Abstract
Purpose of Review
Vascular imaging is a complex field including numerous modalities and imaging markers. This review is focused on important and recent findings in atherosclerotic carotid artery plaque imaging with an emphasis on developments in magnetic resonance imaging (MRI) and computed tomography (CT).
Recent Findings
Recent evidence shows that carotid plaque characteristics and not only established measures of carotid plaque burden and stenosis are associated independently with cardiovascular outcomes. On carotid MRI, the presence of a lipid-rich necrotic core (LRNC) has been associated with incident cardiovascular disease (CVD) events independent of wall thickness, a traditional measure of plaque burden. On carotid MRI, intra-plaque hemorrhage (IPH) presence has been identified as an independent predictor of stroke. The presence of a fissured carotid fibrous cap has been associated with contrast enhancement on CT angiography imaging.
Summary
Carotid artery plaque characteristics have been associated with incident CVD events and advanced plaque imaging techniques may gain additional prominence in the clinical treatment decision process.
Keywords: atherosclerosis, atherosclerotic carotid artery disease, high-risk plaques, computerized tomography, magnetic resonance imaging, plaque characteristics
Introduction
Cardiovascular disease (CVD) remains the number one cause of mortality in the United States. Approximately 809,000 people in the United States died from heart disease, stroke and other cardiovascular diseases in 2019 [1]. CVD is also a leading cause of mortality worldwide that affected 422.7 million people and caused 17.9 million deaths in 2015, accounting for 31% of all deaths [2, 3]. It is estimated that by 2030, approximately 23.6 million people will die from cardiovascular diseases annually [4].
Atherosclerosis, the main underlying cause of CVD, is a multifactorial inflammatory disorder characterized by the accumulation of plaque in multiple arterial beds, most commonly observed in the carotid, coronary, and femoral arteries [5] [6]. The underlying cause of stroke, most heart attacks and sudden cardiac deaths has been attributed to inflamed, active, and growing atherosclerotic plaques, often referred to as vulnerable plaques, and more recently referred to as high-risk plaques which are often discussed in the context of the vulnerable patient [7] [8]. Plaque characteristics, sometimes also referred to as plaque components, have been associated with the risk of plaque rupture, highlighting the importance of atherosclerotic plaque imaging [9, 10].
Various invasive and non-invasive vascular imaging approaches have been utilized to assess atherosclerotic plaques, where recent developments of the former in addition to traditional X-ray angiography have been reported with a primary focus on high-risk coronary atherosclerotic plaques for intravascular ultrasound (IVUS), optical coherence tomography (OCT), OCT-IVUS, intravascular near-infrared fluorescence (NIRF), and intravascular NIRF-OCT systems [11], all of which are described in detail in recent imaging reviews [12, 13]. On the other hand, non-invasive vascular imaging techniques include primarily magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), computed tomography (CT), CT angiography (CTA), ultrasonography (US), and positron emission tomography-CT (PET-CT) [14] [15–17]. Recent findings on vascular US and PET-CT have been recently highlighted by Hussain et al., Nishimiya et al., and Saba et al., and previously by us and others [18] [13] [12] [19] [20–22].
Imaging remains one of the major decision drivers clinically and frequently helps in determining the best path of treatment for patients with vessel diseases [19]. A growing body of evidence suggests that atherosclerotic plaque characteristics play an important and independent role as compared with percent of vessel stenosis, which remains of primary importance clinically and was established by criteria from the North American Symptomatic Carotid Endarterectomy Trial (NASCET) [23]. The large caliber and focused location of atherosclerotic plaque formation in the carotid arteries as compared with the coronary and femoral arteries, affords a high-resolution carotid wall imaging approach to study plaque characteristics, which due to various limitations including in-plane imaging resolution is substantially less feasible for typical atherosclerotic plaques in other arterial beds. A large number of technical and outcome studies have focused on the carotid arteries and advances in non-invasive imaging technologies are typically first applied to the carotids and therefore this review is focused on recent and noteworthy developments in carotid imaging.
Atherosclerotic Carotid Artery Disease
Atherosclerotic plaques are believed to form in the arterial wall due to an injury of the endothelium. The damaged endothelium permits plaque formation where monocyte recruitment and retention play a central role in this complex process [24, 25]. Subsequently, monocytes accumulate lipids in the subendothelial space of the intima and transform into macrophages and appear as fatty streaks. Over time, these processes also promote smooth muscle cell proliferation resulting in the formation of a fibrous capsule between the fatty streak and the intima, the innermost lining of the artery. These atheromatous plaques grow over time and induce enzymes that trigger remodeling of the artery, a compensatory expansion resulting in an eccentric cross section of the artery. Atheromas are composed of an extracellular lipid core, and fibrous connective tissue. The outer parts become increasingly calcified, resulting in hardening of the artery. Eventually atheromas may develop fissures, hematoma, and/or thrombi which are often accompanied by a thin fibrous cap that is prone to rupture [25, 26]. In the event of plaque rupture, clot-promoting material is released which inhibits blood flow and may cause an atherothrombotic event.
Incident CVD amplifies the need for novel non-invasive techniques to assess atherosclerotic plaques. Various reports including those by our group suggest that plaque characteristics may be a more specific predictor to identifying high-risk plaques and future plaque rupture than sole atherosclerotic plaque burden [14, 27–29].
The carotid plaque burden is typically located near the carotid artery bifurcation and predominately increases from the common carotid artery (CCA), the carotid bulb region, and peaks in the internal carotid artery (ICA) and extends to a lesser degree into the external carotid artery (ECA) [30]. Vascular imaging can be utilized to quantify measures of plaque burden and plaque characteristics, where the former typically includes arterial wall volume/area, lumen volume/area, and normalized wall index (NWI) [NWI= wall area/(lumen area + wall area)], and arterial wall thickness. Plaque characteristics typically include calcification, lipid-rich necrotic core (LRNC), intra-plaque hemorrhage (IPH), fibrous cap (FC), plaque ulceration, and inflammation (Table 1). High-risk atherosclerotic plaques have been associated with the presence of one or more plaque characteristics including a LRNC, IPH, fibrous cap thickness, and inflammation [7, 31]. Although plaque burden measures can be obtained with a high degree of accuracy and reproducibility with MRI, CT, and US, plaque characteristics are in part more imaging modality-specific.
Table 1.
