Abstract
Purpose
This study employed meta-analysis to evaluate whether the application of intraoperative wound irrigation (IOWI) with povidone-iodine (PI) in spine surgery effectively reduces the incidence of postoperative surgical site infection (SSI).
Methods
The present study was conducted strictly following the methodological guidance provided by the Cochrane Handbook. The protocol of this work was registered with PROSPERO. Two researchers independently conducted electronic searches in Medline via PubMed, Embase, Cochrane Library, and Web of Science. The bias risk of each included study was evaluated by two assessors. We performed statistical analysis on the dataset using STATA software.
Results
Fourteen studies involving a total of 6777 patients were included in the present work. The risk of bias of six included randomized controlled trials (RCTs) was considered as low-to-moderate risk, and the quality scores of the eight included retrospective cohort studies were rated as high quality. The results of this meta-analysis indicated a significant difference in the incidence of postoperative SSI between the two groups (RR = 0.29, 95% CI: (0.18, 0.47)). Moreover, patients who underwent IOWI with PI had lower rates of deep and superficial infections after spine surgery compared with the controlled group (superficial infection: RR = 0.28, 95%CI: (0.14, 0.54); Deep infection: RR = 0.24, 95%CI: (0.10, 0.60)). The sensitivity analysis results indicated good robustness and high evidence strength after data consolidation in the overall rate of postoperative SSI and the incidence of deep/superficial infection.
Conclusions
IOWI with PI solution during spinal surgery can effectively reduce the incidence of postoperative SSI.
Keywords: intraoperative wound irrigation, povidone-iodine, spine surgery, SSI, surgical site infection
Introduction
Surgical site infection (SSI) represents a pivotal facet of hospital-acquired infection, steadily emerging as a pressing public health concern. The protracted duration of spinal surgery, extensive tissue exposure, and the abundant use of implanted internal fixation materials contribute to an apparent increase in the incidence of SSI (1). Previous studies (2, 3, 4) have reported variable rates of SSI in spinal surgery, ranging from 0.7% to 14%. There exists a current paucity of precise incidence rates for SSI in spinal surgery, primarily attributable to factors such as individual variability and disparities in surgical techniques. Notwithstanding, the certainty persists that SSI after spinal surgery can precipitate severe consequences. For individuals, SSI may result in the failure of the initial surgery, recurrent pain or functional impairments, and even severe complications such as sepsis, ultimately leading to death (5, 6). Moreover, SSI contributes to the extension of hospital stays, necessitates supplementary treatments, and exacts substantial financial burdens on both families and healthcare insurers (7, 8).
Advances have progressed in the practice of perioperative SSI control, including improvement in disinfection methods, operating room environment, surgical techniques, and the use of prophylactic antimicrobial drugs. However, the preventive management of SSI in spine surgery still faces significant challenges (9). The occurrence of spine surgery is influenced by various factors (10, 11, 12), and corresponding preventive measures have been clinically implemented to mitigate these infections. Standardized strategies for preventing SSI during the perioperative period have gained widespread acceptance among clinicians (13, 14). However, there needs to be more emphasis on intraoperative wound irrigation (IOWI) techniques in spine surgery.
The pathogenic bacteria contributing to SSI after spine surgery primarily derive from the skin surface, notably Staphylococcus aureus (15). This establishes a robust theoretical basis for IOWI. The clinical protocols utilized for IOWI in spine surgery mainly encompass normal saline, normal saline mixed with antibiotic, and antimicrobial solutions (16, 17, 18). The critical determinant for optimizing the effectiveness of IOWI resides in selecting an appropriate solution; however, a universally accepted consensus on this matter still needs to be discovered.
Povidone-iodine (PI), a versatile disinfectant solution, served as an adjunctive measure in preventing the occurrence of SSI in spine surgery. Its mechanism of action predominantly involved its key components, polyvinylpyrrolidone and triiodide ions, effectively targeting bacterial cell walls and impeding the release of pathogenic factors (19). Previous studies (20, 21) have indicated that IOWI with PI in spine surgery can significantly reduce the occurrence of SSI. However, these studies were characterized by relatively small sample sizes and varying research quality, leading to insufficient strength of evidence. Therefore, this study was to employ meta-analysis to evaluate whether the application of IOWI with PI in spine surgery effectively reduces the incidence of postoperative SSI.
Materials and methods
The present study was conducted in strict accordance with the methodological guidance provided by the Cochrane Handbook. The reporting of study outcomes conformed to the recommendations of the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) guidelines proposed by the PRISMA workgroup (22). This work constituted a secondary analysis of original studies, and ethical approval was waived by our institutional Ethics Committee. The protocol of this review was registered with PROSPERO.
Eligibility criteria
The inclusion criteria for this study were formulated by three researchers based on the principle of PICOs (Population, Intervention, Comparison, Outcome, and Study design) framework: i) Population: patients undergoing initial spinal surgery, excluding those with inflammatory or infectious diseases, immunological disorders, poorly controlled diabetes, etc. No restrictions on patients’ age, gender, or ethnicity; ii) Intervention: IOWI with diluted PI; iii) Comparison: no or other IOWI techniques; iv) Outcome measures: postoperative SSI rate (SSI identified with bacterial cultures or clinically), adverse events; v) Study design: priority given to randomized controlled trials (RCTs). In instances where the number of RCTs was insufficient, non-RCTs were incorporated. Only English literature was considered for this study. Animal experiments were excluded given the essential differences between animals and humans. Conference abstracts and comments were also excluded due to the fact that they contained fewer details of the study, which did not allow for a quality assessment of the literature and extraction of complete data. Reviews were primarily summaries of the original literature and often did not include complete outcomes for individual original studies; therefore, we also did not include articles lacking comprehensive study details.
Literature search
Two researchers with more than 3 years of literature retrieval experience independently conducted electronic searches for studies on the effectiveness of IOWI with PI in spinal surgery for SSI prevention. The searches were performed according to a predefined strategy and covered databases, including Medline via PubMed, Embase, Cochrane Library, and Web of Science. The search period spanned from the inception of each database to December 2023. The Medical Subject Headings (MeSH) terms or queries utilized included, but were not limited to, ‘spine surgery’, ‘spine’, ‘lumbar’, ‘cervical’, ‘thoracic’, ‘surgical site infection’, ‘povidone-iodine’, ‘irrigation’, ‘lavage’, and ‘wound lavage’. Additionally, we manually scrutinized the included studies and their respective reference lists to detect potential articles that might have eluded the initial electronic searches.
