Abstract
Peripheral artery disease (PAD) causes lower extremity dysfunction and is associated with an increased risk of cardiovascular mortality and morbidity. In this study, we analyzed how non-invasive 2-dimensional-phase-contrast magnetic resonance imaging (2D-PC-MRI) measured velocity markers of the distal superficial femoral artery (SFA) are associated with clinical and functional characteristics of PAD. A total of 70 (27 diabetic and 43 non-diabetic) PAD patients were included in this secondary analysis of data collected from the Effect of Lipid Modification on Peripheral Artery Disease after Endovascular Intervention Trial (ELIMIT). Electrocardiographically (ECG)-gated 2D-PC-MRI was performed at a proximal and a distal imaging location of the distal SFA. Baseline characteristics did not differ between diabetic and non-diabetic PAD patients. Claudication onset time (COT) was shorter in diabetic PAD patients compared to non-diabetics (0.56 (inter quartile range (IQR): 0.3, 2.04) minutes vs. 1.30 (IQR: 1.13, 2.15) minutes, p=0.025). In a pooled analysis of all 70 PAD patients, maximum velocity was significantly higher in the proximal compared with the distal SFA segment (43.97 (interquartile range (IQR): 20.4, 65.2) cm/s; vs. 34.9 (IQR: 16.87, 51.71) cm/s; p<0.001). The maximum velocities in both the proximal and distal SFA segments were significantly higher in diabetic PAD patients compared with non-diabetics (proximal: 53.6 (IQR: 38.73, 89.43) cm/s vs. 41.49 (IQR: 60.75, 15.9) cm/s, p=0.033; distal: 40.8 (IQR: 23.7, 71.90) cm/s vs. 27.4 (IQR: 41.67, 12.54) cm/s, p=0.012). Intra-observer variability, as assessed by intraclass correlation (ICC) analysis, was excellent for SFA mean and maximum velocities (0.996 (confidence interval [CI]: 0.996, 0.997); 0.999 (CI: 0.999, 0.999)). In conclusion, 2D-PC-MRI SFA velocity measures are reproducible and may be of interest in assessing diabetic and non-diabetic PAD patients.
Keywords: peripheral artery disease, magnetic resonance imaging, diabetes mellitus, atherosclerosis, superficial femoral artery, arterial blood flow velocity
1. Introduction
Peripheral artery disease (PAD) is a debilitating illness affecting more than 8.5 million Americans of age 40 and older and 202 million people globally [1–4]. PAD causes hemodynamic dysfunction and is associated with impaired lower extremity function, reduced quality of life, and possibly limb loss [5–7]. Intermittent claudication is a classic PAD symptom that occurs in 40% of symptomatic patients and is associated with a 5-, 10-, and 15-year mortality rate of 30%, 50%, and 70%, respectively [8–10]. Non-invasive imaging remains of central importance in assessing PAD [6, 11]. Among imaging techniques, magnetic resonance imaging (MRI) has been utilized to investigate superficial femoral artery (SFA) plaque burden and vessel morphology [8] [12]. Two dimensional-phase-contrast MRI (2D-PC-MRI) is a validated non-invasive rapid technique utilizing the phase shift of the MR signal produced by blood flowing in a magnetic field to measure blood flow velocity [13, 14]. 2D-PC-MRI has been applied successfully among others to quantify ventricular function, valvular heart disease, pulmonary artery disease, thoracic aortic disease, congenital heart disease, ischemic heart disease and PAD [13–15]. Phase-contrast MRI based coronary sinus blood flow measures have been shown to be useful as a prognostic marker for diabetic patients [16]. Phase-contrast MRI has also been utilized to study leg thermotherapy in patients with symptomatic PAD resulting in an increased peak blood flow velocity [17]. However, it remains unclear if 2D-PC-MRI can be utilized to non-invasively study differences in blood flow velocities in diabetic and non-diabetic PAD patients. In this secondary analysis of data collected from the Effect of Lipid Modification on Peripheral Artery Disease after Endovascular Intervention Trial (ELIMIT; NCT00687076) [8], we analyzed associations of 2D-PC-MRI velocity measurements of the distal SFA with clinical and functional characteristics of PAD. We hypothesized that MRI-based measures of SFA velocity are associated with PAD severity including diabetes status and functional capacity.
2. Materials and Methods
2.1. Study Design
ELIMIT was a double-blind and double-placebo randomized controlled study of PAD patients, results of which were previously published [8]. PAD patients were recruited between 2005 to 2008 at the Ben Taub General Hospital, the Michael E. DeBakey Veterans Affairs Medical Center, and the Houston Methodist Hospital in Houston, TX. The study was approved by the local institutional review board and participants provided informed consent. Briefly, a total of 102 participants with lifestyle-limiting claudication consistent with Fontaine stage IIa/IIb were randomized to either triple lipid-modification therapy consisting of simvastatin (40 mg/day), ezetimibe (10 mg/day), and niacin (1,500 mg/day), or to monotherapy with Simvastatin (40 mg/day) only. In addition to the randomized therapy, patients continued to receive standard of care including medical management and the option of vascular intervention (lower-extremity revascularization), if indicated. PAD was confirmed either clinically using an ankle brachial index (ABI) < 0.90 or via imaging studies that included a duplex ultrasound.