Carotid artery plaque characteristics and measures of carotid plaque burden.
| Variable | Reference | |
|---|---|---|
| MRI | CT | |
| Carotid Artery Plaque Characteristics | ||
| Lipid rich necrotic core, (mL or mm2) | [14], [120], [61], [51] | [76], [78], [108] |
| Fibrous cap thickness, mm | [14], [61], [47], [51] | [111] |
| Intraplaque hemorrhage, (mL or mm2) | [56], [55], [50], [52] | [76], [106], [108] |
| Calcification, (mm2, calcium score [CT only]) | [57], [66] | [76], [78], [79], [108] |
| Soft plaque, HU [CT only] | N/A | [79], [75], [76], [108] |
| Measures of Carotid Artery Plaque Burden | ||
| Lumen, (mL or mm2) | [14], [56], [19] | [76], [19], [78], [79] |
| Wall, (mL, mm2, or mm for wall thickness) | [14], [56], [35], [57], [121] | [76], [19], [78], [79] |
| Maximum stenosis, % | [14], [56], [64], [122] | [76], [79], [123, 124] |
| Categorical Carotid Artery Plaque Characteristics | ||
| Lipid-rich necrotic core | [14], [121], [61], [66], [122] | [109], [125], [108] |
| Calcification | [14], [57], [121] | [19], [109, 110], [126] |
| Intraplaque hemorrhage | [14], [56], [57], [61], [66], [122] | [107, 108] |
| Ulceration | [62], [51], [63], [57] | [76], [111], [127] |
Selected references included are predominately from within the last 5 years or older if no relevant recent publications were available.
N/A: not applicable; HU: Hounsfield Units; MRI: magnetic resonance imaging; CT: computed tomography.
MR Imaging of Carotid Plaques
MRI is recognized as one of the preferred methods to image carotid artery pathologies, including atherosclerotic vascular lesions [32]. High-resolution multi-contrast MRI can detect and quantify with high accuracy atherosclerotic plaque burden and plaque characteristics including calcification, LRNC, IPH, and fibrous cap presence and thickness [33] [32] (Figure 1).
Figure 1. Carotid artery plaque images.
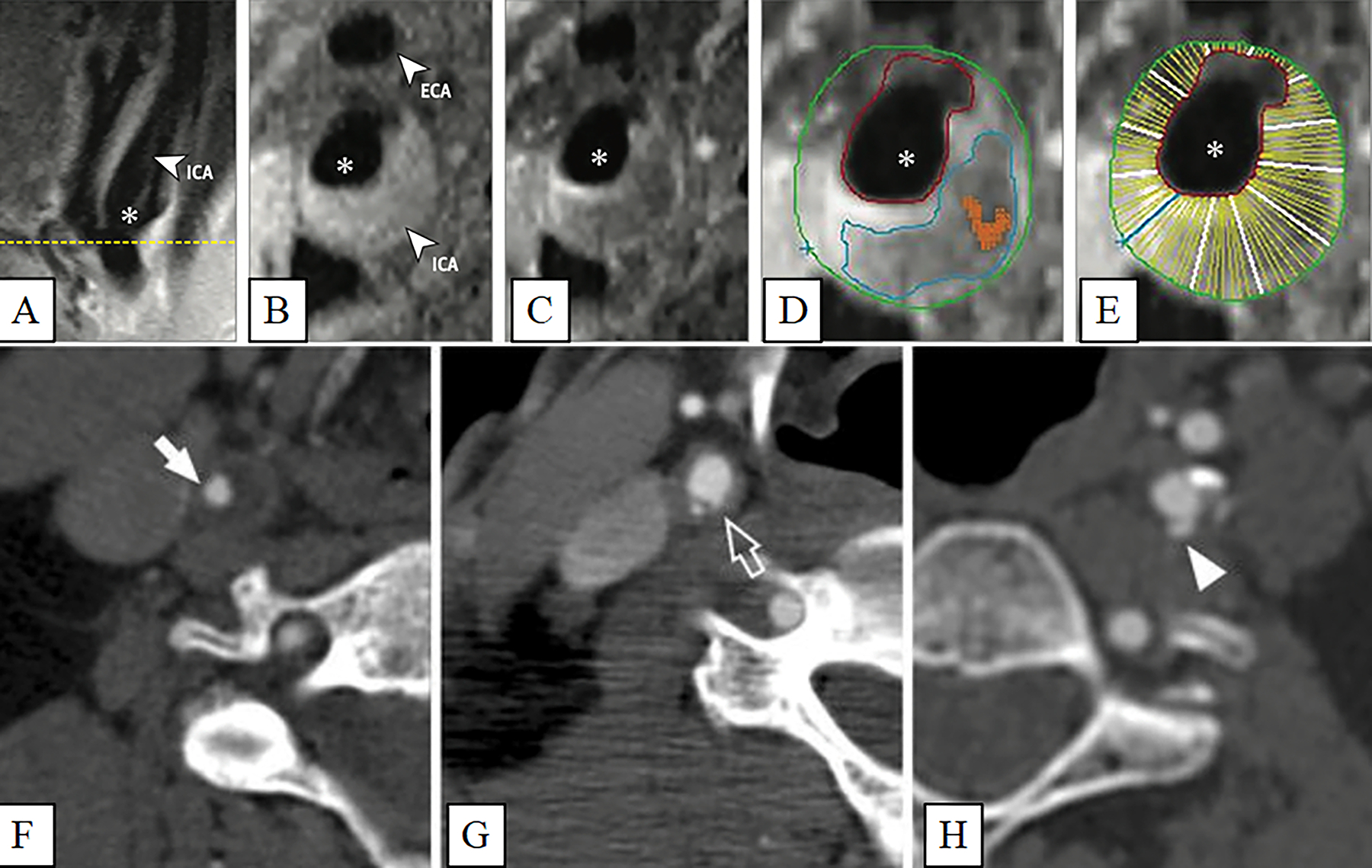
Carotid artery plaque MRI and CT images. Panels A-E: (A) Black blood MRI (BBMRI) images through the carotid artery with atherosclerotic plaque. A long axis BBMRI image through the carotid bifurcation is used for slice positioning. Short axis BBMRI images were then acquired before (B) and after (C) gadolinium contrast administration (asterisk, ICA lumen). Slices shown were acquired at the thickest part of the plaque (yellow line, A). Contours were drawn on the post-contrast image (D) to delineate the lipid core (blue), carotid lumen (red), outer wall (green), and calcification (orange). The wall of the ICA was automatically divided into 12 radial segments (E) to generate thickness measurements. Panels F-H: Various luminal morphology images of carotid artery plaques imaged with CT. (F) Smooth luminal surface (white arrows). (G) Irregular luminal surface (white open arrows). (H) Smooth plaque ulcers (white arrowheads). (Panels A-E: Adapted with permission from: JAMA Cardiol. 2021. 6(1):79–86. Copyright© (2021) American Medical Association. All rights reserved) [14••]. (Panels F-H: Adapted with permission from Springer Nature from: Cardiovasc Intervent Radiol 2014, 37(3):572–585. Copyright© 2014) [8].