Study selection and data extraction
The preliminary search findings were initially imported into EndNote software, version 20 (Thomson Corporation, Connecticut, USA). This literature management system autonomously identified and preserved a single instance of duplicate entries sourced from multiple databases. Two researchers independently screened the remaining studies by reviewing their titles and abstracts, and categorized them as ‘include’, ‘uncertain’, or ‘exclude’. Studies marked as ‘include’ or ‘uncertain’ underwent a comprehensive full-text review to determine their final inclusion in this work. Any disputes arising during the study selection were resolved through internal discussion between the two researchers, with a third researcher with over a decade of research experience available for decision-making if necessary.
Two researchers independently extracted the following data from each included study: i) Study characteristics: first author, publication date, study location, type of study design, and number of cases; ii) Baseline characteristics of cases: age, gender, primary diagnosis, surgical techniques and region; iii) Outcome measures: IOWI techniques and their volumes, postoperative SSI (deep or superficial infection), and adverse events.
Quality assessment
Two assessors independently evaluated each included study’s literature quality or bias risk. Details such as author names, affiliations, and journal names were concealed throughout the assessment. In cases of disagreement, resolution was achieved through internal consultation between the two assessors.
We utilized the risk of bias tool recommended by the Cochrane Handbook to evaluate the risk of bias in the included RCTs (23). This tool comprised 13 items. A study meeting nine or more of the 13 items was classified as low risk; those meeting 5–8 items were categorized as moderate risk, while studies meeting fewer than five criteria were deemed high risk (24).
The Newcastle-Ottawa Quality Assessment Scale (NOQAS) was employed to evaluate the methodological quality of the included retrospective cohort studies (25). This scale consisted of eight items under three main categories (selection of study population, comparability between groups, and assessment of outcomes). An asterisk was applied to indicate satisfaction with each item. Studies accumulating six or more asterisks were classified as high quality, whereas those with fewer were categorized as low quality.
Statistical analysis
In this study, we performed statistical analysis on the dataset using STATA software, version 12 (StataCorp LP, College Station, TX, USA). For dichotomous data, such as the number of postoperative SSI, we utilized the relative risk (RR) and 95% confidence intervals (95% CI) to estimate the pooled effects. Statistical significance was defined as a P-value less than 0.05.
We applied the quantitative I 2 statistic to assess the heterogeneity among studies. An I 2 value over 75% signifies considerable heterogeneity, while a range between 25% and 75% suggests moderate heterogeneity, and a value below 25% indicates low or negligible heterogeneity (26). In instances of notable heterogeneity, a random-effects model was employed for statistical analysis. Subgroup analysis or meta-regression analysis was undertaken as needed to explore the sources of heterogeneity. A stepwise exclusion method was performed for sensitivity analysis to validate the robustness of the pooled effect size of an outcome measure. Moreover, The funnel plot was utilized to identify potential publication bias for outcome measures with an inclusion of more than 10 studies.
Results
Literature search
Following a predefined retrieval strategy, two researchers screened in electronic databases, identifying 1746 potential literature that could meet the inclusion criteria. A total of 1217 duplicate records from multiple databases were excluded. After reviewing titles and abstracts, 492 studies were excluded for various reasons. A thorough examination of the full texts of the remaining 37 articles resulted in the identification of 14 studies that fully met the inclusion criteria (27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40). A manual search of the references of these included studies yielded no any additional records meeting the inclusion criteria. The literature retrieval process is illustrated in Fig. 1.
Figure 1.
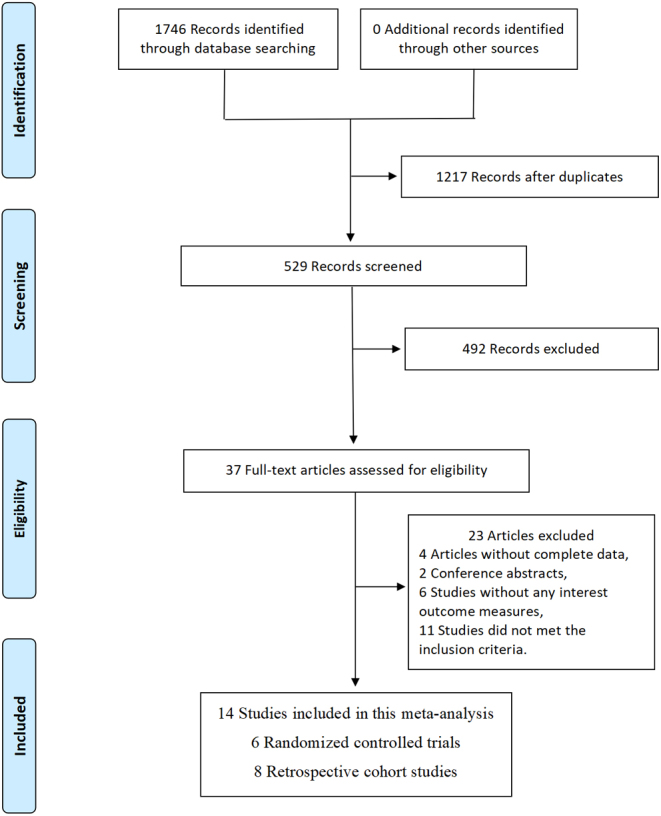
Flow diagram of study selection.
Study characteristics and quality assessment
The 14 included studies (27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40) involved a total of 6777 patients (experimental group: 3117 cases, control group: 3660 cases). Six (27, 28, 31, 32, 36, 40) of these studies were RCTs, while the remaining were retrospective cohort studies (RCS). The articles included in the present study spanned the years 2005 to 2023. The sample sizes in the included studies varied from 50 to 2425 cases. In the experimental group of the included studies, PI was used for IOWI in spine surgery. In contrast, most studies utilized normal saline or vancomycin for IOWI in the control group. For specific details on the essential characteristics of the literature, refer to Table 1.
