Abstract
The indication for femoral stem cementation should be made on a patient-specific basis, taking physical activity, femoral geometry, and bone tissue quality into account. Age alone should not be the sole justification for cementation. The Dorr classification can serve as decision support for whether a cemented fixation should be used. Femoral neck fractures should generally be cemented.
Familiarize yourself with the applied stem philosophy. Force-closed stems typically have a polished surface that allows for subsidence, especially in the first 2 years postoperatively. Stems following the shape-closed philosophy have rougher surfaces and do not allow subsidence.
There are various types of cement that differ in viscosity and can be categorized accordingly. These cement types go through four temperature-dependent phases: mixing phase, waiting phase, working phase, and curing phase. Rough implants should be implanted quickly, using wetter cement. For a polished stem, the cement should be slightly firmer.
To avoid complications like bone cement implantation syndrome, it is essential to adhere to the state-of-the-art retrograde cementation technique, which recommends pulsatile lavage and vacuum mixing of the cement. Additionally, cement restrictors and pressurizers are used.
A thorough understanding of cementation techniques is crucial to ensure a favorable outcome with a uniformly thick cement mantle that encompasses the entire stem. Incorrect cementing can lead to the premature failure of the endoprosthesis.
Keywords: bone cement, bone cement implantation syndrome, cemented stem, cemented hip arthroplasty, cementing technique, force-closed stem, shape-closed stem
Principles of cemented stem implantation in hip arthroplasty
The aim of this article is to summarize the state of the art in cementing femoral stems in primary hip arthroplasty. The ultimate goal is to achieve reliable and stable fixation with a low failure rate. Bone cement serves as the foundation of the stem fixation in the femoral canal. It is essential that the cement is prepared in a standardized manner according to current recommendations. The prosthetic design should meet the biomechanical and technical requirements for optimal performance. Although there is a trend toward cementless fixation, cementation may be required in a specific patient population. Careful evaluation of the indication for cementation is necessary to avoid complications. Maintaining a familiar sequence of intraoperative steps and knowledge of the required instruments is mandatory for efficient and accurate performance. Attention should also be given to the occurrence of bone cement implantation syndrome (BCIS), which can occur during the use of bone cement.
Design features of cemented stems
Biomechanical principles: force-closed vs shape-closed
Stem geometry: tapered vs anatomical
Biomechanical principles
An important factor in biomechanics is the roughness of the stem’s surface. This enables a force-closed or shape-closed principle. The roughness of the surface is described scientifically by the value Ra, which represents the average roughness and is given in μm. Ra describes the distance of the elevations from the so-called mean line. Based on these values, polished stems (Ra < 1.0 μm), matte stems (Ra < 2.0 μm), and rough stems (Ra > 2.0 μm) are distinguished (1).
Force-closed (or taper-slip)
The stems following the force-closed principle have a geometric taper shape and a polished surface with extremely small average roughness values (Ra < 1 μm). The load is transferred from the uncoupled stem to the cement mantle (1, 2). This leads to improved proximal load transfer to the cement and a significant reduction in peak stresses on the proximal and distal cement mantle (3, 4, 5).
The design features allow the stem to subside minimally into the cement mantle, which is crucial for this anchorage philosophy. This is the well-known English principle pioneered by Ling and Lee from Exeter, as well as Charnley from Manchester and Wrightington, which is based on the viscoelastic properties of polymethylmethacrylate (PMMA). PMMA deforms under cyclic loading (creep) and allows for vertical micro-movements of the tapered stem, ultimately resulting in the subsidence (taper slip) of the stem into the cement mantle (1, 2, 3, 4, 5, 6, 7, 8).
The subsidence of the stem in force-closed designs brings three protective effects for biomechanical stability. First, the force causing stem migration does not induce damaging shear forces at the cement–bone interface. Secondly, radial compression is exerted on the cement mantle, providing additional protection against shear forces at the cement–bone interface. Finally, the subsidence enhances the torsional stability of the stem (5, 6).
The subsidence of the stem extends over the entire lifespan but decreases over time, so that after the first 2 years following implantation, no clinically or radiologically relevant effect can be detected (6). If progressive subsidence occurs after 2 years or if the total subsidence exceeds 5 mm, loosening of the implant must be assumed (2). Radiolucent zones around the stem >2 mm are also an indication of loosening, especially if they show progressive changes over time (9).
Stems designed according to the force-closed principle typically do not have a collar, as it would prevent the intended subsidence. To ensure an adequate cement mantle that fully encloses the prosthesis, the option of using a centralizer is available and should be used whenever possible (2, 3, 6).
Force-closed stems are the most commonly used worldwide, with excellent results, and are considered more forgiving than shape-closed stems regarding the surgical technique and the established cement mantle (6). In octogenarians, force-closed stems tend to have slightly higher rates of periprosthetic fractures (2, 10).
Shape-closed (or composite beam)
Stems following the shape-closed philosophy can have an anatomical or straight geometry and may also feature rough surfaces with high roughness values (>1 µm) and possibly a structured surface with additional design features such as flanges, grooves, or collars (2). The rough surface, as well as optional flanges and grooves, achieves a tight cement-implant bond between the stem and cement, which transfers the load to the bone. This bond is primarily based on mechanical rather than chemical adhesive properties (4, 6, 8).
In contrast to the force-closed philosophy, shape-closed shafts do not intentionally undergo subsidence. Subsidence in this case would lead to increased wear and resulting damage to the cement, ultimately resulting in the loosening and failure of the arthroplasty (4, 5, 6, 7).
This design converts the axial forces acting on the femoral stem into tensile and shear forces that are transmitted at the cement–bone interface. These forces, combined with cyclic loading, promote progressive wear. This, in turn, is a significant driver of osteolysis and implant loosening. If the stem subsides by >0.15 mm after 2 years postoperatively due to these forces, an increased revision rate (4% increase in revision rate with every 0.1 mm subsidence) has been described (3, 4). Although the contact between the femoral shaft and the cortical bone increases stability, it also promotes additional wear and loosening (3).
The shaft preparation is also performed using rasps, with particular attention given to preparing the calcar region to ensure an adequate cement mantle. The rasps used aim for impacting rather than removing the spongiosa. In this stem design as well, centralizers are sometimes possible, but there are also self-centering implants (2).
Cassar-Gheiti expanded the classification not only by distinguishing between force-closed and shape-closed stems. Due to the heterogeneous stem designs of the shape-closed stems, a third and fourth type were introduced (2).
The third type of classification includes stems that also follow the shape-closed philosophy. These shafts have a self-centering and a partially press-fit fixation component. A line-to-line cementation with a thin cement mantle (<1 mm) is used (2). The implant, in this case, has the same size as the last used rasp. Another distinguishing feature is that the implant is inserted with hammer blows.
Contrary to the general assumption that a sufficiently thick cement mantle completely surrounding the stem must be present, this class of implants follows a different approach. This technique is also referred to as the ‘French paradox’ (see above under cementation technique) (2, 5).
The fourth type includes curved and anatomical stems that match the natural femoral shaft shape. This way, a potential sagittal malalignment is addressed. Type four also follows the shape-closed design and requires an adequately thick cement mantle of 2 mm (2, 5). All common stem types with their different features and biomechanical philosophies are listed in Table 1. The less forgiving nature of shape-closed designs toward suboptimal cementation techniques should be taken into account in the decision-making process (3, 8).
Table 1.