Zavodni et al. used MRI to identify asymptomatic subjects at risk of cardiovascular events based on measures of carotid plaque component parameters including thickness, outer contour area, lumen area, wall area, remodeling index, presence of a lipid core, calcification, and ulceration [34]. A combination of various MRI pulse sequences can be utilized to distinguish plaque characteristics including LRNC, IPH, calcification, FC and thin or ruptured FC.
MRI based CCA wall thickness, a measure of plaque burden, has been studied in 698 participants free of CVD who were enrolled from the Multi-Ethnic Study of Atherosclerosis (MESA) and the authors compared their findings with ultrasonography based carotid intima-media thickness (CIMT) measurements from the same individuals [35]. MRI carotid wall thickness compared with US CIMT was reported as more consistently associated with incident CVD including stroke.
Black-blood MRI (BBMRI) sequences optimize contrast between the vessel wall and lumen which is of importance to determine measures of plaque burden and plaque characteristics. BBMRI has aided in treatment decisions in a recent study of 18 patients with symptomatic mild stenosis (<50%), as seen on angiography, and were selected from among 175 patients who underwent revascularization [36]. The authors concluded that if imaging findings point to the presence of vulnerable plaques in symptomatic mild carotid artery stenosis patients, surgical treatment may be considered.
T1-weighted (T1W), T2-weighted (T2W), proton density weighted (PDW), time of flight (TOF), and phase-contrast (PC) MRI are frequently used sequences for arterial and vessel wall imaging, and previous reports include details on pulse sequence parameters [37, 38] [39] [40] [19].
Carotid Artery Lumen Segmentation and Plaque Burden
TOF MRI sequences allow to visualize the carotid artery and can be used to determine carotid stenosis. Various methods have been developed to determine the carotid artery geometry, perform carotid lumen segmentation, measure carotid stenosis, and to co-register carotids for longitudinal analyses and across imaging modalities, whereas registration results can vary depending on which 3-D registration technique is deployed [41] [42].
Using CASCADE, a custom-designed image analysis software package (University of Washington, Seattle, Washington, USA), lumen and total vessel areas of the carotid artery were measured on axial image slices. Remodeling patterns were investigated and compared with maximum carotid wall thickness for each segment and adjusted for patient age, sex, and height. The CCA showed a positive remodeling pattern, while the bifurcation demonstrated negative remodeling, and a mixed pattern of outer wall expansion and lumen constriction was observed for the ICA [43].
A semi-automatic carotid lumen segmentation algorithm that builds on the traditional level set method was recently applied to TOF-MRA images from the CARE II study, where manual delineations were used as reference [44]. Compared with traditional level set approaches, the proposed method improved the TOF-MRA segmentation accuracy and computational efficiency [44]. There is a growing number of automated vessel segmentation methods which may over time gain in importance clinically [45] [37] [46].
High-Risk Plaques
In a recent feasibility study of 27 carotid plaques from 20 patients, a new method was reported to identify vulnerable carotid plaques utilizing a multi-modality-based approach using multi-contrast MRI and ultrasound shear wave elastography (SWE) [47]. MR imaging included 3D TOF-MRA, a 3D black-blood magnetization-prepared rapid acquisition gradient echo (MPRAGE) sequence to detect IPH, 2D pre-contrast black-blood T1W, 2D T2W, and contrast-enhanced 2D black-blood T1W (CE-T1W). Carotid MRI slices were analyzed with the well-validated PlaqueView software tool (MRI-PlaqueView; VP Diagnostics, Seattle, WA, USA), which determines carotid plaque components including the LRNC, fibrous cap, IPH, loose matrix, calcification, and measures of plaque burden. In addition, FC measures were obtained including mean, maximum, and minimum FC thickness, FC length, and FC volume. SWE measures comprised of group velocity and frequency-dependent phase velocities which were significantly correlated with MRI LRNC content and IPH volume, known markers of high-risk plaques [47].
Intraplaque Hemorrhage
IPH is an important determinant of progression and destabilization of atherosclerotic plaques and an independent predictor of cardiovascular events [48] [49]. Recent studies showed that IPH is associated with cerebrovascular event risk [50], stroke [51], transient ischemic attack, and amaurosis [50]. IPH can be characterized as a hyperintense signal on pre-contrast 3D TOF-MRA and black-blood T1W double inversion recovery spin echo sequences [52]. IPH as determined by MRI has been well studied and validated histologically showing an excellent agreement for acute or recent hemorrhage [53]. Schindler et al. recently reported an association between IPH and CVD events in asymptomatic patients who presented with carotid stenosis ≥50% [54] [55]. The analysis comprised of pooled data from 7 cohort studies with 560 patients with symptomatic carotid stenosis and 136 patients with asymptomatic carotid stenosis ≥50%. IPH presence was higher in symptomatic (51.6%) compared with asymptomatic patients (29.4%). Ipsilateral stroke rates were higher for those with IPH, and IPH presence was also an independent predictor in multivariate analysis [55].
In a recent carotid MRI investigation of 164 patients from the Plaque At RISK (PARISK) study, the association between IPH and vascular remodeling was reported [56]. Study participants had recent ischemic neurological events and ipsilateral carotid artery stenosis <70%. Carotid remodeling ratio and plaque burden measures including wall, lumen, total vessel area and carotid maximum wall thickness were obtained from the ICA slice with the largest wall area. Plaques containing IPH had a smaller lumen area than plaques without IPH after correcting for plaque size, and there was no difference in arterial remodeling [56].