Table 1.
General characteristics of the included literature.
| Study | Year | Country | Study design | Subjects, n | Population | Primary diagnosis | Operative location | Irrigation techniques and Volumes | ||
|---|---|---|---|---|---|---|---|---|---|---|
| E | C | E | C | |||||||
| Cheng et al. (27) | 2005 | China | RCT | 208 | 206 | Adult | Varied | Varied | 0.35% P-I, 2L | Normal saline, 2L |
| Chang et al. (28) | 2006 | China | RCT | 120 | 124 | Adult | LIS | LS | 0.35% P-I , 2LC: | Normal saline, 2L |
| Tomov et al. (29) | 2015 | USA | RCS | 1173 | 1252 | Adult | Varied | Varied | 0.3% P-I , N/A | N/A |
| van Herwijnen et al. (30) | 2016 | UK | RCS | 71 | 15 | Pediatric | Scoliosis | Varied | 1% P-I , 6L | Saline with 80 mg gentamicin, 7L |
| De Luna et al. (31) | 2017 | Italy | RCT | 25 | 25 | Adult | Scoliosis | Varied | 3% P-I , 2L | Normal saline, 2L |
| Fei et al. (32) | 2017 | China | RCT | 40 | 40 | Adult | PLID | Lumbar | 0.1% P-I , 0.2L | Normal saline, N/A |
| Yamada et al. (33) | 2018 | Japan | RCS | 301 | 741 | Adult | N/A | Varied | 0.38% P-I , 0.52L | Normal saline, N/A |
| Onishi et al. (34) | 2019 | Japan | RCS | 177 | 146 | Adult | N/A | Varied | 1% P-I , N/A | Normal saline, N/A |
| Lemans et al. (35) | 2019 | Netherlands | RCS | 217 | 257 | Adult | N/A | Varied | 500mL P-I solution; concentration: 1.3g/L | Normal saline, N/A |
| Sigari et al. (36) | 2020 | Germany | RCT | 468 | 468 | Adult | Scoliosis, DD | Varied | 3% P-I , N/A | Normal saline, N/A |
| Carballo Cuello et al. (37) | 2021 | USA | RCS | 144 | 134 | Adult | Varied | Lumbar | 0.35% P-I , 1L | Normal saline, 1L |
| Roberto et al. (38) | 2021 | USA | RCS | 13 | 88 | Pediatric | Spinal deformity | Varied | 3.5% w/v P-I , N/A | No irrigation techniques |
| Lin et al. (39) | 2022 | China | RCS | 120 | 124 | Adult | Varied | Lumbar | 0.35% P-I , 1L | Normal saline, N/A |
| Inojie et al. (40) | 2023 | Nigeria | RCT | 40 | 40 | Adult | N/A | Varied | 0.35% P-I , 1L | 1L saline with 80 mg gentamicin |
C, Controlled group; DD, degenerative diseases; E, Experimental group; LIS, lumbar instability; LS, lumbosacral; PLID, prolapsed lumbar intervertebral discs; P-I, povidone-iodine; RCS, Retrospective cohort study; RCT, randomized controlled trial.
According to the bias risk tool recommended by the Cochrane Handbook, four RCTs included (27, 28, 36, 40) met nine or more out of the 13 criteria and were considered low risk. Two studies (31, 32), meeting 5–8 criteria, were deemed to have a moderate risk (Table 2). Based on the NOQAS criteria, the quality scores of the eight included RCS (29, 30, 33, 34, 35, 37, 38, 39) ranged from six to seven, all considered high quality. The quality assessment of the included studies is presented in Table 3.
Table 2.
Methodological quality assessment results according to Cochrane review criteria.
| Items | Cheng et al. (27) | Chang et al. (28) | De Luna et al. (31) | Fei et al. (32) | Sigari et al. (36) | Inojie et al. (40) |
|---|---|---|---|---|---|---|
| Adequate randomization | Y | Y | N | Y | Y | Y |
| Concealed treatment allocation | Y | Y | U | U | Y | Y |
| Patient blinded | Y | Y | U | U | Y | Y |
| Care provider blinded | N | N | U | U | U | Y |
| Outcome assessor blinded | U | U | U | U | Y | U |
| Dropout rate described | Y | Y | N | Y | Y | Y |
| All randomized participants analyzed in the groups | Y | Y | Y | Y | Y | Y |
| No reports of selective outcome reporting | U | Y | Y | U | Y | Y |
| Groups similar at baseline for the most important prognostic indicators | Y | Y | Y | Y | Y | Y |
| Cointerventions avoided or similar | Y | Y | Y | Y | Y | Y |
| Compliance acceptable in all group | Y | Y | Y | Y | Y | Y |
| Time of outcome assessment in all similar groups | Y | Y | Y | Y | Y | Y |
| Other sources of potential bias unlikely | Y | Y | U | Y | Y | Y |
| Score | 10/13 | 11/13 | 6/13 | 8/13 | 12/13 | 12/13 |
N, no; U, unclear, Y, yes.
Table 3.
Quality assessment results of the included retrospective cohort study. A (⭐) indicated satisfaction with each item.
Meta-analysis results
The overall rate of postoperative SSI
The 14 studies included (27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40) all reported the total incidence rate of postoperative SSI in both the experimental and control groups, with moderate heterogeneity among the studies (P = 0.08, I2 = 37.1%). Data were pooled using a random-effects model. The results of this meta-analysis showed a significant difference in the overall rate of postoperative SSI between the two groups (RR = 0.29, 95% CI: (0.18, 0.47), Fig. 2), indicating that IOWI with PI during spine surgery was effective in reducing the overall incidence of SSI. Moreover, according to the study design, we conducted subgroup analyses for this outcome measure. The results demonstrated that regardless of whether it was an RCT or RCS, the incidence of postoperative SSI in patients who underwent IOWI with PI was lower than in the control group (RCT: RR = 0.16, 95% CI: (0.08, 0.34); RCS: RR = 0.41, 95% CI: (0.24, 0.68), Fig. 3), suggesting that the study design had a minimal effect on this outcome.
Figure 2.