2.2. MRI
MR imaging was performed at baseline, 6, 12, and 24 months, as previously reported [8] [12]. MRI scans were acquired using a 3.0T system (Signa Excite, GE Healthcare, Milwaukee, Wisconsin) with a unilateral phased array coil (Pathway Biomedical, Inc.). The coil was centered 8 cm above the patella and secured with a Velcro strap to image the distal SFA territory. SFA plaque burden imaging with fast spin echo proton-density-weighted (FSE-PDW) scans were acquired for both lower extremities (repetition time (TR)= 2575 ms, echo time (TE)= 30 ms, number of slices= 40, field of view (FOV)= 22 cm, flip angle (FA)= 90°, slice thickness (ST)= 2 mm, in-plane pixel spacing= 0.43 × 0.43 mm, echo train length (ETL)= 8, matrix size= 384 × 224). In addition, 2D-PC-MRI scans were acquired during the same exam with ST=6mm, TR=10.6ms, TE=4.97ms, ETL=1, trigger window=20%, bandwidth=244 Hz/pixel, and a phase-contrast encoding velocity (VENC) of 120 cm/sec (through-plane encoding). 2D-PC-MRI scans were electrocardiographically (ECG) gated and obtained at a proximal and a distal slice location within the field of view of the primary FSE-PDW scans.
MRI Analysis
SFA lumen, wall, and total vessel volumes were quantified by two readers with VesselMASS (University of Leiden, The Netherlands) as previously reported [8]. Image analysis was performed for the target limb, defined as the non-intervened limb or the less symptomatic limb in patients who were not scheduled for revascularization at the time of recruitment.
SFA lumen boundaries were traced on the magnitude images and then propagated on the phase images of the 2D-PC-MRI scans to determine velocities at the proximal and distal locations within the FOV of the FSE-PDW scans (Figure 1). Tracings were done with Sante DICOM Editor Version 3.0 (Santesoft LTD, Greece). Velocities were measured within the traced region of interest (ROI) of the SFA lumen for each acquired frame over the cardiac cycle. Subsequently, the maximum velocity was determined as the maximum of all peak velocities across all frames acquired over the cardiac cycle. Similarly, the minimum velocity was determined as the minimum of all lowest velocities across all frames acquired over the cardiac cycle. The reported average velocities were determined as the mean velocities averaged over all frames. Velocity differences were calculated by subtracting the velocity measured at the distal location from the velocity measured at the proximal location. We performed background corrections to compensate for background noise, significant bulk motion including involuntary leg twitching (which is common in PAD patients [18]), and arbitrary phase offset errors. Corrections were applied by subtracting the mean phase information of an adjacent stationary background area from the ROI of the SFA (done with MATLAB, MathWorks Inc., Natick, MA). The background ROI was placed within the adductor muscle or vastus medialis muscle and care was taken to exclude arteries or veins (Figure 1). Background regions were at least as large as luminal ROIs. Reproducibility of velocity profiles and background corrections were determined by intra-reader reproducibility analysis and in addition, inter-reader variability was also determined for the background corrections. Both readers were blinded to patient identifiers. Intra-reader reproducibility was assessed for a single reader for the velocity profile and background correction tracings by choosing 10 randomly selected 2DPC-MRI scans that were re-traced four weeks after the initial reading. In addition, inter-reader variability was assessed for the background correction tracings by choosing a different set of 20 randomly selected 2DPC-MRI scans that were traced by both readers.
Figure 1.
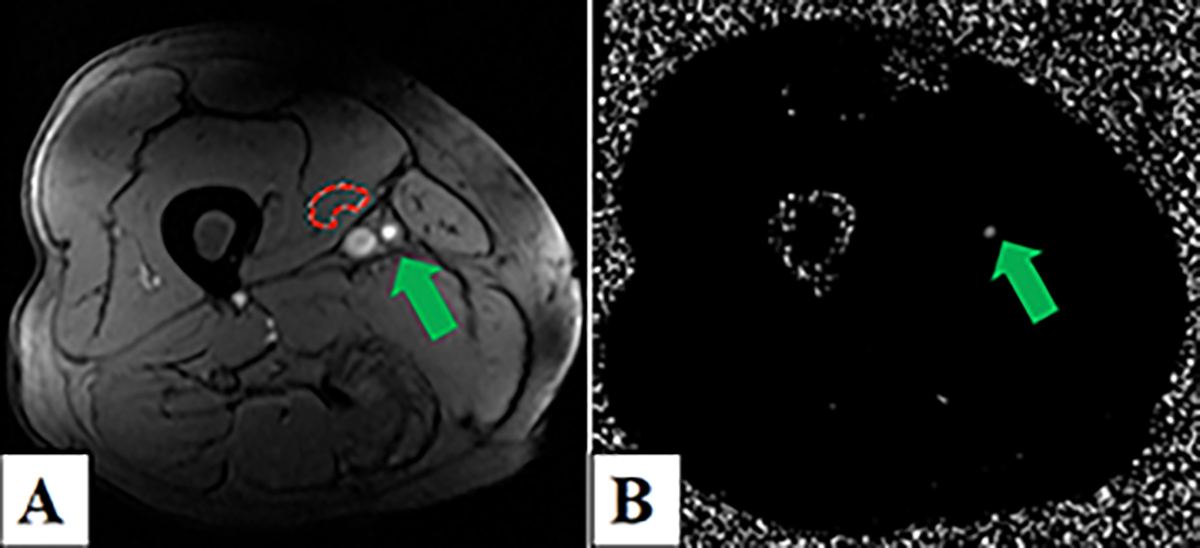
Two-dimensional-phase-contrast magnetic resonance imaging (2D-PC-MRI) of the right distal superficial femoral artery (SFA) in a non-diabetic peripheral artery disease (PAD) patient. Panel A) Magnitude image depicting the background correction region of interest in the vastus medialis (red contour) and the SFA (green arrow). Panel B) Corresponding phase-contrast image showing the SFA (green arrow).
Velocity Pulsatility Index
The velocity pulsatility index (VPI) was calculated by subtracting the minimum velocity from the maximum velocity and dividing by the mean velocity for a specific location of the vessel. Using this approach, VPI at the proximal and distal sites were calculated, separately.