Modified classification system of cemented stem design after Cassar-Gheiti (2).
| Type/subtype | Geometry | General category | Description | Fixation | Cement mantle | Example |
|---|---|---|---|---|---|---|
| 1 | ||||||
| 1a | Double taper, +/− flange, +/− ribs | Polished, collarless | a.p.: thin | Force-closed | 2–4 mm | Exeter, CPCS, CPT, MS–30, Expersus, Pyramid, Sirius |
| m.l.: wide | ||||||
| 2× tapered distally | ||||||
| Straight stem | ||||||
| 1b | Triple taper | Polished, collarless | a.p.: thin + narrows medially | Force-closed | 2–4 mm | C-Stem, Trilliance, TrendHip c, CPCS |
| m.l.: wide | ||||||
| 3× tapered distally | ||||||
| 2 | ||||||
| 2a | Rounded, flanged | +/− polished | Round and thick | Shape-closed | 2–4 mm | Charnley, Excia, Spectron EF, Elite, Echo FX |
| +/− collar | Minimal tapering distally | |||||
| 2b | Tapered, flanged | +/− polished | a.p.: thin | Shape-closed | 2–4 mm | Synergy c |
| +/− collar | m.l.: wide | Summit c | ||||
| Profiled | Straight stem | Bicontact | ||||
| 3 | ||||||
| Mono taper/single wedge | +/− polished | a.p.: thin | Shape-closed + press-fit | 0–1 mm | Mueller, CMK, Taperloc c, Quadra c, Avenir c, Corail c, TwinSys c, Generic | |
| +/− collar | m.l.: wide | 3-P-Fixation | ||||
| +/− profiled | Self centered | Line-to-line | ||||
| Press-fit | Rectangular cross section | |||||
| Flat + straight stem | ||||||
| 4 | Anatomical ‘triple taper’ | Curved | Round + wide | Shape-closed | 2 mm | Lubinus SP I |
| +/− polished | prox.: curved | Lubinus SP II | ||||
| +/− collar | dist.: +/curved | Olympia | ||||
| +/− profiliert | Neck anteversion | |||||
| Right/left version | ||||||
a.p., anteroposterior; m.l., mediolateral.
Stem shape
Straight tapered stems
There are mono-, double-, and triple-tapered stems. The multidimensional tapered stems have better force transmission into the proximal cement mantle through a wider section of the stem. Tapered cemented stems always have a neutral position of the neck.
Anatomical shaped stems
The anatomically designed stems are largely adapted to the inner medullary canal geometry and are characterized by often having two curvatures, an optional collar, and possibly surface profiles. The neck of the implant is inevitably anteverted. Anatomical hip stems necessarily require left and right versions.
Anatomically cemented stems consistently belong to the group of shape-closed stems and form a composite system with the cement (2, 4). For optimized positioning, some anatomical stems have spacing buffers, mostly out of PMMA or centralizers, attached in the proximal neck region. Standard rasps are also used for the preparation of the femur in these cases. The cement mantle should consistently be 2 mm thick (2). In summary, anatomical stems allow for better centralization in the femoral shaft and enable a more even thickness of the cement mantle (4).
There are also polished anatomical stems, but, strictly speaking, they do not fully adhere to the force-closed principle. Compared to tapered stems, anatomical designs result in fewer periprosthetic fractures within the first 2 postoperative years (10, 11).
Collared stems
Another important design feature is the stems with collars. These are located at the proximal end of the stem and align with the resection level of the femoral neck. This exerts an additional pressurizing effect at the end of the stem insertion.
The collar is attributed with two main advantages. First, it allows for direct load transfer from the implant to the calcar region. Therefore, a wide contact area should be created by using a calcar reamer. This relieves stress on the proximal cement mantle when there is direct contact with the calcar. It reduces stress and limits overall migration. On the other hand, this design feature prevents the desired subsidence of the force-closed philosophy. Therefore, this design feature is only used in shape-closed stems. The collar does not significantly reduce wear or micro-movement. The second advantage lies in the insertion of the stem when using the over-reaming technique with a relatively undersized implant. The collar ensures that the implant is placed at the same level as the rasp, providing proper alignment (4).
Cemented short stems typically refer to double-tapered polished stems without a collar. The initial studies show good short to medium-term results with a follow-up period of up to 14 years (12). Short stems are known to conserve bone substance due to their shorter length and ultimately require a shorter femoral cementing distance. This is particularly beneficial for revision surgery in an aging population. In a registry study, 664 cemented Exeter® short stems were compared to 698 standard Exeter® stems. There were no differences in survival rates after 5 or 10 years between the standard and short stem groups (13). In order to draw conclusions about the long-term survival of cemented short stems, studies with a longer follow-up will be necessary.
Bone cement
A self-curing polymer consists of two components: powder (copolymer) and liquid (monomer). Copolymers (such as PMMA) from the powder mixture can only polymerize with the monomer in the liquid at room temperature through the energy generated by a second reaction: benzoyl peroxide (BPO) and dimethylparatoluidine (DmpT) combine during mixing to form radicals. A detailed overview of the components is displayed in Table 2 (3).
Table 2.
Composition of conventional bone cements.
| Type/function | Substance | Abbreviation |
|---|---|---|
| Powder | ||
| Copolymer* | Polymethylmethacrylate | PMMA |
| Methylmethacrylate-methylacrylate | MMA-MA | |
| Ethylmethacrylate | E-MA | |
| Butylmethacrylate | Bu-MA | |
| Styrene-Methacrylate | Styrene-MA | |
| Radiopaque* | Zirconium dioxide | ZrO2 |
| Barium sulfate | BaSO4 | |
| Antibiotic Mono | Gentamicin sulfate | G |
| Tobramycin sulfate | ||
| Antibiotic Duo | Gentamicin + Vancomycin | G + V |
| Gentamicin + Clindamycin | G + C | |
| Erythromycin + Colistin | E + C | |
| Initiator (Catalyst) | Benzoyl peroxide | BPO |
| Colorant* | Chlorophyll-copper complex (E141) | |
| Indigocarmine (E132) | ||
| Liquid | ||
| Monomer | Methylmethacrylate | MMA |
| Activator/Starter/Initiator (Catalyst) | Di-methyl-para-toluidine | DmpT |
| Stabilizer* | Hydroquinone | HQ |
| Vitamin C | ||
| Colorant* | Chlorophyll-copper complex (E 141) | |
*Addition and quantity depend on the respective manufacturer.
This involves an exothermic reaction that is accompanied by a temperature increase, measured in vivo at over 56°C (which denatures collagen). However, this temperature elevation is transient and does not appear to have a significant clinical impact (5).
The setting time to reach strength and the associated polymerization rate of the cement are influenced, among other factors, by the temperature of the cement during mixing, the moisture of the powder, and the amount of air added to the liquid cement during mixing. Three different viscosity forms are distinguished, although they are not consistently defined in the literature (5):
Cement with low viscosity (LV): long waiting phase where the cement is ‘sticky’, followed by a rapid increase in viscosity during the working phase, with the final curing phase typically lasting about 1 to 2 min.
Cement with medium viscosity (MV): similar waiting phase but a slower increase in viscosity during the working phase.
Cement with high viscosity (HV): short waiting phase followed by a long working phase, and the curing phase typically lasts 1.5–2 min (5).