In a recent study, 117 patients with cerebrovascular symptoms and carotid plaques as determined by vascular ultrasound underwent multi-contrast MRI to investigate associations between calcification and IPH in carotid plaques. Carotid plaques with a presence of IPH had a significantly greater prevalence of calcification than those without IPH [57].
Cui et al. investigated associations between MRI measures of carotid IPH of different ages (fresh, recent, old) with minor fibrous cap disruption in 37 patients [58]. The authors reported an independent association between fresh IPH volume and minor fibrous cap disruption, suggesting that IPH properties may be linked to FC stability.
Lipid-Rich Necrotic Core
A LRNC presents as isointense on PDW and 3D TOF-MRA scans, is hypointense on T2W images, and is isointense to slightly hyperintense on T1W scans [14]. Utilizing pre- and post-contrast-enhanced BBMRI scans significantly improves reader reproducibility of LRNC measurements, as shown by Takaya et al. [59]. The presence of a LRNC, a thin or ruptured FC, and IPH is associated with increased risk of future stroke or transient ischemic attack (TIA). Plaques with greater than 40% LRNC with a thin overlying FC are prone to rupture [51].
Carotid plaque characteristics including the presence of a LRNC were studied in 1256 participants of the Atherosclerosis Risk in Communities (ARIC) MRI carotid sub-study [14]. The study participants, who were free of CVD at baseline, were followed on average of 9.1 ± 2.98 years for incident CVD events and the cumulative event rate for this period was 13.7%. Carotid plaques of participants with incident CVD events as compared to those without had a higher percentage of individuals with a LRNC present, a significantly higher NWI, maximum carotid wall thickness, maximum carotid stenosis, and when present, a larger LRNC. The presence of a LRNC was independently associated with incident CVD events after adjusting for typical CVD risk factors and carotid plaque burden [14].
Fibrous Cap
A FC contains fibrous connective tissue, which will more strongly enhance between pre- and post-contrast-enhanced T1W MRI scans [60]. A fibrous cap contour can then be semi-automatically delineated between carotid lumen and the LRNC using multi-contrast MRI sequences [54]. Sun et al. studied associations between carotid plaque characteristics and CVD outcomes in 214 patients with clinical atherosclerotic disease who were recruited from the AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes) study [61]. The authors reported that a thin or ruptured fibrous cap and plaque lipid content, measured as the %-of LRNC of carotid plaques, were strongly associated with fatal and non-fatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome, and symptom-driven revascularization [61].
Ulceration
Carotid plaque ulcerations can be detected by the presence of plaque surface irregularities [62] [51]. Ulceration is visualized as an erosion or irregularity on the luminal surface of the carotid plaque and is considered a feature of high-risk plaques. MRI has been used for the study of ulcerated carotid plaques [63]. Xu et al. reported in a prospective MRI study of 120 asymptomatic patients with 50% to 79% carotid stenosis that a larger LRNC was associated with the development of new carotid ulceration or FC rupture and an increased plaque burden at 3 years after baseline imaging [64].
Calcification
Calcification is depicted as hypointense signal on all MRI contrast weightings. Based on a report from Pugliese et al. macrocalcification is a feature of stable plaques whereas microcalcification has been associated with plaque inflammation [65]. Pletsch-Borba et al. reported on carotid MRI characteristics of 198 participants with carotid wall thickening based on ultrasound who were recruited from the population-based Rotterdam Study [66]. Participants underwent longitudinal MR imaging at baseline and after 4 years and carotid plaque characteristics including presence of IPH, calcification, and LRNC were measured. The authors investigated associations between cardiovascular risk factors and incident carotid plaque characteristics and reported that all plaque components changed significantly over time. Specifically, it was reported that incident MRI plaque calcification was associated with hypertension and incident LRNC with higher cholesterol levels [66].
Limitations of MR Imaging
Overall, MRI has excellent tissue contrast and is highly reproducible. However, MR imaging is more expensive, less widely available compared with CT or ultrasonography imaging, and scan times are longer. Multi-contrast MRI can provide carotid artery plaque characteristics (calcification, LRNC, fibrous tissue, IPH, ulceration) [29]. Plaque calcification usually presents in the form of calcium hydroxyapatite (CHA) [67]. Due to its low spin density, CHA typically exhibits low intensity on multi-contrast MRI scans and hence can be difficult to quantify (hypointense on T1W, T2W, and PDW scans). Artifacts from implants, swallowing motion and dark-blood sequences, that are typically utilized to quantify carotid plaque characteristics, can further impair accuracy of quantification of calcification with MRI. Among other diseases, patients with advanced renal disease are contraindicated to gadolinium-based contrast agents. Ferumoxytol, an ultrasmall superparamagnetic iron oxide (USPIO) particle, is an alternative to standard gadolinium-based MRI contrast agents that is approved for clinical use in patients with renal failure [68]. Ferumoxytol also has macrophage-selective properties which could prove useful in evaluating inflammation, a hallmark of atherosclerosis. Patients with implants are often contraindicated to MRI, especially in high-field systems.
CT Imaging of Carotid Plaques
It is estimated that over 70 million CT scans are performed annually in the U.S. CT is a non-invasive and cost-effective imaging modality that can detect, quantify, and distinguish details of atherosclerotic lesions [69]. CT provides a high spatial resolution and imaging protocols are established and standardized resulting in short scan times effectively limiting motion artifacts and operator variability [70]. Tissue characteristics can be quantified with CT imaging through Hounsfield units (HU) that are obtained from linear transformation of the computed attenuation coefficients [71]. Multi-detector-row CT (MDCT) allows for multiplanar reconstruction across various planes with a high spatial and contrast resolution [72]. Whereas dual-source CT (DSCT) applies two different sources of x-rays with different energies to achieve Hounsfield units for the same tissue which results in improved tissue differentiation and postprocessing [73]. The advantages of DSCT over MDCT include the power to differentiate calcified plaque from iodinated contrast, which allows for a more accurate assessment [74]. In the context of carotid plaque morphology, HU density values have been suggested to categorize plaque structures as fatty (lipid-rich) <60 HU, mixed or fibrous 60–130 HU, and calcified >130 HU [75], or alternatively as calcified (>130 HU) and non-calcified or soft-plaque (≤130 HU) [75].
CT imaging has a high accuracy for detecting plaque calcification, ulceration and neovascularization. In terms of reliability, Chrencik and colleagues conducted a recent study showing that carotid plaque morphology and tissue characteristics including calcification, lipid-rich regions, and intraplaque hemorrhage can be measured reliably using CTA images [76] (Figure 1, Table 1).