2.3. Statistical Analysis
Baseline patient characteristics were expressed as mean (standard deviation), median, and interquartile range (IQR, 25 % and 75%) for non-normal variables, percentages, and frequencies, as appropriate. Non-parametric and parametric continuous variables were compared using the Mann-Whitney-Wilcoxon test and the independent sample student’s t-test, as appropriate. Categorical data were analyzed by Chi-square tests. Data normality was determined using the Shapiro-Wilk test. Pooled data were analyzed separately. Linear regression analyses were performed to determine associations between MRI measured volumes and velocities with known clinical markers of PAD. All tests were two-tailed and the statistical significance level was determined at a p-value of < 0.05. Intra-reader reproducibility and inter-reader variability was assessed by intraclass correlation (ICC) analysis. ICC analysis was performed using a 2-way random-effects model, in which ICC>0.7 was considered an excellent agreement. All statistical analyses were performed using Stata Statistical Software (College Station, Texas, StataCorp LP) and SAS (SAS Institute, Inc., Cary, NC).
3. Results
3.1. Baseline Characteristics
Out of 102 randomized participants, 87 completed baseline MR imaging (1 participant withdrew, 6 participants declined blood draws, 8 participants opted out of MRI), of whom 17 did not have 2D-PC-MRI scans (14 did not undergo 2D-PC-MRI scans, and 3 had a missing proximal 2D-PC-MRI scan). Therefore, a total of 70 patients was included for the analyses. Among the 70 PAD patients, 43 were non-diabetic and 27 were diabetic. The baseline characteristics, including lipids, did not differ between diabetic and non-diabetic PAD patients except for body mass index (BMI), which was marginally significant (p= 0.051, Table 1).
Table 1.
Baseline patient characteristics.
| Variables | All Patients (Total, N=70) | Diabetic PAD Patients (N=27) | Non-Diabetic PAD Patients (N=43) | P-value |
|---|---|---|---|---|
|
| ||||
| Age (years) | 63.15 ± 6.33 | 62.49 ± 6.08 | 63.56 ± 6.5 | 0.92 |
| Male sex, n (%) | 64 (91.43) | 24 (34.29) | 40 (57.14) | 0.67 |
| Black race, n (%) | 13 (18.57) | 4 (5.71) | 9 (12.86) | 0.52 |
| Body mass index (kg/m²) | 29.9 (25.2, 35.5) | 31.3 (28.5, 39) | 27.7 (23.8, 33.8) | 0.051 |
| Aspirin, n (%) | 69 (98.57) | 27 (38.57) | 42 (60.00) | 1.00 |
| Statin, n (%) | 68 (97.14) | 26 (37.14) | 42 (60.00) | 1.00 |
| Current smoking, n (%) | 32 (45.71) | 9 (12.86) | 23 (32.86) | 0.10 |
| Diabetes mellitus, n (%) | 27 (38.57) | 13 (18.57) | 14 (20.00) | 0.53 |
| Hypertension, n (%) | 57 (81.43) | 23 (32.86) | 34 (48.57) | 0.52 |
| Hyperlipidemia, n (%) | 64 (95.52) | 24 (35.82) | 40 (59.70) | 1.00 |
| Coronary artery disease, n (%) | 21 (32.31) | 10 (15.38) | 11 (16.92) | 0.22 |
| History of revascularization, n (%) | 27 (38.57) | 10 (14.29) | 17 (24.29) | 0.83 |
| Triglyceride (mg /dl) | 134.0 (80) | 145 (102, 192) | 128 (95, 177) | 0.63 |
| Non-HDL cholesterol (mg /dl) | 123.5 (100, 152) | 113 (84, 164) | 130 (109, 151) | 0.28 |
| LDL cholesterol (mg /dl) | 96.0 (73, 121) | 84.50 (61, 126) | 101 (79, 120) | 0.18 |
| HDL cholesterol (mg /dl) | 40.5 (34, 47) | 35.0 (32, 47) | 42 (35, 47) | 0.11 |
| C-reactive protein (mg /dl) | 3.0 (1.7, 5.4) | 4.26 (1.9, 8.08) | 279 (1.7, 4.7) | 0.08 |
| Total cholesterol (mg /dl) | 168.0 (137, 200) | 157.73 (127, 200) | 173 (146, 200) | 0.18 |
Values are reported as mean (standard deviation), medians and interquartile range (IQR), and as frequencies (percentage). PAD: peripheral artery disease. For hyperlipidemia, LDL cholesterol, and coronary artery disease: total n=26.
In this secondary analysis of ELIMIT data which included 70 PAD patients, baseline characteristics between the lipid-modifying mono- (n= 37) and triple-therapy (n= 33) groups did not differ (data not shown).
3.2. Intra-Reader Reproducibility And Inter-Reader Variability
Both intra-reader reproducibility for SFA mean and maximum velocities (as assessed by ICC analyses) and background correction tracings were excellent (0.996 (confidence interval [CI]: 0.996, 0.997); 0.999 (CI: 0.999, 0.999); and 0.99 (CI: 0.986, 0.992), respectively); Table 2). Inter-reader variability was excellent for the background correction tracings (0.988 CI: 0.986, 0.989, Table 2).
Table 2.
Intra-reader reproducibility and inter-reader variability.
| Intra-reader ICC for SFA Velocities |
Intra-reader ICC for Background Correction Tracings |
|||||
|---|---|---|---|---|---|---|
| N (patients) | ICC | CI (95%) | N (patients) | ICC | CI (95%) | |
|
|
|
|||||
| Individual ICC, mean | 10 | 0.996 | 0.996 – 0.997 | 10 | 0.99 | 0.986 – 0.992 |
| Average ICC, mean | 10 | 0.998 | 0.998 – 0.998 | 10 | 0.995 | 0.993 – 0.996 |
| Individual ICC, maximum | 10 | 0.999 | 0.999 – 0.999 | 10 | 0.972 | 0.961 – 0.980 |
| Average ICC, maximum | 10 | 0.999 | 0.999 – 0.999 | 10 | 0.986 | 0.980 – 0.989 |
|
| ||||||
| Inter-reader ICC for background correction tracings |
||||||
| Individual ICC, mean | 20 | 0.988 | 0.986 – 0.989 | |||
| Average ICC, mean | 20 | 0.994 | 0.993 – 0.994 | |||
| Individual ICC, maximum | 20 | 0.948 | 0.921 – 0.964 | |||
| Average ICC, maximum | 20 | 0.973 | 0.959 – 0.981 | |||
ICC and confidence interval were calculated using a two-way model. SFA: Superficial Femoral Artery. ICC: intra-class correlation. CI: Confidence interval.