Conflicting results in the literature make it difficult to determine the best viscosity. Ultimately, no general statement can be made. However, there is a trend towards medium and high viscosity. It should be noted that higher intramedullary peak pressure is achieved during pressurization and stem insertion with medium and high-viscosity cement, which carries potential risks such as a weakened cement–implant interface, fat embolism, and BCIS. Ultimately, the decision lies with the surgeon (5).
There is no uniform recommendation for the widespread use of antibiotic-loaded bone cement in hip arthroplasty, particularly as there is a lack of evidence regarding adverse effects with widespread use. However, antibiotics should be considered in high-risk patients who are at risk of periprosthetic infection (5). Risk factors for a periprosthetic infection include body mass index ≥ 35 kg/m2, diabetes mellitus, active smoking, chronic kidney disease, autoimmune disease, and nasal colonization with methicillin-resistant and/or methicillin-sensitive Staphylococcus aureus (14). Modern cementing techniques utilize mixture systems with a vacuum, ensuring a consistent and homogeneous material density free from air inclusions (voids), resulting in exceptional long-term strength (3, 15). As a result, cement porosity is reduced, and mechanical properties are improved (5). A detailed overview of the modern fourth-generation cementing technique is displayed in Table 3.
Table 3.
Modern cementing technique – 4th generation (3).
| Item/instrument/implant | Note |
|---|---|
| Cement restrictor | Absorbable or non-absorbable |
| Cooling at 4°C | >24 h (follow instructions for use) |
| Vacuum mixing | Follow working phases |
| Brush | Single-use item can also be used with machines |
| Pulsatile lavage (with brush attachment) | Warmed without additives |
| Compresses | Watch out for debris |
| Adrenaline compresses | In Anglo-American literature |
| Cement gun | Retrograde filling (‘bottom to top’) |
| Pressurizer | End of marrow space filling |
| Centralizer | Not for line-to-line technique, e.g. ‘Müller straight stem’ |
| Not for very narrow marrow spaces <10 mm |
The preparation and processing of the cement are divided into four temperature-dependent phases:
Mixing phase
Waiting phase
Working phase
Curing phase
Each phase has a specific time requirement. Mixing is done with a lifting frequency of 1–2 s. The consistency of the cement depends on various variables such as ambient temperature and humidity. Therefore, in addition to time, the consistency of the cement should be considered before application (13). During the working phase, the cement should no longer be sticky. The slightly higher viscosity facilitates the penetration (indentation) into the cancellous gaps after compression with the seals (pressurizer) (3, 5). This is further facilitated by the subsequent insertion of the prosthetic stem. Gloves should be changed after contamination with cement. Rough implants should be implanted quickly, using wetter cement. Cement intrusion into small grooves and gaps of the implant promotes a mechanical bond. For a polished stem, the cement should be slightly firmer (5). ‘The cement is not a chemical adhesive but a mechanically load-bearing filling material!’ (16, 17).
Pressurization
This creates a strong cement–bone interface (by deep cement penetration into the cancellous bone, thus increasing the contact area) that resists shear and tensile forces. When pressure is maintained for an extended period, the backflow of fluid from the cancellous bone is reduced, resulting in an intact cement mantle (5).
Bone cement implantation syndrome
BCIS is a circulatory disorder that occurs due to the chemical composition of the bone cement and the pressure increase in the medullary canal, particularly during stem insertion. It is characterized by a drop in blood pressure, decreased oxygen saturation, and/or cardiopulmonary collapse (3, 15, 18, 19, 20).
During the introduction of the cement, a significant pressure increase of up to 1447 kPa occurs in the medullary canal. This increase in pressure may favor air, fat, and bone marrow embolism (19, 21). This highlights the importance of pulsed lavage, which flushes out the fatty marrow from the intertrabecular spaces of the cancellous bone and provides a significant contribution to prevention, although it does not completely eliminate the risk.
Different explanations see vasodilatation caused by methyl methacrylate particles or histamine as a possible contributing factor, as well as complement activation. There is no definitive explanation for the development of BCIS, and it may be a combination of the mechanisms mentioned above (19, 21).
In order to mitigate BCIS as effectively as possible, in addition to using pulsed lavage, the preoperative cardiopulmonary situation and comorbidities should be optimized. The anesthesia procedure should be adjusted in cases of increased BCIS risk (for risk factors of BCIS, see Table 4). Intraoperatively, volume loss should be compensated, and a high oxygen saturation level should be aimed for. Systolic blood pressure should remain within 20% of baseline values. Intraoperative monitoring through capnography and pulse oximetry is mandatory (3, 19).
Table 4.
Risk factors for developing a bone cement implantation syndrome during cemented stem implantation (3, 19, 20, 22, 23).
| Risk factors for BCIS |
|---|
| ASA 3 and 4 |
| Age >65 years |
| Male |
| Diuretics |
| Anticoagulants (warfarin) |
| Chronic obstructive pulmonary disease |
| Severe cardiopulmonary diseases |
| Pulmonary hypertension |
| Osteoporosis |
| Bone metastases |
| Proximal femur fractures |
| Wide femoral medullary canal (≥21 mm) |
| Cemented long stems |
| Excessive pressure during application |
| Revision surgeries |
BCIS is classified into three severity grades (Table 5) and is associated with increased postoperative cardiovascular complication rates and higher mortality rates. In particular, severe BCIS (grades 3 and 4) significantly increases 30-day mortality compared to a milder grade (grade 1) or when BCIS does not occur at all. Factors that predispose to severe BCIS include age >75 years, high ASA classification (ASA III or IV), and the presence of renal insufficiency (18, 22, 23). A femoral drill hole appears to reduce the likelihood of BCIS (23).
Table 5.
| Grade | Criteria |
|---|---|
| Grade 1 | Moderate hypoxia (SpO2 <94%) or |
| Hypotension (drop of SBP >20%) | |
| Grade 2 | Severe hypoxia (SpO2 <88%) or |
| Hypotension (drop of SBP >40%) or | |
| Unexpected loss of consciousness | |
| Grade 3 | Cardiovascular collapse requiring CPR |
The overall incidence of BCIS (grades 1–3) in hemiarthroplasty is 31%, while in total hip arthroplasty, it is 24%. Severe BCIS (grades 2 and 3) is much less common, with an incidence of 9% in hemiarthroplasty and 5% in total hip arthroplasty (22).
Indication
According to the authors, factors such as cost, surgical time, or chronological age of the patient should play a subordinate role in the indication for cementation. Characteristics such as physical activity, femoral geometry, or bone tissue quality provide a patient-specific approach and should receive greater attention (24).
Chronological age alone appears to be an inadequate primary decision-making factor. It does not take into account pre-existing conditions or individual bone quality (which may be influenced by age). It also does not take into account the ultimate clinical outcome and financial burden. The level of training and clinical experience should also influence the choice of fixation method, as they also impact the results. Therefore, age alone does not adequately address the complexity of the indication (25). A direct cost comparison is inevitably flawed because financial expenses do not solely depend on the implant price, but also on factors such as surgical time, comorbidities, individual risks, hospitalization duration, and revision rates, all of which significantly impact the overall financial burden (25, 26).