CT and CTA imaging are reliable imaging techniques for longitudinal monitoring of carotid plaque progression and quantifying changes in plaque characteristics with high intra- and interobserver agreement [77] [78] [79].
The American Society of Neuroradiology delineates the application of CT along with other imaging modalities in their recent consensus recommendations regarding vessel wall imaging and how plaque imaging may be useful in the decision-making process for vascular care [19].
Calcification
CT imaging is the gold standard for quantifying arterial calcification. The presence of arterial calcification is a prominent feature in advanced atherosclerotic lesions and arterial calcification has been associated with inflammatory markers [80, 81] [82]. In particular, coronary calcification has been studied in detail. The Agatston score or coronary artery calcium score is the clinical standard method to quantify calcified plaque in the coronaries and its association with adverse CVD outcomes, and additive predictive-value when added to traditional cardiovascular risk factors is well-documented [83–85]. In a re-analysis of CT imaging scans from the epidemiologic MESA reported that categorical density information of coronary calcification (weighted 1–4 of peak HU: 100, 200, 300, >400) identifies patients with a higher from those with a lower number of cardiovascular events [86]. Conversely, several studies have reported that non-calcified soft, lipid-rich coronary plaques are rupture-prone and that extensive calcification is a marker of plaque stability [87–89]. However, spotty calcification and microcalcification may adversely impact stability in these plaques, highlighting the controversial role of plaque calcification [90]. Calcification in carotid plaques were studied in the Diabetes Heart Study which identified carotid artery calcification as a significant predictor of cardiovascular events [91]. Other studies suggested that carotid artery plaque calcification predicts atherosclerosis burden and subsequent vascular events [40, 92, 93]. Nandalur et al. found a significant association between raw carotid calcium scores and luminal stenosis in 49 patients of whom 43 presented with calcifications [94]. The quantification of carotid artery calcification on high-resolution micro-CT has been histologically validated, showing strong agreement with von Kossa stained sections [95].
Microcalcification
The early stage of calcification has been associated with mast cells and macrophages [96]. Electron microscopy of calcific lesions in aortae and mitral valves have identified crystalline hydroxyapatite among micro-spherical particles in tissues, suggesting that calcium- and phosphate-rich spherical particles are the first mineralized structures to appear in ectopic tissues [97, 98]. In carotid plaques, intra-plaque calcification has been found to be distributed heterogeneously and histologically determined calcification size in carotid endarterectomy (CEA) tissues extend across several orders of magnitude under bright-field microscopy [99]. It has been suggested that the action of lipoprotein-associated phospholipase A2 (Lp-PLA2) on oxidized low-density lipoprotein (oxLDL) produce lysophosphatidylcholine, an atherogenic agent which can, in turn, induce vascular smooth muscle cells (VSMCs) to differentiate into calcifying vascular cells in vitro [67, 100]. Levels of oxLDL and Lp-PLA2 activities have been shown to be high in the bifurcation segment of CEA tissues which may give rise to microcalcifications observed by our group previously [99].
Although previous analyses have associated calcified lesions with plaque stability [101, 102], recent studies indicate that calcification size, density, and location may be important determinants of plaque rupture risk [90, 103] [86]. However, the exact mechanism of calcification including spatial distribution and size (including microcalcifications) within atherosclerotic lesions remains incompletely understood and additional studies will need to be conducted in carotid plaques and in other vascular beds [104] [30].
Intraplaque Hemorrhage
There are conflicting reports in terms of identifying IPH on CT. Some findings suggest that CT density is higher in fatty plaques with IPH, while other results show no difference in HUs. U-King-Im et al. studied IPH in 167 consecutive patients with carotid MRI and CTA within 3-weeks [105]. The group reported a considerable overlap in the distribution of HUs for plaques with compared to those without IPH, suggesting a limited usefulness of plaque density in identifying carotid IPH [105]. Eisenmenger et al. reported on detecting carotid IPH in a retrospective cohort study of 96 patients who underwent carotid MRA and CTA within 1 month [106]. Carotid CTA-based measures of adventitial calcification and soft plaque could predict MRA-defined IPH using mixed-effects multivariable Poisson regression [106]. In addition, two more studies have shown that IPH can be identified using CT [107, 108]. However, more evidence and validation studies are need to determine the accuracy of CTA imaging to detect carotid IPH.
Lipid-Rich Plaques, Plaque Ulceration, and Fibrous Cap
Carotid fatty soft plaque and calcified plaque have been identified with CT imaging based on HU values [109, 110]. MDCT allows for an accurate identification of plaque ulcerations and plaque enhancement after contrast injection and the results are comparable to histopathology [111]. However, assessment of plaque ulceration using CT can be limited for small ulceration due to halo or edge blur artifacts. Saba et al. studied 47 consecutively enrolled patients with symptomatic carotid disease who underwent CTA imaging preprocedurally [111]. Histopathology comparisons were made using CEA tissues and preprocedure imaging which revealed that specimen with a fissured fibrous cap had a significantly larger plaque contrast-enhancement as compared to CEAs with non-fissured fibrous caps.
Adventitial neovascularization and inflammation have been studied by CT, and it has been suggested that the presence of carotid microvascular networks are related to symptomatic patients with higher degree of stenosis [112]. However, it has to be noted that 18F-fluorodeoxyglucose (18F-FDG) PET-CT is the gold-standard to assess carotid plaque inflammation, also referred to as plaque activity. A recent study of 130 individuals by Joshi et al. showed that arterial segments with subsequent incident calcification, including carotids, had the highest baseline 18F-FDG PET/CT uptake, a marker of plaque inflammation, suggesting that inflammation precedes plaque calcification [80].
Carotid CT Imaging and CVD Risk Assessment
A recent retrospective study by Mosleh et al. described the efficacy of CTA for carotid artery disease to assess the risk of adverse cardiovascular events [113]. The study suggested that the presence of carotid calcification and soft plaque can be both significant predictors of increased risk of acute myocardial infarction (AMI) and, or >50% coronary artery stenosis within a year. Similarly, a study by Magee et al. presented a calculative approach to determining stroke risk by combining clinical features and CT findings [114]. The results highlighted that a maximum carotid wall thickness of >4mm on CT, along with older age, and hypertension could help to identify patients at a higher risk of developing incident ischemic stroke.