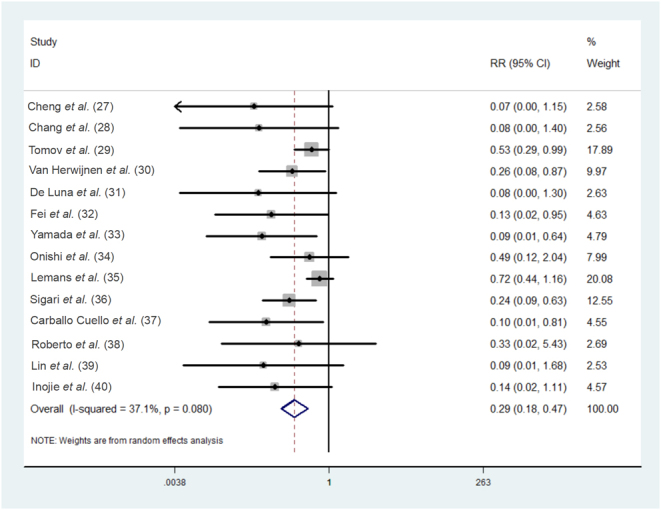
Forest plot of the meta-analytic estimate for the overall rate of postoperative SSI.
Figure 3.
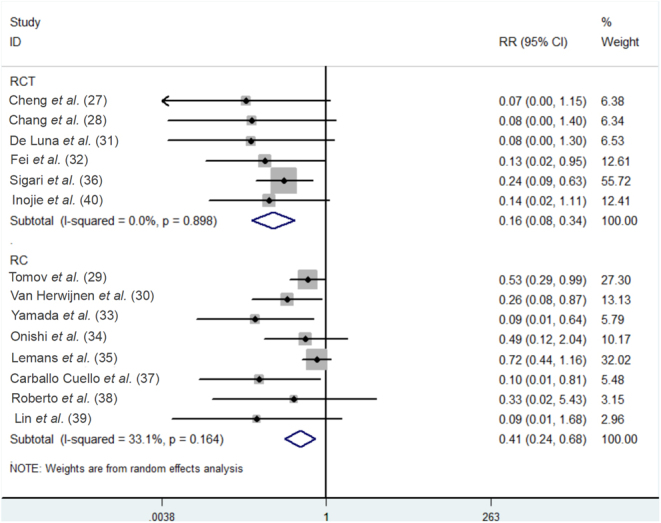
Subgroup analysis of the meta-analytic estimate for the overall rate of postoperative SSI according to study design.
Deep and superficial infection
The incidence rate of superficial infection following different irrigation techniques was reported in 10 studies (27, 30, 31, 32, 33, 34, 35, 36, 39, 40), with no significant heterogeneity among the studies (P = 0.782, I2 = 0); while the incidence of deep infection postoperatively was documented in a total of 11 studies (27, 28, 30, 31, 32, 33, 34, 35, 36, 39, 40), with significant heterogeneity among the studies (P = 0.006, I2 = 59.8%). Data were pooled using a random-effects model. The results of this meta-analysis indicated significant differences in the incidence rates of postoperative SSI between the two irrigation techniques (superficial infection: RR = 0.28, 95%CI: (0.14, 0.54); deep infection: RR = 0.24, 95%CI: (0.10, 0.60), Fig. 4), demonstrating that IOWI with PI in spine surgery had the significant advantage over the control group in reducing both deep and superficial infections.
Figure 4.
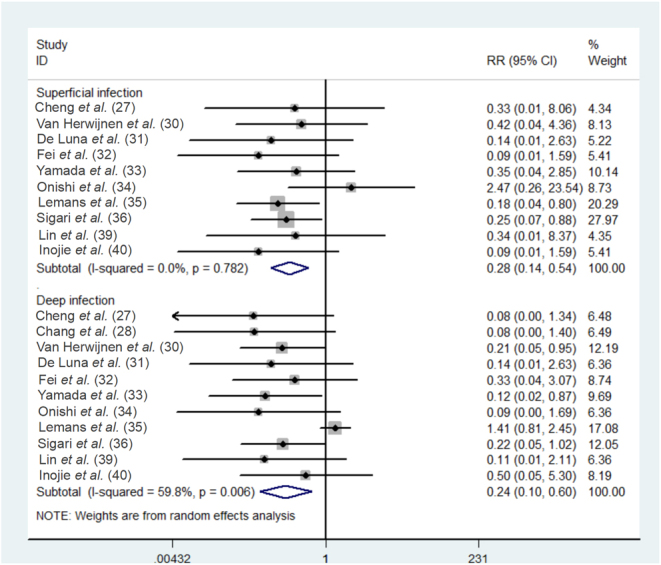
Forest plot of the meta-analytic estimate for the incidence of deep and superficial infection.
Sensitivity analysis and publication bias
We employed a stepwise exclusion method to conduct sensitivity analysis for studies with more than eight included for individual outcome measures. The results demonstrated no significant changes in the statistical differences in the overall rate of postoperative SSI, and the incidence of deep or superficial infection throughout the analysis process (Fig. 5). This indicates good robustness and high evidence strength after consolidation. The funnel plot suggests no potential presence of publication bias (Fig. 6).
Figure 5.