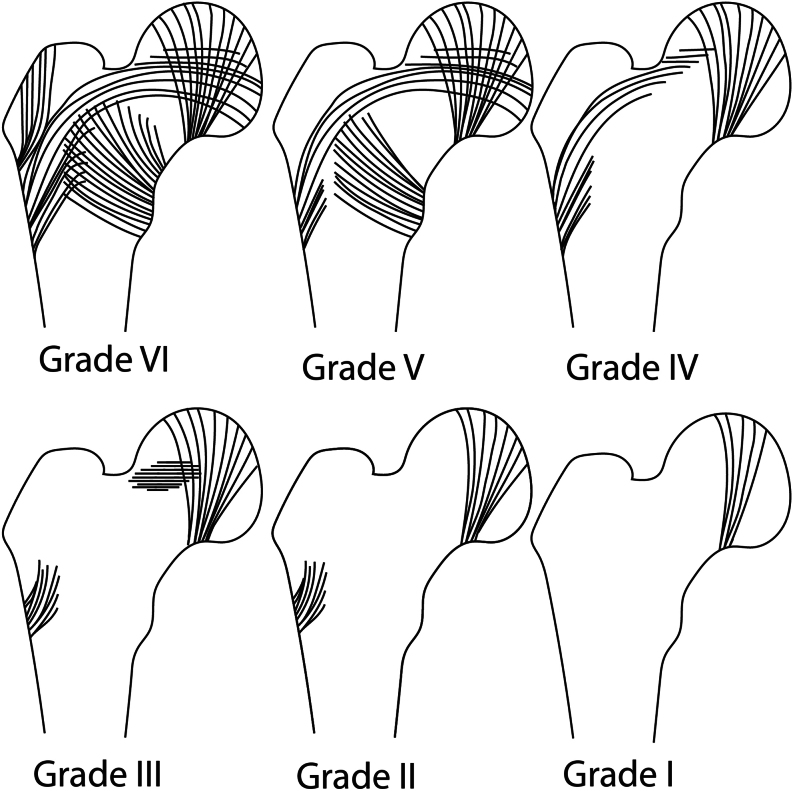
Singh et al. developed a radiomorphological index of osteoporosis using pelvic radiographs, which has also been incorporated into other scores (27, 28). The density and distribution of tension and compression trabeculae in the femoral neck and trochanteric region help assess bone quality, with a grading scale from 1 to 6. Grade 1 represents the weakest bone quality, while grade 6 describes a healthy normal bone quality (Fig. 1). Advanced osteoporosis suggests a preference for cemented surgical techniques (3, 24, 28).
Figure 1.
The Singh index grade, describing the DEXA-based trabecular pattern of the femoral neck in an osteoporotic bone stock, from grade I poor bone quality) to grade VI (normal trabecular pattern) (27).
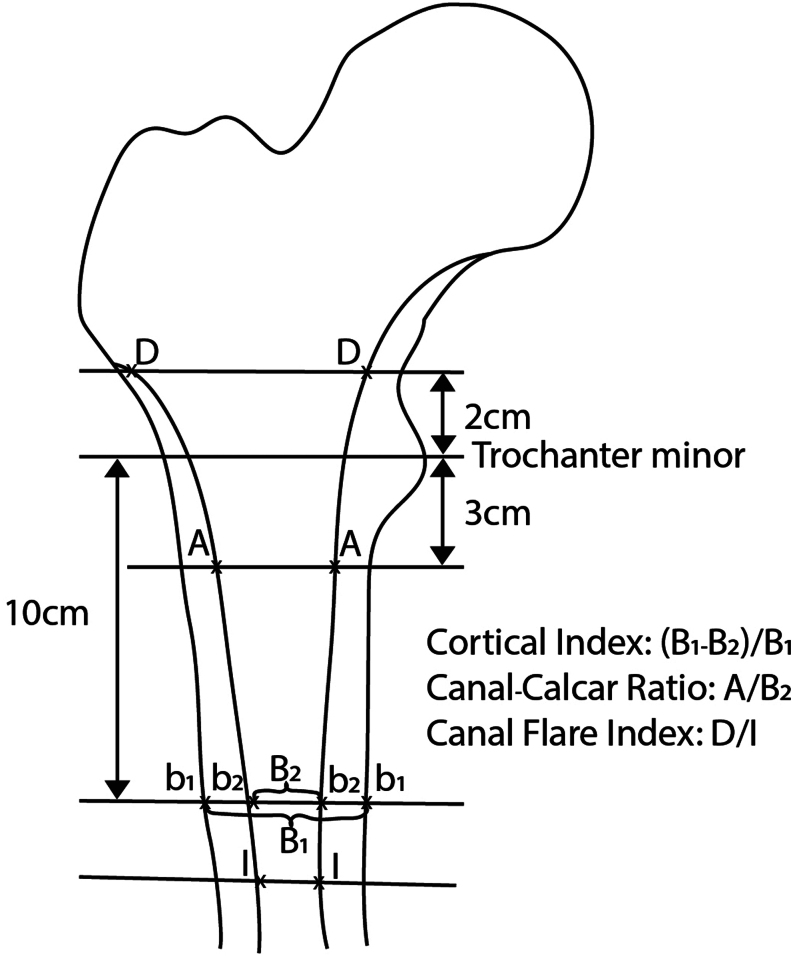
Considering osteoporotic bone changes, Dossick and Dorr have classified the shape of the femur into three classes: tube-like morphology, also known as group C (‘stove pipe’), is associated with certain bone loss and presents challenges in fitting cementless prosthetic types, thus suggesting the decision for a cemented solution. This classification can be quantified using the cortical index (Fig. 2) (3, 29, 30).
Figure 2.
Evaluation and classification of osteoporotic bone changes after Dossick and Dorr (Cortical Index and Canal-Calcar Ratio) and Nobel (CFI).
Further descriptions of the proximal femoral medullary canal geometry are provided by flare indices, which serve as measurement parameters for better score interpretation. The Canal Flare Index (CFI) developed by Philip Noble provides a numerical value for the femoral geometry by calculating the ratio between the diameter 2 cm proximal to the lesser trochanter and the isthmus. A smaller value indicates a more cylindrical shape of the femur (Fig. 2) (31). The different classifications of the medullary canal geometry are displayed in Table 6.
Table 6.
| Dorr classification | Canal Flare Index | Cortical Index ap | Radiographic features | Cement |
|---|---|---|---|---|
| Type A | >4.7 | 0.48 ± 0.01 | Thick cortices ap + lat | Uncemented |
| Type B | 3.0–4.7 | 0.39 ± 0.01 | Thin cortices posterior | Uncemented |
| Type C | <3.0 | 0.30 ± 0.02 | Very thin cortices posterior and medial | Cemented |
Poor bone quality is the primary indication for cemented femoral stem fixation. To assess bone quality, the Dorr classification, as well as other measures such as the CFI and Cortical Index, are available. Female patients of advanced age are often associated with poorer bone quality. In these cases, particular attention should be given to radiological and intraoperative findings. Patients with poorer bone quality and older women tend to have fewer aseptic loosening cases, fewer intraoperative complications, and fewer revisions with better 10-year survival rates if a cemented stem fixation is used. Furthermore, in cases of a narrow proximal femoral canal, periprosthetic fractures, and dysplasia-caused secondary arthritis, the indication for cemented femoral stem fixation should be critically evaluated (3, 26).