A recent study from MESA [115] included 2673 participants with zero coronary artery calcium (CAC) who also underwent carotid plaque ultrasonography, and were followed prospectively for 16.1 years to ascertain associations between carotid plaque measures and CVD events. Carotid plaque presence and burden were independently associated with subsequent coronary heart disease risk and incident CAC among asymptomatic individuals without coronary artery calcification, highlighting the importance of carotid imaging to assess CVD risk.
Limitations of CT Imaging
Overall, CT is an advantageous imaging modality for visualizing atherosclerotic plaque morphology, and plaque characteristics. The main limitation of CT imaging is ionizing radiation [116]. A recent study including 12 million youth found an increase in overall cancer incidence after adjusting for age and sex among individuals who underwent diagnostic low-dose ionizing radiation compared to those who were not exposed [117]. These findings indicate that diagnostic techniques including but not limited to CT imaging should be utilized when there is a clear clinical need. CT radiocontrast agents are associated with various side effects. Andreucci et al. describes various adverse outcomes of the radiocontrast dye used in CT imaging which can range from thyroid dysfunction, nephropathy, and hypersensitivity reactions [118]. Other limitations include beam hardening artifact due to dense calcification, and mixed findings on detecting plaque characteristics due to an overlap of HU values of plaque components and low contrast for lipid-rich tissues (IPH, LRNC and fibrous tissues) [62, 119].
Conclusion
Vascular imaging remains of central importance in assessing and quantifying of cardiovascular disease. Non-invasive atherosclerotic plaque imaging and in particular carotid artery imaging have advanced substantially in recent years. Recent evidence shows that carotid plaque characteristics and not only established measures of carotid plaque burden and stenosis are independently associated with CVD outcomes. The field of carotid imaging is undergoing a major transition from carotid plaque burden imaging toward imaging of carotid plaque characteristics. A growing number of reports including several large studies have shown that the presence of at least one of the prominent carotid plaque characteristics including the presence of a lipid core, intraplaque hemorrhage, thin fibrous cap, or calcification is associated with incident CVD events. More studies are needed to clearly differentiate the impact of individual, and multiple plaque characteristics including their presence versus volume on CVD risk assessment and adverse outcomes.
Funding
This work was supported in part with funding from the National Institutes of Health (R01HL137763, K25HL121149, both to GB).
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent
This article is a review article in the field of vascular imaging. All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References
Papers of particular interest, published recently, have been highlighted as:
* Of importance
** Of major importance
- 1.Xu J, Murphy SL, Kochanek KD, Arias E: National Vital Statistics Reports. US DEPARTMENT OF HEALTH AND HUMAN SERVICES, Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System; 2021, 70(8):1–87. [Google Scholar]
- 2.Roth GA, Johnson C, Abajobir A, et al. : Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. Journal of the American College of Cardiology 2017, 70(1):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozaffarian D: Global Scourge of Cardiovascular Disease: Time for Health Care Systems Reform and Precision Population Health. Journal of the American College of Cardiology 2017, 70(1):26–28. [DOI] [PubMed] [Google Scholar]
- 4.WHO I: cardiovascular diseases (CVDs). In.: WHO; 2017. [Google Scholar]
- 5.Wolf D, Ley K: Immunity and Inflammation in Atherosclerosis. Circ Res 2019, 124(2):315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libby P, Buring JE, Badimon L, et al. : Atherosclerosis. Nat Rev Dis Primers 2019, 5(1):56. [DOI] [PubMed] [Google Scholar]
- 7.Naghavi M, Falk E, Hecht HS, et al. : From vulnerable plaque to vulnerable patient-part III: executive summary of the screening for heart attack prevention and education (SHAPE) task force report. Am J Cardiol 2006, 98(2A):2H–15H. [DOI] [PubMed] [Google Scholar]
- 8.Saba L, Anzidei M, Marincola BC, et al. : Imaging of the carotid artery vulnerable plaque. Cardiovasc Intervent Radiol 2014, 37(3):572–585. [DOI] [PubMed] [Google Scholar]
- 9.Hellings WE, Moll FL, De Vries JP, et al. : Atherosclerotic plaque composition and occurrence of restenosis after carotid endarterectomy. JAMA 2008, 299(5):547–554. [DOI] [PubMed] [Google Scholar]
- 10.Tomaniak M, Katagiri Y, Modolo R, et al. : Vulnerable plaques and patients: state-of-the-art. European Heart Journal 2020, 41(31):2997–3004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara T, Jaffer FA: Intravascular NIRF Molecular Imaging Approaches in Coronary Artery Disease. Curr Cardiovasc Imaging Rep 2016, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tarkin JM, Dweck MR, Evans NR, et al. : Imaging Atherosclerosis. Circ Res 2016, 118(4):750–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nishimiya K, Matsumoto Y, Shimokawa H: Recent Advances in Vascular Imaging. Arteriosclerosis, thrombosis, and vascular biology 2020, 40(12):e313–e321. [DOI] [PubMed] [Google Scholar]
- 14. Brunner G, Virani SS, Sun W, et al. : Associations Between Carotid Artery Plaque Burden, Plaque Characteristics, and Cardiovascular Events: The ARIC Carotid Magnetic Resonance Imaging Study. JAMA Cardiol 2021, 6(1):79–86. ** This paper showed that the presence of a carotid lipid core is associated with incident CVD events independent of carotid wall thickness, a measure of plaque burden.