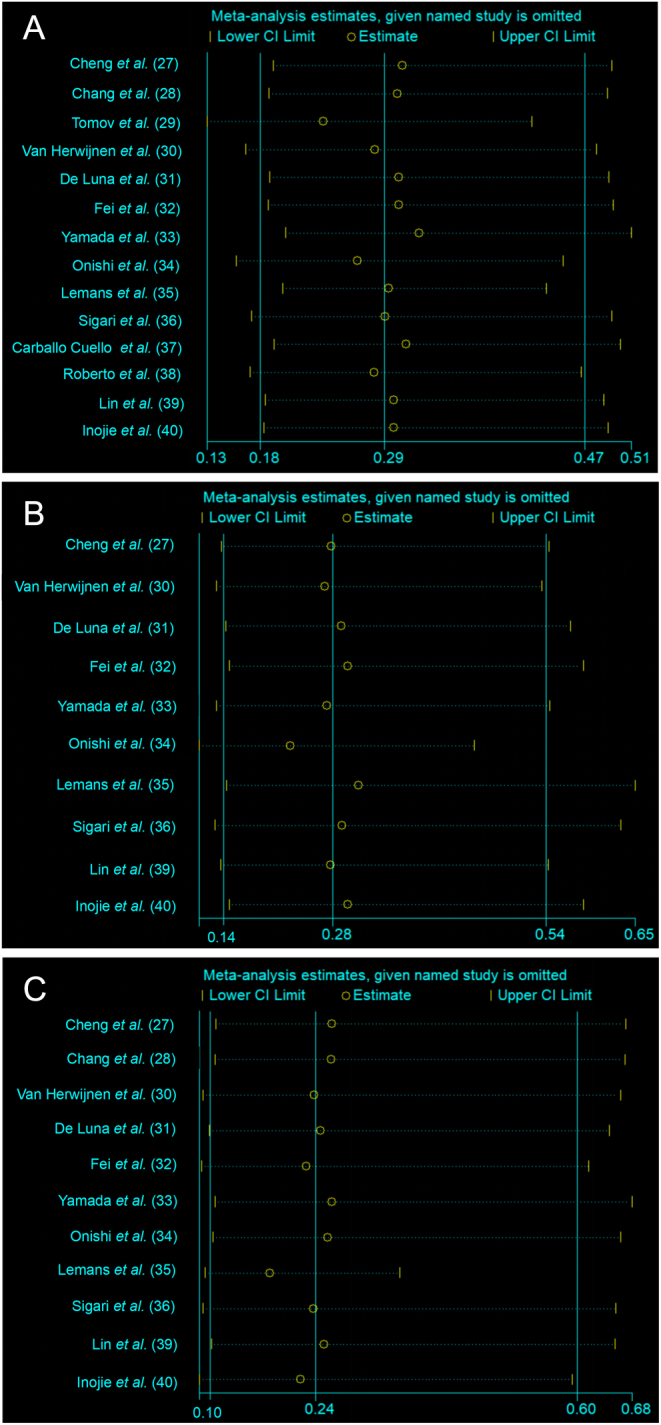
Sensitive analysis of the overall rate of postoperative SSI (5A), the incidence of deep infection (5B) or superficial infection (5C).
Figure 6.
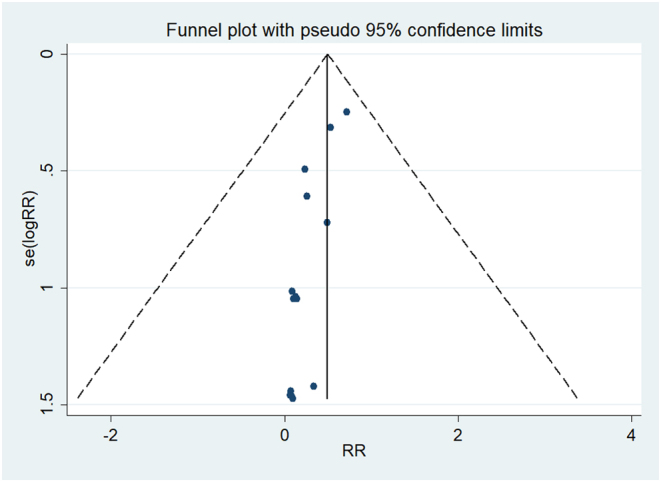
Funnel plot of the overall rate of postoperative SSI.
Discussion
SSI persists as one of the common and challenging complications in spine surgery. Despite the clinical implementation of various perioperative management to prevent the occurrence of SSI after spine surgery, there still needs to be standardized protocols. While IOWI techniques have gained widespread acceptance among clinicians, the choice of irrigation techniques continues to be influenced by individual clinician experience and preferences. The present study aims to conduct a meta-analysis to evaluate the impact of IOWI with PI on the incidence of SSI following spine surgery. The results of this study demonstrate that IOWI with PI effectively reduces the total rate of SSI, and the incidence of deep or superficial wound infections after spinal surgery.
To the best of our knowledge, this is the first meta-analysis focused on the use of IOWI with PI on the incidence of SSI in spine surgery. The interventions included in this study exclusively entailed the application of PI solution during the operative procedure, eliminating potential interference from other confounding factors. Furthermore, the present study conducted comprehensive electronic and manual searches, ensuring the thoroughness of literature retrieval. These meticulous measures facilitated a comprehensive and in-depth examination of the topic, thereby ensuring the robustness of the research findings. The conclusions drawn from our study demonstrated significant concordance with relevant published research (27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40), indicating a favorable impact of IOWI with PI in preventing SSI after spine surgery.
In accordance with the global guidelines for preventing SSI provided by the World Health Organization, the use of antibiotics for intra-wound irrigation in clean wounds to prevent SSI is discouraged. Instead, consideration should be given to employing IOWI with PI solution (41). This establishes a robust foundation for using intraoperative PI irrigation in spinal procedures. Staphylococcus aureus and coagulase-negative staphylococci are prevalent pathogens in spinal surgeries, contributing to SSI (42, 43). With the improper use of antibiotics, there is a continuous increase in bacterial resistance, and SSI caused by methicillin-resistant Staphylococcus aureus (MRSA) has become more prevalent (44, 45). PI demonstrates outstanding antibacterial efficacy against a broad spectrum of bacteria and exhibits bactericidal activity against vancomycin-resistant enterococcus (VRE) and multidrug-resistant gram-negative bacteria, as documented in previous literature (45). This better explains the reasons for the observed reduction in postoperative SSI rates in spine surgery with the use of IOWI with PI in our study.
Compared to hydrogen peroxide and chlorhexidine digluconate, diluted PI solution retains potent bactericidal properties (46). However, they may also adversely affect human osteoblasts’ proliferation, metabolism, and mineralization (47). Theoretically, IOWI with PI solution could predispose to postoperative graft nonunion in osteotomy or bone grafting surgeries. Nevertheless, this assertion predominantly relied on reports from in vitro studies, lacking corroboration from in vivo investigations to date (39). Our present investigation, including the study by Chang et al. (28), demonstrated that IOWI with diluted PI solution and saline before bone grafting does not compromise postoperative bone fusion or clinical outcomes. Regrettably, the studies incorporated into this work primarily focused on the incidence of deep or superficial infection following IOWI with PI during spinal surgery, with most overlooking the report and analysis of related adverse events and prognoses. Therefore, further inquiry is imperative to comprehensively evaluate the safety profile of PI irrigation in spinal surgical settings.