Femoral neck fractures should generally be cemented (3, 18, 24, 32, 33, 34). Cementless fixation, on the other hand, is associated with a higher risk of implant-related complications such as intra- and post-operative periprosthetic fractures, aseptic loosening, and dislocations (34, 35, 36, 37, 38). Risk factors for periprosthetic femoral fractures in cementless fixation (i.e. poor bone quality, female gender) must be carefully weighed against the risk factors for BCIS (Table 4) (22, 25). Furthermore, cemented hemiarthroplasty appears to be associated with lower residual pain and improved postoperative mobility compared to cementless hemiarthroplasty (34). Regarding the mortality rate, a slightly increased rate is reported within the first few postoperative days, but this effect seems to diminish over time (37). In the majority of studies conducted on this topic, no significant impact on mortality rate seems to be demonstrated (34, 38).
Cementation preparation
Modern techniques aim to improve the bone–cement interface through bone preparation and cleaning. Bone bed preparation by rasping, pulsatile jet lavage, using a distal cement restrictor, pressurization of the cement, and insertion of the stem with a distal centralizer (depending on the stem design) are considered standard procedures (37).
Cement restrictor
By using a cement restrictor, cement containment (limiting distal cement spread) is achieved, ensuring better pressurization (2, 3, 5). This requires reliable sealing of the medullary canal. During the planning phase, the position and size of the cement restrictor can be selected or at least estimated (39). Typically, the cement restrictor should be placed approximately 1 cm distal to the tip of the stem, respectively the tip of the centralizer. The diameter is slightly oversized in relation to the femoral canal (5). A separate set of instruments for the selection and application of cement restrictors is indispensable for cemented stems. Intraoperatively, tactile probes (olives) assist in determining the size and position of these restrictors. The application should always be done in consultation with the anesthesia team and only after re-suctioning the medullary canal, preferably without the use of hammering, as intramedullary pressure increases can be significant (3, 5, 39).
Cement restrictors can be made from PMMA, polyethylene, absorbable sutures, collagen type I (porcine), or autologous bone, based on three main designs: universal, press-fit, and expandable. Press-fit restrictors are most commonly used in routine cases (39). Biodegradable restrictors are gaining popularity as they do not require removal during revision. Biodegradable expandable restrictors result in half the number of fat emboli compared to normal biodegradable restrictors. However, other studies show poor results for absorbable restrictors as they migrate further distally than non-biodegradable restrictors (5, 40, 41). Considering these findings, it is recommended to use non-absorbable restrictors in routine cases where insertion at the isthmus is possible, with cautious consideration of an expandable type. Typically, these do not extend distal to the isthmus (5). Fixing restrictors distal to the isthmus, such as in long-stem revision prostheses, is challenging. Multiple stoppers in succession (stacking technique) or the Rex cement stop™ (39) can be used. The REX Cement Stop™ is a gelatin-based plug that expands through a screw mechanism. Expandable cement restrictors withstand the highest pressure during cement application (5, 39).
Lavage
The use of pulsatile jet lavage is essential for the irrigation of the femoral medullary canal. This is necessary to improve cement penetration into the marrow-free intertrabecular spaces and reduce the risk of BCIS (2, 5, 15, 16, 39). Repeated flushing and suctioning between rasping procedures reduce the risk of embolism (5).
Additives are unnecessary, but the warming of the solutions should be considered to minimize circulatory reactions (42). We prefer to use Ringer’s solution or Ringer’s lactate. After lavage, dry the medullary canal.
Centralizer
The philosophy of ‘taper slips’ with polished surfaces requires a homogeneous and uninterrupted cement mantle with an average thickness of 2–4 mm, which cannot be achieved without centralizing aids (3, 15, 16). These so-called ‘centralizers’ are best made from PMMA and should be placed at the tip of the stem, creating a chemical bond with the fixation cement.
Coronal and especially varus malalignment are associated with stem migration, poor functional outcomes, and a high failure rate. This is primarily due to stress peaks and deficiencies in the cement mantle (5). Centralizers prevent these issues. Additionally, cracks in the cement mantle are attributed to malalignment or malrotation. Furthermore, wear particles can enter the cement–bone interface and promote aseptic loosening. However, one should not rely solely on the centralizer, as the surgeon should always ensure that the stem is properly positioned (5).
Cementation technique for stems in hip arthroplasty
The bone–cement interface is the interface that ensures stable anchoring (16). The cement mantle should have no interruptions, a thickness of 2–5 mm, a homogeneous density, and fill the designated spaces in the prepared bone and the intended gaps in the prosthetic stem (15, 43). A thin cement mantle is not sufficient to absorb energy, and there is an increased risk of fissures/fractures in the cement mantle and aseptic loosening. A thick cement mantle (5–10 mm) allows for increased micromotions and is suboptimal regarding radial stresses, favoring radial cement creeping and potentially leading to stem migration (4, 5).
The optimal cement mantle thickness is the subject of current debates; different results regarding the cement mantle thickness are reported in the literature. The following two common techniques, the ‘over-reaming’ technique, and the ‘line-to-line’ (= ‘side-to-side’ technique), will be explained in more detail.
The standard technique is the ‘over-broaching’ technique. Here, the implant is smaller than the last used rasp. This technique allows a cement mantle of 2 mm or more. In contrast to this technique, there is the ‘line-to-line’ technique. In this technique, almost the entire spongy bone is removed in the coronal plane, and the implanted stem has the same size as the last used rasp. The largest possible rasp is used for stem preparation. As a result, a thin cement mantle is achieved. This technique is also referred to as the ‘French paradox’ (2, 4, 5, 16, 44).
The ‘line-to-line technique’ shows partly good results and sometimes even better than the ‘over-reaming’ technique with a cement mantle of 2 mm or more (5, 44). The ‘French paradox’ is an overall successful procedure, even if the individual measures do not always seem entirely comprehensible. These results contradict the previous conventional assumptions that a cement mantle is only durable when it has a minimum thickness of 2 mm without interruption and completely surrounds the entire prosthesis stem (15, 45). Evidence for a successful and durable ‘line-to-line’ technique (French paradox) is provided, among others, by the Müller-Straight-Stem and its considerable number of copies. The Müller-Straight-Stem divides the cement mantle into an anterior and posterior section, and medially and laterally, the simple conical stem should have contact with the cortical bone. The stem self-centers and the rasps need to be designed accordingly for the intramedullary preparation of the cortical bone. Additional equipment with reamers is advised.
Current cementing techniques involve retrograde (‘bottom to top’) filling of the dried medullary cavity (2, 3, 16). Cement guns are indispensable for application (2, 3, 15), and conical nozzles can be used in narrow medullary spaces. Revisions and wide medullary spaces often require two portions of cement, and it is advisable to simultaneously mix with two teams. Several factors can accelerate the setting time of the cement, including higher temperatures, longer mixing, manual handling, and excessive humidity (3, 5).
Gruen’s zones (16, 19) are well suited for assessing biological tissue reactions and their localization. The four-stage classification of the quality of the cement mantle around the femoral stem is credited to Barrack and Mulroy (Table 7) (3, 45).
Table 7.
Grading of the femoral cement mantle according to Barrack et al. (45).
| Grade | Description | Note |
|---|---|---|
| A | Complete filling of the medullary canal; no discernible boundary between bone and cement. | ‘White out’ |
| B | Stable cement mantle; thin boundary layer between bone and cement | ‘Slight radiolucency’ (<50%) |
| C | ||
| C1 | Visible boundary zone; small defects (voids) in the cement mantle; incomplete cement mantle. | ‘Radiolucency incomplete’ (>50%) |
| C2 | Localized thin cement mantle (<1 mm), defects, direct metal contact; endostal. | |
| D | Radiographically visible boundary layer in both planes; incomplete or interrupted cement mantle; large defects (voids). | ‘Radiolucency complete’ (=100%); ‘incomplete coverage of the stem’ |
Biomechanically, there are high loads at the level of the femoral neck and the tip of the stem, making these regions particularly vulnerable to damage. If these cracks propagate, implant failure can occur. To create a high-quality cement mantle, it is important to remove most of the mechanically unstable cancellous bone, especially at the level of the greater trochanter, only leaving a minimal amount of cancellous bone to allow for cement interlocking (4).
