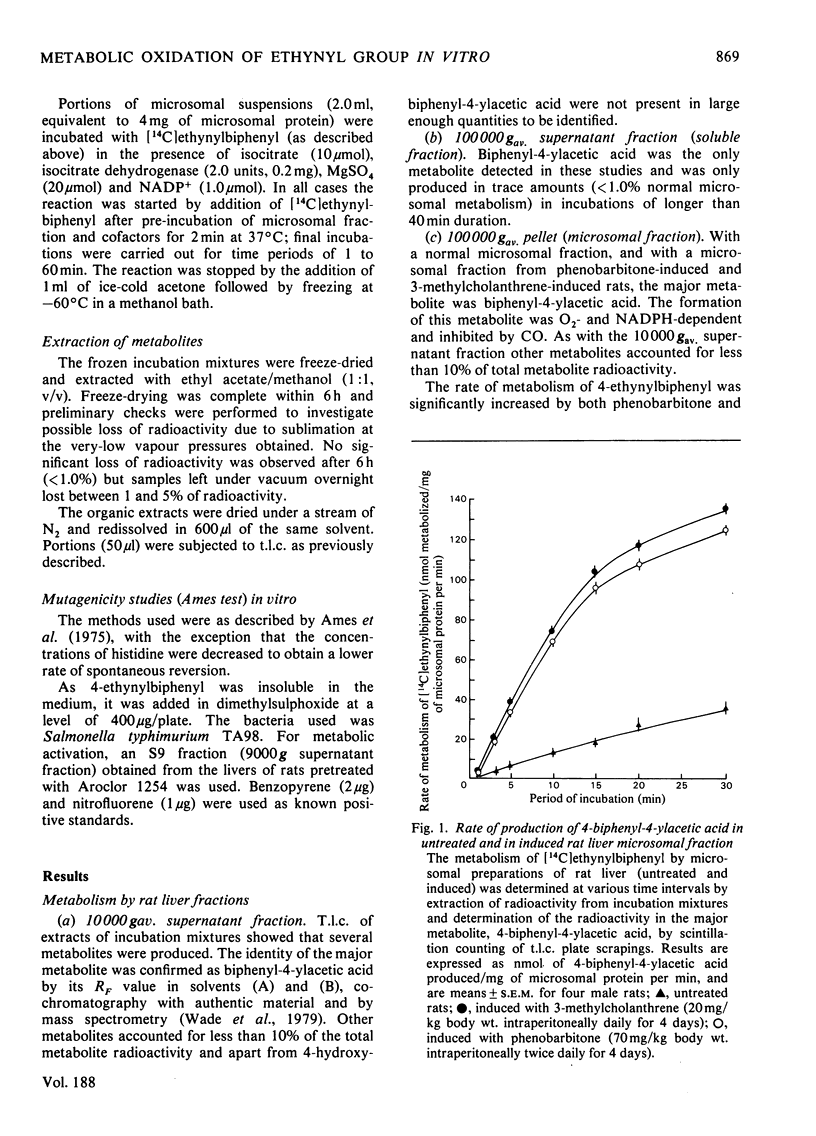
Abstract
1. The metabolism of 4-ethynylbiphenyl has been studied in vitro with subcellular fractions of normal and induced rat liver, and rat intestinal microflora (caecal contents). 2. Oxidation was NADPH-dependent, was inhibited by CO and stimulated by pretreatment with phenobarbitone or 3-methylcholanthrene. 3. Oxidation of the ethynyl group occurred in washed microsomal preparations, but not significantly in soluble fractions. Oxidation of the ethynyl group by a microsomal fraction preceded aromatic hydroxylation and no metabolites containing the intact ethynyl group were detected. 4. The major metabolite in liver fractions was biphenyl-4-ylacetic acid. This was the only product produced by a modified Udenfriend system. 5. Metabolism of 4-ethynylbiphenyl by rat caecal contents under anaerobic conditions produced very small amounts of 4-vinylbiphenyl. 6. In a modified Ames test with Salmonella typhimurium TA98, 4-ethynylbiphenyl gave a weak positive result that was doubled after 'activation' with an induced rat S9 fraction.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Mccann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975 Dec;31(6):347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Jerina D. M., Witkop B. Arene oxides and the NIH shift: the metabolism, toxicity and carcinogenicity of aromatic compounds. Experientia. 1972 Oct 15;28(10):1129–1149. doi: 10.1007/BF01946135. [DOI] [PubMed] [Google Scholar]
- Dilworth M. J. Acetylene reduction by nitrogen-fixing preparations from Clostridium pasteurianum. Biochim Biophys Acta. 1966 Oct 31;127(2):285–294. doi: 10.1016/0304-4165(66)90383-7. [DOI] [PubMed] [Google Scholar]
- EL MASRI A. M., SMITH J. N., WILLIAMS R. T. Studies in detoxication. 73. The metabolism of alkylbenzenes: phenylacetylene and phenylethylene (styrene). Biochem J. 1958 Feb;68(2):199–204. doi: 10.1042/bj0680199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeke B., Hallström G., Anderson E. Enzymic oxidation alpha to the acetylenic group in the metabolism of N-(5-pyrrolidinopent-3-ynyl)-succinimide (BL 14) in vitro. Xenobiotica. 1978 Jun;8(6):341–348. doi: 10.3109/00498257809070017. [DOI] [PubMed] [Google Scholar]
- Oesch F. Mammalian epoxide hydrases: inducible enzymes catalysing the inactivation of carcinogenic and cytotoxic metabolites derived from aromatic and olefinic compounds. Xenobiotica. 1973 May;3(5):305–340. doi: 10.3109/00498257309151525. [DOI] [PubMed] [Google Scholar]
- Scheline R. R. Decarboxylation and demethylation of some phenolic benzoic acid derivatives by rat caecal contents. J Pharm Pharmacol. 1966 Oct;18(10):664–669. doi: 10.1111/j.2042-7158.1966.tb07780.x. [DOI] [PubMed] [Google Scholar]
- UDENFRIEND S., CLARK C. T., AXELROD J., BRODIE B. B. Ascorbic acid in aromatic hydroxylation. I. A model system for aromatic hydroxylation. J Biol Chem. 1954 Jun;208(2):731–739. [PubMed] [Google Scholar]
- Wade A., Symons A. M., Martin L., Parke D. V. Metabolic oxidation of the ethynyl group in 4-ethynylbiphenyl. Biochem J. 1979 Dec 15;184(3):509–517. doi: 10.1042/bj1840509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White I. N. Metabolic activation of acetylenic substituents to derivatives in the rat causing the loss of hepatic cytochrome P-450 and haem. Biochem J. 1978 Sep 15;174(3):853–861. doi: 10.1042/bj1740853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P., English J., Chakraborty J., Hinton R. The use of non-ionic industrial detergents in liquid scintillation counting. Lab Pract. 1975 Nov;24(11):739–740. [PubMed] [Google Scholar]