In the 14 studies (27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40) included in the present work, the concentration of PI ranged from 0.10% to 3.5%. The volume of PI and saline used for intraoperative irrigation primarily depends on the surgeon’s preference and experience, although many clinicians prefer to use a PI concentration of approximately 0.35% during the procedure (27, 28, 29, 33, 37, 39, 40). Regarding the timing of IOWI with PI application in spinal surgery, most studies (27, 28, 33, 35, 36, 37, 40) recommend applying the PI solution to soak the wound for 2–3 minutes before wound closure, followed by normal saline irrigation. De Luna et al. (31) advocated for the use of IOWI with PI before bone graft. For prolonged spinal surgery, additional irrigation every 1–2 h is also advisable (33, 34). In this study, only three studies (31, 32, 38) used a pulsatile lavage device for intraoperative irrigation, but the pressure settings of the device were not specified.
As with the other similar studies, the current study still has some limitations. i) According to the Cochrane Collaboration guidelines, RCTs are recommended in meta-analysis to eliminate potential biases that may impact research outcomes, making them the most suitable for assessing the effects of interventions on disease health. Six were RCTs among the 14 studies included in the present work. RCS tend to exaggerate accurate results, although most of them are of high quality, according to NOQAS. More high-quality RCTs are still needed to obtain convincing conclusions; ii) A standardized formulation for the PI solution used in spine surgery is still lacking. Moreover, there has yet to be a consensus on the volume of saline for secondary irrigation. However, the appropriate concentration is one of the crucial factors for the antimicrobial solution to be effective. Therefore, different configuration schemes of PI used in the included studies may have led to differences in the incidence of postoperative SSI, thereby affecting the results of this study. Further assessment is needed to evaluate the impact of PI usage methods on the occurrence of SSI after spine surgery; iii) The present work encompasses a diverse study population, including those with spinal degenerative diseases, spinal tumors, and spinal trauma, with surgical sites covering the cervical, thoracic, and lumbosacral spine. The findings of this study highlight the effectiveness and safety of the IOWI technique in reducing the incidence of SSIs in spine surgery. Although there is no evidence indicating adverse effects of IOWI on nerves or the spinal cord, the studies reviewed in this work specifically excluded cases involving intraoperative dural breaches and cerebrospinal fluid leaks. Consequently, we advise caution when applying the IOWI technique in patients with compromised neural structures; iv) The study population included adult and child cohorts, encompassing degenerative spinal diseases, spinal deformities, and different operative regions of the spine. The lack of an adequate sample size precludes subgroup analyses, limiting the adaptability of the study results for reducing spinal SSI.
Conclusion
Based on data from 14 studies, we conclude that IOWI with PI solution during spine surgery can effectively reduce the incidence of postoperative SSI. While this study cannot address clinical questions such as ‘What is the optimal formulation for the intraoperative PI solution?’ and ‘Which fluid and what volume should be used for secondary irrigation?’ it is recommended, particularly for patients undergoing spinal surgery with internal fixation devices, to consider employing intra-wound therapies, especially PI irrigation.
ICMJE Conflict of Interest Statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the study reported.
Funding Statement
This work was supported by the Scientific Research and Technology Development Program of Guangxi (AD22035004), Guangxi Healthcare Appropriate Technology Development and Promotion Project (S2023024).
Data Availability
The datasets analyzed during the present study are available from the corresponding author on reasonable request.
Author contribution statement
JW and MW conceived and designed the study. XM and XW performed the experiments. XM and ZL interpreted the data. XM, XW, and ZL contributed reagents, materials, analysis tools. XM wrote the first draft of the manuscript.
References
- 1.Fanous AA Kolcun JPG Brusko GD Paci M Ghobrial GM Nakhla J Eleswarapu A Lebwohl NH Green BA & Gjolaj JP. Surgical site infection as a risk factor for long-term instrumentation failure in patients with spinal deformity: a retrospective cohort study. World Neurosurgery 2019. 132 132, ., e514–132.e. ( 10.1016/j.wneu.2019.08.088) [DOI] [PubMed] [Google Scholar]
- 2.Gerometta A Rodriguez Olaverri JC & Bitan F. Infections in spinal instrumentation. International Orthopaedics 2012. 36 457–464. ( 10.1007/s00264-011-1426-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pull ter Gunne AF & Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine 2009. 34 1422–1428. ( 10.1097/BRS.0b013e3181a03013) [DOI] [PubMed] [Google Scholar]
- 4.Zhou J Wang R Huo X Xiong W Kang L & Xue Y. Incidence of surgical site infection after spine surgery: a systematic review and meta-analysis. Spine 2020. 45 208–216. ( 10.1097/BRS.0000000000003218) [DOI] [PubMed] [Google Scholar]
- 5.Campbell PG Yadla S Malone J Maltenfort MG Harrop JS Sharan AD & Ratliff JK. Complications related to instrumentation in spine surgery: a prospective analysis. Neurosurgical Focus 2011. 31 E10. ( 10.3171/2011.7.FOCUS1134) [DOI] [PubMed] [Google Scholar]
- 6.Wickramasinghe ND Horton J Darshika I Galgamuwa KD Ranasinghe WP Agampodi TC & Agampodi SB. Productivity cost due to postpartum ill health: a cross-sectional study in Sri Lanka. PLoS One 2017. 12 e0185883. ( 10.1371/journal.pone.0185883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edmiston CE Jr Leaper DJ Chitnis AS Holy CE & Chen BPH. Risk and economic burden of surgical site infection following spinal fusion in adults. Infection Control and Hospital Epidemiology 2023. 44 88–95. ( 10.1017/ice.2022.32) [DOI] [PubMed] [Google Scholar]