The modern fourth-generation cementing technique aims to centralize the stem within the femoral canal to ensure an adequate cement mantle. A detailed overview of the fourth-generation cementing technique is provided in Table 3 (5).
In cases of revision surgery with appropriate indications, a good, mechanically reliable, high-quality cement mantle can be left in place using a technique known as ‘cement-in-cement’ (6). However, this topic is beyond the scope of this overview and will not be further discussed.
Recommendations for the over-reaming technique
Never skip preoperative planning (3).
Tilt the oscillating saw slightly caudally during the resection of the femoral neck (anteversion). Position approximately one finger above the lesser trochanter to ensure sufficient support for the calcar. Collarless stems tolerate minor deviations from the resection line and allow for small corrections regarding penetration depth. With collared stems, precise resection is necessary (offset). If too much is resected, the gap of the missing calcar can be filled with cement (3).
Digital examination of the mechanical quality of the femoral neck cancellous bone (‘doctor’s finger’) to draw conclusions about bone quality and the fixation method.
Place a marked compress in the acetabulum.
Open the medullary canal with a box chisel (3).
Initial preparation with a thin channel-finding rasp (finger rasp, rat-tail rasp) (3).
Use a medium-sharp spoon of sufficient length (e.g. Halle spoon, 21 cm, or Volkmann spoon, 27 cm) to tactually assess the geometry of the medullary canal.
Curved, anatomical, modular rasp handles (double offset) are comfortable even for experienced surgeons.
Always start with the smallest rasp size and gradually increase (3).
Clear the medullary canal with a suction device between each rasp step to reduce the risk of embolism (3, 5).
Avoid direct endomedullary contact of the rasp with the calcar corticalis; strong calcar cancellous bone protects against loosening. However, most of the cancellous bone is removed because it is not mechanically sufficient; only a thin border is left for cement–bone interface interlocking (3, 43).
Insert the rasp slightly deeper (1–2 mm) below the resection level of the femoral neck.
If that is not possible despite careful insertion of the rasp, choose a smaller size.
Good rasps have side markings that indicate the future leg length at this ‘rasp depth’. Optionally, mark the depth and antetorsion on the greater trochanter (3).
Always pay attention to the ‘sound’ of the rasp.
Adjust the rasp more towards ‘valgus’; this will create a wider cement mantle medially on the calcar femoris and reduce the risk of dorsolateral perforation (3).
Do not radically remove the proxiomedial cancellous bone, leaving approximately 3–4 mm of metaphyseal cancellous bone (3).
For stems with a collar, use a planing calcar reamer (‘calcarreamer’).
Brush the medullary canal (3).
Trial reduction provides more safety regarding stem position, leg length, neck length, and dislocation prevention.
Familiarize yourself with the neck lengths, which may not be the same for every product (what does 0 mm mean? Is it ‘s’ or ‘m’? There are also negative measurements, e.g. −3.5. Some companies offer neck lengths up to +16!).
Possibly secure the trial head.
Suction even in the depth of the medullary canal (if necessary, use suction extension with disposable bladder catheter Ch 18 or 21) (3).
Carefully implant the pre-measured marking plug into the intramedullary canal (intramedullary pressure!) (39), avoid using hammer blows if possible. Approximately 1–1.5 cm below the prosthesis tip (3).
Warm the irrigation fluid (Ringer’s solution or Ringer’s lactate), no additives (42).
Do not use H2O2 in an unvented medullary canal as there is a risk of causing an air embolism. Moreover, it might destruct the cement at the cement–bone interface and has erosive and cytotoxic properties (46).
Optionally, place an adrenaline-soaked compress in the medullary canal (3).
Cement is generally ready for use 2–4 min after mixing (3).
Retrograde filling of the medullary canal.
Apply a pressurizer (silicone seal); pressure should be applied for 30–60 s. Beware of BICS.
Possibly administer intraoperative heparin to reduce BICS/thrombi (3, 47, 48).
Keep the temperature-dependent duration of working phases in mind (use a stopwatch).
The finished cement surface should have a slightly wrinkled (‘skinny’) appearance (non-sticky) (3).
Take a small sample before cement application to check when the cement hardens (3).
Do not push cement that has leaked out of the canal back into the canal (3).
Centralizers should only be placed distal to the stem tip or at the very proximal position to avoid cement displacement during stem insertion.
If available, implant with a free, spherical inserter (stem driver).
Do not heat or cool the stems; pre-warmed stems are said to prevent near-implant bubbles and lead to higher strength/stability in the cement–implant interface. However, this could not be conclusively proven in experimental studies (3, 49).
Keep the cone protection cap in place.
Stems with a profiled and rough surface may be inserted slightly earlier (to utilize the remaining ‘adhesive effect’) while stems with a polished surface may be inserted slightly later (to prevent ‘adhesion’) (3).
Pro for early implantation: the cement does not become too hard and therefore does not prevent penetration depth and reduces bleeding at the cement–bone interface (3).
Pro for late implantation: additional pressure exerted by the stem implantation leads to improved interlocking at the bone–cement interface (3).
Always introduce the prosthesis laterally and with slight neck antetorsion (3).
Avoid using a hammer if possible; at most, apply 1–2 cm before the final position with 2–3 controlled hammer blows.
Do not make corrections to the position once the stem is inserted definitively (this may cause voids in the cement mantle). Check the positioning when approximately 2/3 of the stem is inserted (3).
Hold the position of the stem until final polymerization (3).
Carefully remove cement residue with a plastic spatula (3).
Change gloves.
Secure trial heads (e.g. with a suture).
Thoroughly clean and dry the cone.
The dimensioning of the rasps does not always create a corresponding minimum thickness of the cement mantle for the corresponding stems (4). Nowadays, many stem models are offered in both cementless and cemented variations, and the rasps developed for them are the same. The difference in diameter between cementless and cemented stems is often only 1 mm on each side and should not be less. The surgeon should always be aware of the respective dimensions of the prepared bed, and it may be possible to implant a size below the dimension of the last rasp size used.
Not all rasps have cutting edges or blades in the calcar region, which allows spongiosa to be preserved. However, this increases the risk of a blunt impact on the medial femoral neck cortex. Therefore, the rasps should always be inserted slightly laterally and gently pushed towards the trochanter major with caution. Less stable spongiosa in the proximal medullary canal, which is not captured by the rasps, can be curetted (4).
Modern designs of femoral rasps displace and compress the spongiosa to achieve biologically stable primary stability in the cementless stem variation. For the cemented version, thorough jet lavage is essential to reopen the intertrabecular gaps to a greater extent (16). Proper femoral canal preparation and appropriate jet lavage are key points to ensure a stable bone–cement interface, as well as using a distal cement restrictor, cement pressurization, and a distal centralizer if necessary (5). Preserve stable spongiosa, especially in the calcar region!
ICMJE Conflict of Interest Statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the study reported.