- 15.Carr JJ, Nelson JC, Wong ND, et al. : Calcified coronary artery plaque measurement with cardiac CT in population-based studies: standardized protocol of multi-ethnic study of atherosclerosis (MESA) and coronary artery risk development in young adults (CARDIA) study. Radiology 2005, 234(1):35–43. [DOI] [PubMed] [Google Scholar]
- 16.Fayad ZA, Fuster V, Nikolaou K, Becker C: Computed tomography and magnetic resonance imaging for noninvasive coronary angiography and plaque imaging: current and potential future concepts. Circulation 2002, 106(15):2026–2034. [DOI] [PubMed] [Google Scholar]
- 17.DeMaria AN, Narula J, Mahmud E, Tsimikas S: Imaging vulnerable plaque by ultrasound. Journal of the American College of Cardiology 2006, 47(8 Suppl):C32–39. [DOI] [PubMed] [Google Scholar]
- 18.Hussain A, Brunner G, Nambi V: Ultrasound and MRI Assessment of Cardiovascular Risk. In: ASPC Manual of Preventive Cardiology. Edited by Wong ND, Amsterdam EA, Toth PP. Cham: Springer International Publishing; 2021: 391–415. [Google Scholar]
- 19.Saba L, Yuan C, Hatsukami TS, et al. : Carotid Artery Wall Imaging: Perspective and Guidelines from the ASNR Vessel Wall Imaging Study Group and Expert Consensus Recommendations of the American Society of Neuroradiology. AJNR American journal of neuroradiology 2018, 39(2):E9–E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Yang EY, Brunner G, et al. : Plaque volume of carotid endarterectomy (cea) specimens measured using a novel 3-dimensional ultrasound (3dus) technology. JACC: Cardiovascular Imaging 2016, 9(9):1118–1119. [DOI] [PubMed] [Google Scholar]
- 21.Brunner Gerd, Yang Eric, Morrisett Joel, Garami Zsolt, Nambi V: Image fusion technology. In: Multi-Modality Atherosclerosis Imaging and Diagnosis. Edited by Saba Luca, Sanches João, Pedro Luís Mendes, Suri Jasjit S.: Springer; 2013: 385–398. [Google Scholar]
- 22.Nambi V, Brunner G, Ballantyne CM: Ultrasound in cardiovascular risk prediction: don’t forget the plaque! J Am Heart Assoc 2013, 2(2):e000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke; a journal of cerebral circulation 1991, 22(6):711–720. [DOI] [PubMed] [Google Scholar]
- 24.Gerszten RE, Lim YC, Ding HT, et al. : Adhesion of monocytes to vascular cell adhesion molecule-1-transduced human endothelial cells: implications for atherogenesis. Circ Res 1998, 82(8):871–878. [DOI] [PubMed] [Google Scholar]
- 25.Andres V: Control of vascular cell proliferation and migration by cyclin-dependent kinase signalling: new perspectives and therapeutic potential. Cardiovasc Res 2004, 63(1):11–21. [DOI] [PubMed] [Google Scholar]
- 26.Stary HC, Chandler AB, Glagov S, et al. : A definition of initial, fatty streak, and intermediate lesions of atherosclerosis. A report from the committee on vascular lesions of the council on arteriosclerosis, american heart association. Circulation 1994, 89(5):2462–2478. [DOI] [PubMed] [Google Scholar]
- 27.Polsani V, Kerwin W, Xu D, et al. : Comparison of Right vs Left Carotid Artery Atherosclerotic Plaque Dimensions and Composition by High-Resolution Magnetic Resonance Imaging (MRI). Arteriosclerosis Thrombosis and Vascular Biology 2009, 29(7):E117–E117. [Google Scholar]
- 28.Morrisett J, Vick W, Sharma R, et al. : Discrimination of components in atherosclerotic plaques from human carotid endarterectomy specimens by magnetic resonance imaging ex vivo. Magn Reson Imaging 2003, 21(5):465–474. [DOI] [PubMed] [Google Scholar]
- 29.Karmonik C, Basto P, Vickers K, et al. : Quantitative segmentation of principal carotid atherosclerotic lesion components by feature space analysis based on multicontrast MRI at 1.5 T. IEEE Trans Biomed Eng 2009, 56(2):352–360. [DOI] [PubMed] [Google Scholar]
- 30.Ababneh B, Rejjal L, Pokharel Y, et al. : Distribution of calcification in carotid endarterectomy tissues: comparison of micro-computed tomography imaging with histology. Vasc Med 2014, 19(5):343–350. [DOI] [PubMed] [Google Scholar]
- 31.Aidi HE, Mani V, Weinshelbaum KB, et al. : MRI plaque burden of the cartoid arteries and aorta : Reproducibility, Age, Sex and Systemic Distribution. Nature Clinical Practice Cardiovascular Medicine 2009, 6(3):219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kassem M, Florea A, Mottaghy FM, van Oostenbrugge R, Kooi ME: Magnetic resonance imaging of carotid plaques: current status and clinical perspectives. Ann Transl Med 2020, 8(19):1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwee RM, van Oostenbrugge RJ, Hofstra L, et al. : Identifying vulnerable carotid plaques by noninvasive imaging. Neurology 2008, 70(24 Pt 2):2401–2409. [DOI] [PubMed] [Google Scholar]
- 34.Zavodni AE, Wasserman BA, McClelland RL, et al. : Carotid artery plaque morphology and composition in relation to incident cardiovascular events: the Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2014, 271(2):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Guallar E, Malhotra S, et al. : Carotid Artery Wall Thickness and Incident Cardiovascular Events: A Comparison between US and MRI in the Multi-Ethnic Study of Atherosclerosis (MESA). Radiology 2018, 289(3):649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takai H, Uemura J, Yagita Y, et al. : Plaque Characteristics of Patients with Symptomatic Mild Carotid Artery Stenosis. J Stroke Cerebrovasc Dis 2018, 27(7):1930–1936. [DOI] [PubMed] [Google Scholar]
- 37.Arias Lorza AM, van Engelen A, Petersen J, van der Lugt A, de Bruijne M: Maximization of regional probabilities using Optimal Surface Graphs: Application to carotid artery segmentation in MRI. Medical physics 2018, 45(3):1159–1169. [DOI] [PubMed] [Google Scholar]
- 38.van den Bouwhuijsen QJ, Vernooij MW, Hofman A, Krestin GP, van der Lugt A, Witteman JC: Determinants of magnetic resonance imaging detected carotid plaque components: the Rotterdam Study. Eur Heart J 2012, 33(2):221–229. [DOI] [PubMed] [Google Scholar]