3.3. MRI-Based Measures Of SFA
The maximum and average velocities were significantly higher in the proximal compared with the distal SFA segment (maximum velocity: 43.97 (IQR: 20.4, 65.2) cm/s; vs. 34.9 (IQR: 16.87, 51.71) cm/s; p<0.001, Table 3). Conversely, the minimum velocities were similar between the proximal and distal SFA segments (p=0.91). The VPI was higher at the proximal compared to the distal SFA segment (maximum velocity: 1.65 (IQR: 1.03, 1.83) cm/s; vs. 1.5 (IQR: 0.74, 1.8) cm/s; p=0.015, Table 3).
Table 3.
Magnetic resonance imaging measured superficial femoral artery velocities at the proximal and distal imaging locations.
| Proximal Location |
Distal Location |
||||
|---|---|---|---|---|---|
| Pooled Data (N=70) | N | Median (IQR) | N | Median (IQR) | P-value |
|
| |||||
| Average velocity, cm/s | 70 | 26.8 (16.4, 37.1) | 69 | 18.88 (11.1, 28.6) | <0.001 |
| Maximum velocity, cm/s | 70 | 43.97 (20.4, 65.2) | 69 | 34.9 (16.9, 51.7) | <0.001 |
| Minimum velocity, cm/s | 70 | 4.13 (1.99, 9.18) | 69 | 4.31 (2.47, 7.4) | 1.00 |
| Velocity pulsatility index (VPI) | 70 | 1.65 (1.02, 1.83) | 69 | 1.5 (0.74, 1.8) | 0.015 |
The proximal and distal maximum velocities were significantly higher in diabetic-PAD patients compared with non-diabetics (proximal: 53.6 (IQR: 38.73, 89.43) cm/s vs. 41.49 (IQR: 60.75, 15.9) cm/s, p=0.033; distal: 40.8 (IQR: 23.7, 71.90) cm/s vs. 27.4 (IQR: 41.67, 12.54) cm/s, p=0.012; (Table 4). Neither the minimal velocities nor the velocity differences between the proximal and distal SFA segments differed between the groups.
Table 4.
Magnetic resonence imaging measured superficial femoral artery velocities and measures of plaque burden, and clinical markers of PAD of diabetic and non-diabetic PAD patients.
| Diabetic PAD Patients |
Non-Diabetic PAD Patients |
||||
|---|---|---|---|---|---|
| N | Median (IQR) | N | Median (IQR) | P-value | |
|
| |||||
| A. MRI Parameters | |||||
|
| |||||
| SFA Velocities | |||||
|
| |||||
| Proximal SFA average velocity, cm/s | 27 | 28.0 (20.53, 44.83) | 43 | 24.75 (24.75, 34.45) | 0.08 |
| Proximal SFA maximum velocity, cm/s | 27 | 53.6 (38.73, 89.43) | 43 | 41.49 (15.9, 60.75) | 0.033 |
| Proximal SFA minimum velocity, cm/s | 27 | 4.05 (2.13, 8) | 43 | 7.29 (1.5, 9.2) | 0.50 |
| Distal SFA average velocity, cm/s | 27 | 25.85 (15.2, 51.96) | 42 | 15.89 (9.92, 24.65) | 0.021 |
| Distal SFA maximum velocity, cm/s | 27 | 40.8 (23.7, 71.90) | 42 | 27.4 (12.54, 41.67) | 0.012 |
| Distal SFA minimum velocity, cm/s | 27 | 4.92 (5.73, 7.34) | 42 | 4.33 (4.33, 7.75) | 0.53 |
| Delta minimum velocity, cm/s | 27 | −0.02 (−1.59, 1.71) | 42 | 0.159 (−1.7, 2.18) | 0.93 |
| Delta maximum velocity, cm/s | 27 | 2.81 (−5.6, 20.17) | 42 | 4.88 (1.5, 20.14) | 0.49 |
| Delta average velocity, cm/s | 27 | 1.21 (−2.14, 10.8) | 42 | 6.53 (0.56, 12.63) | 0.26 |
|
| |||||
| SFA Plaque Burden Measures | |||||
|
| |||||
| SFA wall volume, cc | 27 | 0.042 (0.030, 0.050) | 43 | 0.037 (0.030, 0.05) | 0.41 |
| SFA lumen volume, cc | 27 | 0.016 (0.008, 0.024) | 43 | 0.017 (0.009, 0.023) | 0.95 |
| SFA total volume, cc | 27 | 0.059 (0.041, 0.074) | 43 | 0.056 (0.043, 0.065) | 0.59 |
| 24Mo Δ of SFA wall volume, cc | 14 | 0.041 (0.031, 0.051) | 24 | 0.038 (0.031, 0.045) | 0.62 |
| 24Mo Δ of SFA lumen volume, cc | 14 | 0.019 (0.012, 0.032) | 24 | 0.012 (0.007, 0.025) | 0.14 |
| 24Mo Δ of SFA total volume, cc | 14 | 0.062 (0.047, 0.082) | 24 | 0.059 (0.039, 0.068) | 0.27 |
|
| |||||
| B. Clinical Markers of PAD | |||||
|
| |||||
| Ankle brachial index | 22 | 0.78 (0.64, 0.89) | 31 | 0.8 (0.66, 1.0) | 0.32 |
| Claudication onset time, (min) | 21 | 0.56 (0.3, 2.04) | 33 | 1.30 (1.13, 2.15) | 0.025 |
| Peak walking time, (min) | 22 | 3.05 (1.19, 4.16) | 33 | 2.49 (1.48, 4.02) | 0.67 |
| Initial distance walked, (miles) | 19 | 0.02 (0.0, 0.06) | 33 | 0.04 (0.03, 0.08) | 0.003 |
| Absolute distance walked, (miles) | 20 | 0.09 (0.05, 0.135) | 33 | 0.09 (0.06, 0.18) | 0.69 |
PAD: peripheral artery disease; SFA: superficial femoral artery; MRI: magnetic resonance imaging; Delta (Δ) refers to the velocity difference between the distal and proximal SFA locations. Mo: month.