Funding Statement
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
References
- 1.Verdonschot N. Implant choice: stem design philosophies. In The Well-Cemented Total Hip Arthroplasty, Theory and Practice, pp. 168–179. Eds Breusch SJ & Malchau H. US: Springer, 2005. [Google Scholar]
- 2.Cassar-Gheiti AJ McColgan R Kelly M Cassar-Gheiti TM Kenny P & Murphy CG. Current concepts and outcomes in cemented femoral stem design and cementation techniques: the argument for a new classification system. EFORT Open Reviews 2020. 5 241–252. ( 10.1302/2058-5241.5.190034) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Emara AK Ng M Krebs VE Bloomfield M Molloy RM & Piuzzi NS. Femoral stem cementation in hip arthroplasty: the know-how of a “lost” art. Current Reviews in Musculoskeletal Medicine 2021. 14 47–59. ( 10.1007/s12178-020-09681-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheerlinck T & Casteleyn PP. The design features of cemented femoral hip implants. Journal of Bone and Joint Surgery 2006. 88 1409–1418. ( 10.1302/0301-620X.88B11.17836) [DOI] [PubMed] [Google Scholar]
- 5.El-Othmani MM Zalikha AK Cooper HJ & Shah RP. Femoral stem cemen tation in primary total hip arthroplasty. JBJS Reviews 2022. 10 1–10. ( 10.2106/JBJS.RVW.22.00111) [DOI] [PubMed] [Google Scholar]
- 6.Timperley AJ Howell JR Hubble MJW Gie GA & Whitehouse SL. Cemented femoral components. In Musculoskeletal Key, Fastest Musculoskeletal Insight Engine (Internet). 2016. Available at: https://musculoskeletalkey.com/cemented-femoral-components/. [Google Scholar]
- 7.Huiskes R Verdonschot N & Nivbrant B. Migration, stem shape, and surface finish in cemented total hip arthroplasty. Clinical Orthopaedics and Related Research 1998. (355) 103–112. ( 10.1097/00003086-199810000-00011) [DOI] [PubMed] [Google Scholar]
- 8.Howell JR Hubble MJW & Ling RSM. Implant choice : stem design – the Surgeon’s perspective. In The Well-Cemented Total Hip Arthroplasty, Theory and Practice, pp. 180–189. Eds Breusch SJ & Malchau H. US: Springer, 2005. [Google Scholar]
- 9.Gruen TA McNeice GM & Amstutz HC. “Modes of Failure” of cemented stem-type femoral components : a radiographic analysis of loosening. Clinical Orthopaedics and Related Research 1979. (141) 17–27. [PubMed] [Google Scholar]
- 10.Mukka S Mellner C Knutsson B Sayed-Noor A & Sköldenberg O. Substantially higher prevalence of postoperative periprosthetic fractures in octogenarians with hip fractures operated with a cemented, polished tapered stem rather than an anatomic stem. Acta Orthopaedica 2016. 87 257–261. ( 10.3109/17453674.2016.1162898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohammed J Mukka S Hedbeck CJ Chammout G Gordon M & Sköldenberg O. Reduced periprosthetic fracture rate when changing from a tapered polished stem to an anatomical stem for cemented hip arthroplasty: an observational prospective cohort study with a follow-up of 2 years. Acta Orthopaedica 2019. 90 427–432. ( 10.1080/17453674.2019.1624339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santori N Falez F Potestio D & Santori FS. Fourteen-year experience with short cemented stems in total hip replacement. International Orthopaedics 2019. 43 55–61. ( 10.1007/s00264-018-4205-3) [DOI] [PubMed] [Google Scholar]
- 13.Clement ND Yapp LZ Baxendale-Smith LD MacDonald D Howie CR & Gaston P. Standard versus short stem cemented Exeter® when used for primary total hip arthroplasty: a survivorship analysis. Arthroplasty (Internet) 2023. 5 47. ( 10.1186/s42836-023-00200-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inabathula A Dilley JE Ziemba-Davis M Warth LC Azzam KA Ireland PH & Meneghini RM. Extended oral antibiotic prophylaxis in high-risk patients substantially reduces primary total hip and knee arthroplasty 90-day infection rate. Journal of Bone and Joint Surgery 2018. 100 2103–2109. ( 10.2106/JBJS.17.01485) [DOI] [PubMed] [Google Scholar]
- 15.Bühler A. Einfluss der 3. Generationszementiertechnik auf das Auftreten des Bone Cement Implantation Syndrome bei der Impla ntation von zementierten Duokopfprothesen. Medizinischen Fakultät der Universität Ulm 2018. [Google Scholar]
- 16.Learmonth ID Young C & Rorabeck C. The operation of the century: total hip replacement. Lancet 2007. 370 1508–1519. ( 10.1016/S0140-6736(0760457-7) [DOI] [PubMed] [Google Scholar]
- 17.Kazi HA Whitehouse SL Howell JR & Timperley AJ. Not all cemented hips are the same: a register-based (NJR) comparison of taper-slip and composite beam femoral stems. Acta Orthopaedica 2019. 90 214–219. ( 10.1080/17453674.2019.1582680) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen F Hård Af Segerstad M Nellgård B Houltz E & Ricksten SE. The role of bone cement for the development of intraoperative hypotension and hypoxia and its impact on mortality in hemiarthroplasty for femoral neck fractures. Acta Orthopaedica 2020. 91 293–298. ( 10.1080/17453674.2020.1745510) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hines CB. Understanding bone cement implantation syndrome. AANA Journal 2018. 86 433–441. [PubMed] [Google Scholar]
- 20.Herrenbruck T Erickson EW Damron TA & Heiner J. Adverse clinical events during cemented long-stem femoral arthroplasty. Clinical Orthopaedics and Related Research 2002. 395 154–163. ( 10.1097/00003086-200202000-00017) [DOI] [PubMed] [Google Scholar]
- 21.Clarius M Heisel C & Breusch SJ. Pulmonary embolism in cement ed total hip arthroplasty. In The Well-Cemented Total Hip Arthroplasty, Theory and Practice, pp. 320–331. Springer: Berlin, Heidelberg,2005. ( 10.1007/3-540-28924-0_43) [DOI] [Google Scholar]
- 22.Rassir R Schuiling M Sierevelt IN van der Hoeven CWP & Nolte PA. What are the frequency, related mortality, and factors associated with bone cement implantation syndrome in arthroplasty surgery? Clinical Orthopaedics and Related Research 2021. 479 755–763. ( 10.1097/CORR.0000000000001541) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weingärtner K Störmann P Schramm D Wutzler S Zacharowski K Marzi I & Lustenberger T. Bone cement implantation syndrome in cemented hip hemiarthroplasty—a persistent risk. European Journal of Trauma and Emergency Surgery (Internet) 2022. 48 721–729. ( 10.1007/s00068-020-01587-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen KK Nayyar S Davidovitch RI Vigdorchik JM Iorio R & Macaulay W. Cemented compared with uncemented femoral fixation in the arthroplasty treatment of displaced femoral neck fractures: a critical analysis review. JBJS Reviews 2018. 6 e6. ( 10.2106/JBJS.RVW.17.00119) [DOI] [PubMed] [Google Scholar]