- 8.Dietz N Sharma M Adams S Ugiliweneza B Wang D Bjurström MF Karikari I Drazin D & Boakye M. Health care utilization and associated economic burden of postoperative surgical site infection after spinal surgery with follow-up of 24 months. Journal of Neurological Surgery. Part A, Central European Neurosurgery 2023. 84 21–29. ( 10.1055/s-0040-1720984) [DOI] [PubMed] [Google Scholar]
- 9.Phillips BT Sheldon ES Orhurhu V Ravinsky RA Freiser ME Asgarzadeh M Viswanath O Kaye AD & Roguski M. Preoperative versus extended postoperative antimicrobial prophylaxis of surgical site infection during spinal surgery: a comprehensive systematic review and meta-analysis. Advances in Therapy 2020. 37 2710–2733. ( 10.1007/s12325-020-01371-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xie J Du Y Tan Z & Tang H. Association between malnutrition and surgical site wound infection among spinal surgery patients: a meta-analysis. International Wound Journal 2023. 20 4061–4068. ( 10.1111/iwj.14297) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Rudic TN Althoff AD Kamalapathy P & Bachmann KR. Surgical site infection after primary spinal fusion surgery for adolescent idiopathic scoliosis: an analysis of risk factors from a nationwide insurance database. Spine 2023. 48 E101–E106. ( 10.1097/BRS.0000000000004591) [DOI] [PubMed] [Google Scholar]
- 12.Lian J Wang Y Yan X Xu G Jia M Yang J Ying J & Teng H. Development and validation of a nomogram to predict the risk of surgical site infection within 1 month after transforaminal lumbar interbody fusion. Journal of Orthopaedic Surgery and Research 2023. 18 105. ( 10.1186/s13018-023-03550-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tkatschenko D, Hansen S, Koch J, Ames C, Fehlings MG, Berven S, Sekhon L, Shaffrey C, Smith JS, Hart R, et al. Prevention of surgical site infections in spine surgery: an international survey of clinical practices among expert spine surgeons. Global Spine Journal 2023. 13 2007–2015. ( 10.1177/21925682211068414) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson DJ. Surgical site infections. Infectious Disease Clinics of North America 2011. 25 135–153. ( 10.1016/j.idc.2010.11.004) [DOI] [PubMed] [Google Scholar]
- 15.Hickmann AK, Bratelj D, Pirvu T, Loibl M, Mannion AF, O'Riordan D, Fekete T, Jeszenszky D, Eberhard N, Vogt M, et al. Management and outcome of spinal implant-associated surgical site infections in patients with posterior instrumentation: analysis of 176 cases. European Spine Journal 2022. 31 489–499. ( 10.1007/s00586-021-06978-y) [DOI] [PubMed] [Google Scholar]
- 16.Agrawal A Ramachandraiah MK Shanthappa AH & Agarawal S. Effectiveness of gentamicin wound irrigation in preventing surgical site infection during lumbar spine surgery: a retrospective study at a rural teaching hospital in India. Cureus 2023. 15 e46094. ( 10.7759/cureus.46094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumoto H, Bonsignore-Opp L, Warren SI, Hammoor BT, Troy MJ, Barrett KK, Striano BM, Roye BD, Lenke LG, Skaggs DL, et al. Strategies reducing risk of surgical-site infection following pediatric spinal deformity surgery. Spine Deformity 2023. 11 71–86. ( 10.1007/s43390-022-00559-9) [DOI] [PubMed] [Google Scholar]
- 18.Hsiung W Yao YC Lin HH Wang ST Hsiung L Chen KJ Chang MC & Chou PH. Reducing surgical site infections after spine surgery: the optimal amount of normal saline for intra-wound irrigation. Spine Journal 2023. 23 1580–1585. ( 10.1016/j.spinee.2023.07.011) [DOI] [PubMed] [Google Scholar]
- 19.Gruenberg MF Campaner GL Sola CA & Ortolan EG. Ultraclean air for prevention of postoperative infection after posterior spinal fusion with instrumentation: a comparison between surgeries performed with and without a vertical exponential filtered air-flow system. Spine 2004. 29 2330–2334. ( 10.1097/01.brs.0000142436.14735.53) [DOI] [PubMed] [Google Scholar]
- 20.Mallet C Meissburger V Caseris M Happiette A Chinnappa J Bonacorsi S Simon AL & Ilharreborde B. Does the use of intrawound povidone-iodine irrigation and local vancomycin powder impact surgical site infection rate in adolescent idiopathic scoliosis surgery? European Spine Journal 2022. 31 3020–3028. ( 10.1007/s00586-022-07340-6) [DOI] [PubMed] [Google Scholar]
- 21.Ulivieri S Toninelli S Petrini C Giorgio A & Oliveri G. Prevention of post-operative infections in spine surgery by wound irrigation with a solution of povidoneiodine and hydrogen peroxide. Archives of Orthopaedic and Traumatic Surgery 2011. 131 1203–1206. ( 10.1007/s00402-011-1262-0) [DOI] [PubMed] [Google Scholar]
- 22.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021. 372 n71. ( 10.1136/bmj.n71) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo RA, Schoene M, Bronfort G, van Tulder MW. & Editorial Board of the Cochr ane Back , Neck Group. 2015 updated method guideline for systematic reviews in the Cochrane Back and Neck Group. Spine 2015. 40 1660–1673. ( 10.1097/BRS.0000000000001061) [DOI] [PubMed] [Google Scholar]
- 24.Li BZ Tang WH Li Y Zhou L Liu MG & Bao SX. Clinical efficacy of epidural injections of local anesthetic alone or combined with steroid for neck pain: a systematic review and meta-analysis. BioMed Research International 2022. 2022 8952220. ( 10.1155/2022/8952220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology 2010. 25 603–605. ( 10.1007/s10654-010-9491-z) [DOI] [PubMed] [Google Scholar]
- 26.Higgins JPT Thompson SG Deeks JJ & Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003. 327 557–560. ( 10.1136/bmj.327.7414.557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng MT Chang MC Wang ST Yu WK Liu CL & Chen TH. Efficacy of dilute Betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine 2005. 30 1689–1693. ( 10.1097/01.brs.0000171907.60775.85) [DOI] [PubMed] [Google Scholar]