SFA total, wall, and lumen volumes did not differ between diabetic and non-diabetic PAD patients (Table 4).
Claudication onset time (COT) was shorter in diabetic PAD patients when compared to non-diabetics (0.56 (IQR: 0.3, 2.04) minutes vs. 1.30 (IQR: 1.13, 2.15) minutes, p=0.025), while peak walking time (PWT) did not differ (p=0.67). Additionally, ABIs were similar between diabetic and non-diabetic PAD patients.
The results of sub-group analysis showed that the maximum and average velocities were higher in the proximal compared to the distal SFA segment in non-diabetics, but not in diabetic PAD patients (Table 5). The analysis identified a significant difference in the VPI between the proximal and distal locations among the non-diabetic PAD patients, but not among the diabetic PAD patients (p=0.043 vs. p=0.24, respectively).
Table 5.
MRI velocity differences between the proximal and distal imaging locations for diabetic and non-diabetic PAD patients.
| Proximal SFA Location | Distal SFA Location | P-value | |||
|---|---|---|---|---|---|
|
|
|||||
| Diabetic PAD Patients | N | Median (IQR) | N | Median (IQR) | |
|
| |||||
| Average velocity, cm/s | 27 | 27.98 (20.532, 44.83) | 27 | 25.85 (15.21, 39.55) | 0.25 |
| Maximum velocity | 27 | 53.63 (38.73, 89.43) | 27 | 40.78 (23.71, 71.91) | 0.25 |
| Minimum velocity | 27 | 4.056 (2.13, 7.98) | 27 | 4.31 (0.77, 7.34) | 1.00 |
| Velocity pulsatility index | 27 | 1.74(1.43, 1.91) | 27 | 1.73 (1.20, 1.90) | 0.24 |
|
| |||||
| Non-Diabetic PAD Patients | |||||
|
| |||||
| Average velocity | 43 | 24.753 (9.76, 34.45) | 42 | 15.90 (9.92, 24.66) | <0.003 |
| Maximum velocity | 43 | 39.72 (15.9, 60.3) | 42 | 27.4 (12.54, 41.67) | <0.003 |
| Minimum velocity | 43 | 4.43 (1.55, 9.20) | 42 | 4.33 (2.47, 7.76) | 0.88 |
| Velocity pulsatility index | 43 | 1.52 (0.66, 1.74) | 42 | 1.38 (0.60, 1.73) | 0.043 |
PAD: peripheral artery disease; SFA: superficial femoral artery; MRI: magnetic resonance imaging.
Results of pooled analysis revealed a significant association between the ABI and the proximal maximum and average velocities, as well as velocity differences (p<0.005, Table 6). In a sub-group analysis among non-diabetics, the ABI was significantly associated with the proximal maximum and average velocities and velocity differences, whereas no significant associations were found in a separate analysis of diabetic PAD patients.
Table 6.
Associations between the ankle brachial index (ABI) and magnetic resonance imaging velocity parameters.
| Independent Variable | N | BETA | SE | R2 | Adjusted R2 | P-value | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Pooled Analysis | |||||||
|
| |||||||
| ABI | Proximal SFA average velocity, cm/s | 53 | 0.005 | 0.002 | 0.156 | 0.139 | 0.004 |
| Proximal SFA maximum velocity, cm/s | 53 | 0.003 | 0.001 | 0.152 | 0.135 | 0.004 | |
| Proximal SFA minimum velocity, cm/s | 53 | 0 | 0.004 | 0 | −0.019 | 0.90 | |
| Distal SFA average velocity, cm/s | 52 | 0.003 | 0.002 | 0.044 | 0.025 | 0.13 | |
| Distal SFA maximum velocity, cm/s | 52 | 0.002 | 0.001 | 0.052 | 0.033 | 0.10 | |
| Distal SFA minimum velocity, cm/s | 52 | −0.003 | 0.005 | 0.007 | −0.013 | 0.56 | |
| Delta SFA average velocity, cm/s | 52 | 0.009 | 0.003 | 0.133 | 0.116 | 0.008 | |
| Delta SFA maximum velocity, cm/s | 52 | 0.005 | 0.002 | 0.132 | 0.114 | 0.008 | |
| Delta SFA minimum velocity, cm/s | 52 | 0.004 | 0.005 | 0.01 | −0.01 | 0.49 | |
|
| |||||||
| Diabetic PAD Patients | |||||||
|
| |||||||
| ABI | Proximal SFA average velocity, cm/s | 22 | 0.006 | 0.003 | 0.155 | 0.112 | 0.07 |
| Proximal SFA maximum velocity, cm/s | 22 | 0.003 | 0.001 | 0.168 | 0.126 | 0.06 | |
| Proximal SFA minimum velocity, cm/s | 22 | −0.005 | 0.009 | 0.013 | −0.037 | 0.61 | |
| Distal SFA average velocity, cm/s | 22 | 0.002 | 0.003 | 0.028 | −0.021 | 0.46 | |
| Distal SFA maximum velocity, cm/s | 22 | 0.002 | 0.001 | 0.048 | 0.001 | 0.32 | |
| Distal SFA minimum velocity, cm/s | 22 | −0.01 | 0.008 | 0.072 | 0.025 | 0.23 | |
| Delta SFA average velocity, cm/s | 22 | 0.007 | 0.004 | 0.106 | 0.061 | 0.14 | |
| Delta SFA maximum velocity, cm/s | 22 | 0.003 | 0.002 | 0.09 | 0.044 | 0.18 | |
| Delta SFA minimum velocity, cm/s | 22 | 0.039 | 0.018 | 0.183 | 0.142 | 0.047 | |
|
| |||||||
| Non-Diabetic PAD Patients | |||||||
|
| |||||||
| ABI | Proximal SFA average velocity, cm/s | 31 | 0.007 | 0.002 | 0.227 | 0.201 | 0.007 |
| Proximal SFA maximum velocity, cm/s | 31 | 0.003 | 0.001 | 0.230 | 0.203 | 0.006 | |
| Proximal SFA minimum velocity, cm/s | 31 | 0.001 | 0.005 | 0.001 | −0.034 | 0.89 | |
| Distal SFA average velocity, cm/s | 30 | 0.008 | 0.003 | 0.189 | 0.160 | 0.016 | |
| Distal SFA maximum velocity, cm/s | 30 | 0.004 | 0.002 | 0.183 | 0.154 | 0.018 | |
| Distal SFA minimum velocity, cm/s | 30 | 0.002 | 0.007 | 0.004 | −0.032 | 0.76 | |
| Delta SFA average velocity, cm/s | 30 | 0.011 | 0.005 | 0.141 | 0.111 | 0.041 | |