- 25.Konan S Abdel MP & Haddad FS. Cemented versus uncemented hip implant fixation: should there be age thresholds? Bone and Joint Research 2019. 8 604–607. ( 10.1302/2046-3758.812.BJR-2019-0337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schönherr R Hansen P Lorch R & Parsch D. Zement in der Hüftendoprothetik – ein Update. OUP 2017. 4 202–206. ( 10.3238/oup.2017.0202-0206) [DOI] [Google Scholar]
- 27.Singh M Nagrath A & Maini P. Changes in trabecular pattern of the upper end of the femur as an index of osteoporosis. Journal of Bone and Joint Surgery 1970. 53 1063–1067. [PubMed] [Google Scholar]
- 28.Grevenstein D Vidovic B Baltin C Eysel P Spies CK Unglaub F & Oppermann J. The proximal femoral bone geometry in plain radiographs. Archives of Bone and Joint Surgery 2020. 8 675–681. ( 10.22038/abjs.2020.44937.2226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dossick PH Dorr LD Gruen T & Saberi MT. Techniques for preoperative planning and postoperative evaluation of noncemented hip arthroplasty. Techniques in Orthopaedics 1991. 6 1–6. ( 10.1097/00013611-199109000-00002) [DOI] [Google Scholar]
- 30.Dorr LD Faugere MC Mackel AM Gruen TA Bognar B & Malluche HH. Structural and cellular assessment of bone quality of proximal femur. Bone 1993. 14 231–242. ( 10.1016/8756-3282(9390146-2) [DOI] [PubMed] [Google Scholar]
- 31.Noble PC Alexander JW Lindahl LJ Yew DT Granberry WM & Tullos HS. The anatomic basis of femoral component design. Clinical Orthopaedics and Related Research 1988. (235) 148–165. ( 10.1097/00003086-198810000-00015) [DOI] [PubMed] [Google Scholar]
- 32.Andersen MF Jakobsen T Bensen AS & Krarup N. Lower reoperation rate for cemented femoral stem than for uncemented femoral stem in primary total hip arthroplasty following a displaced femoral neck fracture. SICOT-J 2015. 1 26. ( 10.1051/sicotj/2015028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kannan A Kancherla R McMahon S Hawdon G Soral A & Malhotra R. Arthroplasty options in femoral-neck fracture: answers from the national registries. International Orthopaedics 2012. 36 1–8. ( 10.1007/s00264-011-1354-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robertson GAJ & Wood AM. Hip hemi-arthroplasty for neck of femur fracture: what is the current evidence? World Journal of Orthopedics 2018. 9 235–244. ( 10.5312/wjo.v9.i11.235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kristensen TB Dybvik E Kristoffersen M Dale H Engesæter LB Furnes O & Gjertsen JE. Cemented or uncemented hemiarthroplasty for femoral neck fracture? Data from the Norwegian hip fracture register. Clinical Orthopaedics and Related Research 2020. 478 90–100. ( 10.1097/CORR.0000000000000826) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lindberg-Larsen M, Petersen PB, Jørgensen CC, Overgaard S, Kehlet H. & Lundbeck Foundation Center for Fast-track Hip and Knee Arthroplasty Collaborating Group. Postoperative 30-day complications after cemented/hybrid versus cementless total hip arthroplasty in osteoarthritis patients > 70 years. Acta Orthopaedica 2020. 91 286–292. ( 10.1080/17453674.2020.1745420) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yli-Kyyny T Sund R Heinänen M Venesmaa P & Kröger H. Cemented or uncemented hemiarthroplasty for the treatment of femoral neck fractures? Acta Orthopaedica 2014. 85 49–53. ( 10.3109/17453674.2013.878827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veldman HD Heyligers IC Grimm B & Boymans TAEJ. Cemented versus cementless hemiarthroplasty for a displaced fracture of the femoral neck. Bone and Joint Journal 2017. 99–B 421–431. ( 10.1302/0301-620X.99B4.BJJ-2016-0758.R1) [DOI] [PubMed] [Google Scholar]
- 39.Moran M Heisel C Rupp R Simpson AHRW & Breusch SJ. Cement restrictor function below the femoral isthmus. Clinical Orthopaedics and Related Research 2007. 458 111–116. ( 10.1097/BLO.0b013e3180316caa) [DOI] [PubMed] [Google Scholar]
- 40.Schauss SM Hinz M Mayr E Bach CM Krismer M & Fischer M. Inferior stability of a biodegradable cement plug: 122 total hip replacements randomized to degradable or non-degradable cement restrictor. Archives of Orthopaedic and Trauma Surgery 2006. 126 324–329. ( 10.1007/s00402-006-0132-7) [DOI] [PubMed] [Google Scholar]
- 41.Agrawal P Chacko VJ Divecha H & Board TN. Canal occlusion in cemented primary total hip replacement: autologous compacted bone block compared to a commercially available gelatine plug. Hip International 2021. 31 342–347. ( 10.1177/1120700019881586) [DOI] [PubMed] [Google Scholar]
- 42.Bastawros DS. 5 things you need to know about: pulsed lavage. Advances in Skin and Wound Care 2003. 16 282. ( 10.1097/00129334-200311000-00007) [DOI] [Google Scholar]
- 43.Massoud SN Hunter JB Holdsworth BJ Wallace WA & Juliusson R. Early femoral loosening in one design of cemented hip replacement. The Journal of Bone and Joint Surgery 1997. 79-B 603–608. ( 10.1302/0301-620x.79b4.7131) [DOI] [PubMed] [Google Scholar]
- 44.Langlais F Kerboull M Sedel L & Ling RSM. The “French paradox.” Journal of Bone and Joint Surgery 2003. 85 17–20. ( 10.1302/0301-620x.85b1.13948) [DOI] [PubMed] [Google Scholar]
- 45.Barrack RL Mulroy RD Jr & Harris WH. Improved cementing techniques and femoral component loosening in young patients with hip arthroplasty. Journal of Bone and Joint Surgery 1992. 74 385–389. ( 10.1302/0301-620X.74B3.1587883) [DOI] [PubMed] [Google Scholar]
- 46.Zhou AK Girish M Thahir A An Lim J Tran C Patel S & Krkovic M. The role of hydrogen peroxide in hip arthroplasty: a narrative review. Journal of Perioperative Practice 2022. 32 178–182. ( 10.1177/1750458921996259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westrich GH Salvati EA Sharrock N Potter HG Sánchez PM & Sculco TP. The effect of intraoperative heparin administered during total hip arthroplasty on the incidence of proximal deep vein thrombosis assessed by magnetic resonance venography. Journal of Arthroplasty 2005. 20 42–50. ( 10.1016/j.arth.2004.03.022) [DOI] [PubMed] [Google Scholar]
- 48.Nassif JM Ritter MA Meding JB Keating EM & Faris PM. The effect of intraoperative intravenous fixed-dose heparin during total joint arthroplasty on the incidence of fatal pulmonary emboli. Journal of Arthroplasty 2000. 15 16–21. ( 10.1016/s0883-5403(0091025-8) [DOI] [PubMed] [Google Scholar]
- 49.Damron LA Kim DG & Mann KA. Fatigue debonding of the roughened stem-cement interface: effects of surface roughness and stem heating conditions. Journal of Biomedical Materials Research. Part B, Applied Biomaterials 2006. 78 181–188. ( 10.1002/jbm.b.30470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mulroy WF Estok DM & Harris WH. Total hip arthroplasty with use of so-called second-generation cementing techniques: a fifteen-year-average follow-up study. Journal of Bone and Joint Surgery 1995. 77 1845–1852. ( 10.2106/00004623-199512000-00008) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a