- 28.Chang FY Chang MC Wang ST Yu WK Liu CL & Chen TH. Can povidone-iodine solution be used safely in a spinal surgery? European Spine Journal 2006. 15 1005–1014. ( 10.1007/s00586-005-0975-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tomov M Mitsunaga L Durbin-Johnson B Nallur D & Roberto R. Reducing surgical site infection in spinal surgery with betadine irrigation and intrawound vancomycin powder. Spine 2015. 40 491–499. ( 10.1097/BRS.0000000000000789) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Herwijnen B Evans NR Dare CJ & Davies EM. An intraoperative irrigation regimen to reduce the surgical site infection rate following adolescent idiopathic scoliosis surgery. Annals of the Royal College of Surgeons of England 2016. 98 320–323. ( 10.1308/rcsann.2016.0132) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Luna V Mancini F De Maio F Bernardi G Ippolito E & Caterini R. Intraoperative disinfection by pulse irrigation with povidone-iodine solution in spine surgery. Advances in Orthopedics 2017. 2017 7218918. ( 10.1155/2017/7218918) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fei J & Gu J. Comparison of lavage techniques for preventing incision infection following posterior lumbar interbody fusion. Medical Science Monitor 2017. 23 3010–3018. ( 10.12659/msm.901868) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamada K, Abe H, Higashikawa A, Tonosu J, Kuniya T, Nakajima K, Fujii H, Niwa K, Shinozaki T, Watanabe K, et al. Evidence-based care bundles for preventing surgical site infections in spinal instrumentation surgery. Spine 2018. 43 1765–1773. ( 10.1097/BRS.0000000000002709) [DOI] [PubMed] [Google Scholar]
- 34.Onishi Y Masuda K Tozawa K & Karita T. Outcomes of an intraoperative povidone-iodine irrigation protocol in spinal surgery for surgical site infection prevention. Clinical Spine Surgery 2019. 32 E449–E. ( 10.1097/BSD.0000000000000908) [DOI] [PubMed] [Google Scholar]
- 35.Lemans JVC Öner FC Wijdicks SPJ Ekkelenkamp MB Vogely HC & Kruyt MC. The efficacy of intrawound vancomycin powder and povidone-iodine irrigation to prevent surgical site infections in complex instrumented spine surgery. Spine Journal 2019. 19 1648–1656. ( 10.1016/j.spinee.2019.05.592) [DOI] [PubMed] [Google Scholar]
- 36.Sigari RA & Abdolhoseinpour H. Operative site irrigation with povidone-iodine solution in spinal surgery for surgical site infection prevention: can it be used safety? Anaesthesia, Pain and Intensive Care 2020. 24 314–319. ( 10.35975/apic.v24i3.1282) [DOI] [Google Scholar]
- 37.Carballo Cuello CM Fernández-de Thomas RJ De Jesus O De Jesús Espinosa A & Pastrana EA. Prevention of surgical site infection in lumbar instrumented fusion using a sterile povidone-iodine solution. World Neurosurgery 2021. 151 151, ., e700–151.e. ( 10.1016/j.wneu.2021.04.094) [DOI] [PubMed] [Google Scholar]
- 38.Roberto RF Rowan FA Nallur D Durbin-Johnson B Javidan Y & Klineberg EO. Povidone-iodine irrigation combined with vancomycin powder lowers infection rates in pediatric deformity surgery. Spine Deformity 2021. 9 1315–1321. ( 10.1007/s43390-021-00333-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin HH Chou PH Ma HH Chang YW Wang ST & Chang MC. Efficacy of povidone iodine solution in the prevention of surgical site infections in minimally invasive instrumented spinal fusion surgery. Global Spine Journal 2022. 12 1058–1065. ( 10.1177/2192568220975385) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inojie MO Okwunodulu O Ndubuisi CA Campbell FC & Ohaegbulam SC. Prevention of surgical site infection following open spine surgery: the efficacy of intraoperative wound irrigation with normal saline containing gentamicin versus dilute povidone-iodine. World Neurosurgery 2023. 173 e1–e. ( 10.1016/j.wneu.2022.12.134) [DOI] [PubMed] [Google Scholar]
- 41.Global guidelines for the prevent ion of surgical site infection. Geneva: World Health O; rganization, 2018. (https://www.who.int/publications/i/item/9789241550475) [Google Scholar]
- 42.Mangram AJ Horan TC Pearson ML Silver LC & Jarvis WR. Guideline for prevention of surgical site infection, 1999. Centers for disease control and prevention (CDC) hospital infection control practices advisory committee. American Journal of Infection Control 1999. 27 9, 6–13. [PubMed] [Google Scholar]
- 43.Lee CS Kang KC Chung SS Kim KT & Shin SK. Incidence of microbiological contamination of local bone autograft used in posterior lumbar interbody fusion and its association with postoperative spinal infection. Journal of Neurosurgery. Spine 2016. 24 20–24. ( 10.3171/2015.3.SPINE14578) [DOI] [PubMed] [Google Scholar]
- 44.Massie JB Heller JG Abitbol JJ McPherson D & Garfin SR. Postoperative posterior spinal wound infections. Clinical Orthopaedics and Related Research 1992. (284) 99–108. ( 10.1097/00003086-199211000-00013) [DOI] [PubMed] [Google Scholar]
- 45.Durani P & Leaper D. Povidone-iodine: use in hand disinfection, skin preparation and antiseptic irrigation. International Wound Journal 2008. 5 376–387. ( 10.1111/j.1742-481X.2007.00405.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Meurs SJ Gawlitta D Heemstra KA Poolman RW Vogely HC & Kruyt MC. Selection of an optimal antiseptic solution for intraoperative irrigation: an in vitro study. Journal of Bone and Joint Surgery 2014. 96 285–291. ( 10.2106/JBJS.M.00313) [DOI] [PubMed] [Google Scholar]
- 47.Newton Ede MP Philp AM Philp A Richardson SM Mohammad S & Jones SW. Povidone-iodine has a profound effect on in vitro osteoblast proliferation and metabolic function and inhibits their ability to mineralize and form bone. Spine 2016. 41 729–734. ( 10.1097/BRS.0000000000001332) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed during the present study are available from the corresponding author on reasonable request.



 This work is licensed under a
This work is licensed under a