| Delta SFA maximum velocity, cm/s | 30 | 0.006 | 0.003 | 0.161 | 0.131 | 0.028 | |
| Delta SFA minimum velocity, cm/s | 30 | 0.000 | 0.005 | 0.000 | −0.036 | 0.96 | |
BETA: parameters estimate; SE: standard error; PAD: peripheral artery disease; SFA: superficial femoral artery; ABI: ankle brachial index. Delta refers to the velocity difference between the distal and proximal SFA locations.
In a pooled analysis of all patients and in a separate analysis among diabetic PAD patients, the VPI at the distal SFA was significantly associated with the SFA lumen volume, but no association was seen among non-diabetic PAD patients (Supplementary Table 1).
4. Discussion
In this study, we have analyzed 2D-PC-MRI based measures of velocity of the distal SFA territory in PAD patients with and without diabetes. We identified four primary findings. First, in a pooled analysis of all PAD patients, 2D-PC-MRI based measures of velocity decreased significantly between proximal and distal SFA imaging locations. Second, the maximum velocities in both the proximal and distal SFA segments were significantly higher in diabetic PAD patients compared with non-diabetics. Third, intra-observer variability was excellent for SFA mean and maximum velocities, as well as for background correction tracings. Fourth, COT was shorter in diabetic PAD patients compared to non-diabetics, as anticipated.
PAD is typically characterized by atherosclerotic lesions in the lower extremities. The femoral and the popliteal arteries are the most common sites affected by atherosclerosis, followed by the distal aorta and the iliac arteries [19]. The poor circulation leads to transient limb ischemia and calf pain following walking or exertion [20].
This study demonstrated a decrease in blood flow velocity from a proximal to a distal location of the distal SFA, which is consistent with results of previous studies that confirmed reduced blood flow more distally in PAD patients with a history of claudication due to luminal narrowing from atherosclerosis [20].
Several studies have demonstrated an association between diabetes and the development of atherosclerotic lesions in the lower limbs. Impaired glucose tolerance alone is associated with 2 to 4-fold increased risk of having intermittent claudication (in men and women, respectively) [21, 22]. Diabetic PAD patients are reported to have a higher risk of mortality, morbidity, and poor outcomes than non-diabetics, as evidenced by results of previous studies in which PAD patients with diabetes demonstrated a seven-fold increased risk of lower extremity amputation ) [21, 23, 24]. In this study, subgroup analysis was performed based on diabetes status, and a similar pattern of higher velocity at the proximal site followed by a decrease in velocity distally was observed among the non-diabetic PAD patients. However, in diabetic PAD patients, the difference in maximum and average velocities between the proximal and distal SFA locations did not differ significantly.
Vascular remodeling in diabetic PAD patients, affecting vascular compliance and eventually blood flow velocity, may differ from than in non-diabetics. A study by Zamin et al. established that vascular remodeling in PAD patients is mostly associated with atherosclerosis, a condition of low-grade chronic inflammation and arterial calcification [25]. Their study also reported a higher prevalence of severe medial calcification with or without an occlusive arterial disease in PAD patients with diabetes [25, 26]. In our study, higher median velocity at the proximal and distal sites was observed among the diabetic PAD patients compared to non-diabetic PAD patients. Thus, increased arterial stiffness in the diabetic PAD cohort may be associated with an increased velocity as measured by MRI. The lack of a significant difference in the velocities between the two locations might be due to reduced arterial wall compliance and lack of sensitivity from arterial wall stiffness including medial calcification [11, 27, 28]. Medial arterial calcification, which is common in diabetic patients, reduces arterial wall compliance and elasticity [23, 29, 30]. However, pulse wave velocity measurements were not part of this study [31]. In that context, the applicability of an ABI in patients with calcified vessels (which is common in diabetics) is markedly limited, as the peripheral arteries often become incompressible when calcifications are severe, resulting in an inaccurate or non-diagnostic test [9, 32].
2D-PC-MR imaging has been used previously to assess hemodynamic characteristics in PAD patients [33]. Mohajer et al. found a positive correlation between 2D-PC-MRI SFA mean peak flow velocity and PAD severity [33]. The mean peak velocity was also significantly lower distally to SFA lesions, which is in agreement with our findings.
A comparative analysis of the volumes, however, did not show any significant difference in the vascular total volume, wall, and luminal volumes between diabetic and non-diabetic groups. This finding further supports the assumption that the increase in proximal and distal maximum velocities among diabetic PAD patients is not due to any apparent changes in the vascular morphology, but possibly due to arterial wall pathologies including increased wall stiffening and reduced vascular compliance, which is known to be affected by diabetes [25].
Diabetic PAD patients may have falsely elevated or near normal ABI values, possibly due to arterial stiffness caused by medial calcification, contributing to arterial stiffness [25, 26]. However, our analyses did not identify any significant differences in ABI between diabetic and non-diabetic PAD patients. Among the other clinical markers, claudication onset time and initial distance walked were significantly higher among the non-diabetic PAD patients than in the diabetic PAD group. The early onset of claudication and lower initial distance walked in the diabetic group further supports our above discussion regarding more progressive disease in diabetic PAD patients. The results of this study further provide support for the notion that 2D-PC-MRI derived velocity measurements maybe of value in assessing disease severity, especially in diabetic PAD patients [8].
The VPI is a measure of arterial occlusive disease which has been validated in previous studies [34–36]. The VPI is usually increased in stenotic vessels or in the presence of high vascular resistance [34, 35]. The higher VPI values in diabetic PAD patients observed in this study suggest higher arterial resistance in diabetic patients and might explain the similar VPI at the proximal and distal imaging locations [34, 36].
The present study has limitations. As this is a secondary analysis of data collected from ELIMIT, all limitations of the primary study apply. The gender distribution was imbalanced in this study. Additionally, this study was not powered to detect 2D-PC-MRI derived velocities between diabetic and non-diabetic PAD patients. 2DPC-MRI tracings were done by two readers. Duplex ultrasonography is one of the most widely used diagnostic tools for detecting disease severity and location or length of stenosis in the aortoiliac or femoropopliteal obstructions. Studies have shown that contrast-enhanced MR imaging has excellent sensitivity and specificity for the diagnosis of PAD. A comparative analysis between ultrasonography and MRI, two-gold standard diagnostic modalities, is beyond the scope of the present study [37]. Although the diabetic and non-diabetic PAD groups were unbalanced, the overall percentage of diabetics in this study is in line with national disease statistics. This study focused on MRI and therefore may not be applicable to patients with MR contraindications.
5. Conclusions
In conclusion, 2D-PC-MRI SFA velocity measures are reproducible and may be of interest in assessing diabetic and non-diabetic PAD patients.
Supplementary Material
Acknowledgements:
We thank all study patients for their participation. This work received support from the National Institutes of Health (R01HL137763, K25HL121149 both to GB), and the American Heart Association (13BGIA16720014 to GB).
Footnotes
Conflicts of Interest: Gerd Brunner reports financial support was provided by National Institutes of Health. Gerd Brunner reports financial support was provided by American Heart Association.
References
- [1].Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2015 update: a report from the american heart association. Circulation 2015;131(4):e29–e322. [DOI] [PubMed] [Google Scholar]
- [2].Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res 2015;116(9):1509–26. [DOI] [PubMed] [Google Scholar]
- [3].Rooke TW, Hirsch AT, Misra S, Sidawy AN, Beckman JA, Findeiss LK, et al. 2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2011;58(19):2020–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC-II). Journal of vascular surgery 2007;45 Suppl S:5. [DOI] [PubMed] [Google Scholar]
- [5].Lumsden AB, Rice TW, Chen C, Zhou W, Lin PH, Bray P, et al. Peripheral arterial occlusive disease: magnetic resonance imaging and the role of aggressive medical management. World J Surg 2007;31(4):695–704. [DOI] [PubMed] [Google Scholar]
- [6].Criqui MH, Langer RD, Fronek A, Feigelson HS, Klauber MR, McCann TJ, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. The New England journal of medicine 1992;326(6):381–6. [DOI] [PubMed] [Google Scholar]
- [7].Regensteiner JG, Hiatt WR, Coll JR, Criqui MH, Treat-Jacobson D, McDermott MM, et al. The impact of peripheral arterial disease on health-related quality of life in the Peripheral Arterial Disease Awareness, Risk, and Treatment: New Resources for Survival (PARTNERS) Program. Vasc Med 2008;13(1):15–24. [DOI] [PubMed] [Google Scholar]
- [8].Brunner G, Yang EY, Kumar A, Sun W, Virani SS, Negi SI, et al. The Effect of Lipid Modification on Peripheral Artery Disease after Endovascular Intervention Trial (ELIMIT). Atherosclerosis 2013;231(2):371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Potier L, Abi Khalil C, Mohammedi K, Roussel R. Use and utility of ankle brachial index in patients with diabetes. European Journal of Vascular and Endovascular Surgery 2011;41(1):110–6. [DOI] [PubMed] [Google Scholar]
- [10].Jang SY, Park SW, Kim Y-W, Kim D-K. Survival Rates in Peripheral Artery Disease. Journal of Lipid and Atherosclerosis 2017;6(1):39–45. [Google Scholar]
- [11].Gimnich OA, Zil EAA, Brunner G. Imaging Approaches to the Diagnosis of Vascular Diseases. Curr Atheroscler Rep 2022;24(2):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kamran H, Nambi V, Negi S, Yang EY, Chen C, Virani SS, et al. Magnetic Resonance Venous Volume Measurements in Peripheral Artery Disease (from ELIMIT). Am J Cardiol 2016;118(9):1399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Szolar D, Sakuma H, Higgins C. Cardiovascular applications of magnetic resonance flow and velocity measurements. Journal of magnetic resonance imaging : JMRI 1996;6(1):78–89. [DOI] [PubMed] [Google Scholar]
- [14].Rebergen S, van der Wall E, Doornbos J, de Roos A. Magnetic resonance measurement of velocity and flow: technique, validation and cardiovascular applications. Am Heart J 1993;126(6):1439–56. [DOI] [PubMed] [Google Scholar]
- [15].Mathew RC, Kramer CM. Recent advances in magnetic resonance imaging for peripheral artery disease. Vasc Med 2018;23(2):143–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kato S, Fukui K, Kodama S, Azuma M, Iwasawa T, Kimura K, et al. Incremental prognostic value of coronary flow reserve determined by phase-contrast cine cardiovascular magnetic resonance of the coronary sinus in patients with diabetes mellitus. J Cardiovasc Magn Reson 2020;22(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Neff D, Kuhlenhoelter AM, Lin C, Wong BJ, Motaganahalli RL, Roseguini BT. Thermotherapy reduces blood pressure and circulating endothelin-1 concentration and enhances leg blood flow in patients with symptomatic peripheral artery disease. Am J Physiol Regul Integr Comp Physiol 2016;311(2):R392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chernobelsky A, Shubayev O, Comeau CR, Wolff SD. Baseline correction of phase contrast images improves quantification of blood flow in the great vessels. J Cardiovasc Magn Reson 2007;9(4):681–5. [DOI] [PubMed] [Google Scholar]
- [19].Weitz JI, Byrne J, Clagett GP, Farkouh ME, Porter JM, Sackett DL, et al. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation 1996;94(11):3026–49. [DOI] [PubMed] [Google Scholar]
- [20].Zemaitis MR, Boll JM, Dreyer MA. Peripheral arterial disease. 2017. [PubMed]
- [21].Criqui MH, Browner D, Fronek A, Klauber MR, Coughlin SS, Barrett-connor E, et al. Peripheral arterial disease in large vessels is epidemiologically distinct from small vessel disease: an analysis of risk factors. American journal of epidemiology 1989;129(6):1110–9. [DOI] [PubMed] [Google Scholar]
- [22].Riandini T, Pang D, Toh M, Tan CS, Choong A, Lo ZJ, et al. National Rates of Lower Extremity Amputation in People With and Without Diabetes in a Multi-Ethnic Asian Population: a Ten Year Study in Singapore. Eur J Vasc Endovasc Surg 2022;63(1):147–55. [DOI] [PubMed] [Google Scholar]
- [23].Golomb BA, Dang TT, Criqui MH. Peripheral arterial disease: morbidity and mortality implications. Circulation 2006;114(7):688–99. [DOI] [PubMed] [Google Scholar]
- [24].Hughson W, Mann J, Garrod A. Intermittent claudication: prevalence and risk factors. Br Med J 1978;1(6124):1379–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zamin RM. Arterial remodelling and inflammation in peripheral arterial disease. 2013.
- [26].Soor GS, Vukin I, Leong SW, Oreopoulos G, Butany J. Peripheral vascular disease: who gets it and why? A histomorphological analysis of 261 arterial segments from 58 cases. Pathology 2008;40(4):385–91. [DOI] [PubMed] [Google Scholar]
- [27].Rocha-Singh KJ, Zeller T, Jaff MR. Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications. Catheter Cardiovasc Interv 2014;83(6):E212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Han RI, Wheeler TM, Lumsden AB, Reardon MJ, Lawrie GM, Grande-Allen JK, et al. Morphometric analysis of calcification and fibrous layer thickness in carotid endarterectomy tissues. Computers in Biology and Medicine 2016;70:210–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol 2004;15(12):2959–64. [DOI] [PubMed] [Google Scholar]
- [30].Abedin M, Tintut Y, Demer LL. Vascular calcification: mechanisms and clinical ramifications. Arteriosclerosis, thrombosis, and vascular biology 2004;24(7):1161–70. [DOI] [PubMed] [Google Scholar]
- [31].Murray T, Yang EY, Brunner G, Kumar A, Lakkis N, Misra A, et al. Postprandial effects on arterial stiffness parameters in healthy young adults. Vasc Med 2015;20(6):501–8. [DOI] [PubMed] [Google Scholar]
- [32].Stein R, Hriljac I, Halperin JL, Gustavson SM, Teodorescu V, Olin JW. Limitation of the resting ankle-brachial index in symptomatic patients with peripheral arterial disease. Vasc Med 2006;11(1):29–33. [DOI] [PubMed] [Google Scholar]
- [33].Mohajer K, Zhang H, Gurell D, Ersoy H, Ho B, Kent KC, et al. Superficial femoral artery occlusive disease severity correlates with MR cine phase-contrast flow measurements. Journal of magnetic resonance imaging : JMRI 2006;23(3):355–60. [DOI] [PubMed] [Google Scholar]
- [34].Panaritis V, Kyriakidis AV, Pyrgioti M, Raffo L, Anagnostopoulou E, Gourniezaki G, et al. Pulsatility index of temporal and renal arteries as an early finding of arteriopathy in diabetic patients. Ann Vasc Surg 2005;19(1):80–3. [DOI] [PubMed] [Google Scholar]
- [35].Strandness DE Jr., Priest RE, Gibbons GE. COMBINED CLINICAL AND PATHOLOGIC STUDY OF DIABETIC AND NONDIABETIC PERIPHERAL ARTERIAL DISEASE. Diabetes 1964;13:366–72. [DOI] [PubMed] [Google Scholar]
- [36].Petersen L, Peterson J, Talleruphuus U, Ladefoged S, Mehlsen J, Jensen H. The pulsatility index and the resistive index in renal arteries. Associations with long-term progression in chronic renal failure. Nephrol Dial Transplant 1997;12(7):1376–80. [DOI] [PubMed] [Google Scholar]
- [37].Leiner T, Kessels AG, Nelemans PJ, Vasbinder GB, de Haan MW, Kitslaar PE, et al. Peripheral arterial disease: comparison of color duplex US and contrast-enhanced MR angiography for diagnosis. Radiology 2005;235(2):699–708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