- 39.Hagiwara Y, Takao N, Takada T, et al. : Contrast-enhanced carotid ultrasonography and MRI plaque imaging to identify patients developing in-stent intimal hyperplasia after carotid artery stenting. Med Ultrason 2019, 21(2):170–174. [DOI] [PubMed] [Google Scholar]
- 40.Adams GJ, Simoni DM, Bordelon CB Jr., et al. : Bilateral symmetry of human carotid artery atherosclerosis. Stroke; a journal of cerebral circulation 2002, 33(11):2575–2580. [DOI] [PubMed] [Google Scholar]
- 41.Bizopoulos PA, Sakellarios A, Michalis LK, Koutsouris DD, Fotiadis DI: 3-D registration on carotid artery imaging data: MRI for different timesteps. Annu Int Conf IEEE Eng Med Biol Soc 2016, 2016:1159–1162. [DOI] [PubMed] [Google Scholar]
- 42.Zhang R, Zhang Q, Ji A, et al. : Identification of high-risk carotid plaque with MRI-based radiomics and machine learning. Eur Radiol 2021, 31(5):3116–3126. [DOI] [PubMed] [Google Scholar]
- 43.Watase H, Sun J, Hippe DS, et al. : Carotid Artery Remodeling Is Segment Specific: An In Vivo Study by Vessel Wall Magnetic Resonance Imaging. Arteriosclerosis, thrombosis, and vascular biology 2018, 38(4):927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo L, Liu S, Tong X, et al. : Carotid artery segmentation using level set method with double adaptive threshold (DATLS) on TOF-MRA images. Magn Reson Imaging 2019, 63:123–130. [DOI] [PubMed] [Google Scholar]
- 45.Wu J, Xin J, Yang X, et al. : Deep morphology aided diagnosis network for segmentation of carotid artery vessel wall and diagnosis of carotid atherosclerosis on black-blood vessel wall MRI. Medical physics 2019, 46(12):5544–5561. [DOI] [PubMed] [Google Scholar]
- 46.Brunner G, Chittajallu DR, Kurkure U, Kakadiaris IA: Toward the automatic detection of coronary artery calcification in non-contrast Computed Tomography data. Int J Cardiovasc Imaging 2010, 26(7):829–838. [DOI] [PubMed] [Google Scholar]
- 47.Marlevi D, Mulvagh SL, Huang R, et al. : Combined spatiotemporal and frequency-dependent shear wave elastography enables detection of vulnerable carotid plaques as validated by MRI. Sci Rep 2020, 10(1):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Derksen WJ, Peeters W, van Lammeren GW, et al. : Different stages of intraplaque hemorrhage are associated with different plaque phenotypes: a large histopathological study in 794 carotid and 276 femoral endarterectomy specimens. Atherosclerosis 2011, 218(2):369–377. [DOI] [PubMed] [Google Scholar]
- 49.Larson AS, Benson JC, Brinjikji W, et al. : Variations in the Presence of Carotid Intraplaque Hemorrhage Across Age Categories: What Age Groups Are Most Likely to Benefit From Plaque Imaging? Front Neurol 2020, 11:603055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tapis P, El-Koussy M, Hewer E, Mono ML, Reinert M: Plaque vulnerability in patients with high- and moderate-grade carotid stenosis - comparison of plaque features on MRI with histopathological findings. Swiss Med Wkly 2020, 150:w20174. [DOI] [PubMed] [Google Scholar]
- 51.Porambo ME, DeMarco JK: MR imaging of vulnerable carotid plaque. Cardiovasc Diagn Ther 2020, 10(4):1019–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mura M, Della Schiava N, Long A, Chirico EN, Pialoux V, Millon A: Carotid intraplaque haemorrhage: pathogenesis, histological classification, imaging methods and clinical value. Ann Transl Med 2020, 8(19):1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Albuquerque LC, Narvaes LB, Maciel AA, et al. : Intraplaque hemorrhage assessed by high-resolution magnetic resonance imaging and C-reactive protein in carotid atherosclerosis. Journal of vascular surgery 2007, 46(6):1130–1137. [DOI] [PubMed] [Google Scholar]
- 54.Wasserman BA, Astor BC, Sharrett AR, Swingen C, Catellier D: MRI measurements of carotid plaque in the atherosclerosis risk in communities (ARIC) study: methods, reliability and descriptive statistics. Journal of magnetic resonance imaging : JMRI 2010, 31(2):406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schindler A, Schinner R, Altaf N, et al. : Prediction of Stroke Risk by Detection of Hemorrhage in Carotid Plaques: Meta-Analysis of Individual Patient Data. JACC Cardiovasc Imaging 2020, 13(2 Pt 1):395–406. [DOI] [PubMed] [Google Scholar]
- 56.Dilba K, van Dijk AC, Crombag G, et al. : Association between Intraplaque Hemorrhage and Vascular Remodeling in Carotid Arteries: The Plaque at RISK (PARISK) Study. Cerebrovasc Dis 2021, 50(1):94–99. [DOI] [PubMed] [Google Scholar]
- 57.Lin R, Chen S, Liu G, Xue Y, Zhao X: Association Between Carotid Atherosclerotic Plaque Calcification and Intraplaque Hemorrhage: A Magnetic Resonance Imaging Study. Arteriosclerosis, thrombosis, and vascular biology 2017, 37(6):1228–1233. [DOI] [PubMed] [Google Scholar]
- 58.Cui Y, Qiao H, Ma L, et al. : Association of Age and Size of Carotid Artery Intraplaque Hemorrhage and Minor Fibrous Cap Disruption: A High Resolution Magnetic Resonance Imaging Study. J Atheroscler Thromb 2018, 25(12):1222–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takaya N, Cai J, Ferguson MS, et al. : Intra- and interreader reproducibility of magnetic resonance imaging for quantifying the lipid-rich necrotic core is improved with gadolinium contrast enhancement. Journal of magnetic resonance imaging : JMRI 2006, 24(1):203–210. [DOI] [PubMed] [Google Scholar]
- 60.Cai J, Hatsukami TS, Ferguson MS, et al. : In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation 2005, 112(22):3437–3444. [DOI] [PubMed] [Google Scholar]
- 61.Sun J, Zhao XQ, Balu N, et al. : Carotid Plaque Lipid Content and Fibrous Cap Status Predict Systemic CV Outcomes: The MRI Substudy in AIM-HIGH. JACC Cardiovasc Imaging 2017, 10(3):241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brinjikji W, Huston J, 3rd, Rabinstein AA, Kim GM, Lerman A, Lanzino G: Contemporary carotid imaging: from degree of stenosis to plaque vulnerability. J Neurosurg 2016, 124(1):27–42. [DOI] [PubMed] [Google Scholar]
- 63.Rafailidis V, Chryssogonidis I, Tegos T, Kouskouras K, Charitanti-Kouridou A: Imaging of the ulcerated carotid atherosclerotic plaque: a review of the literature. Insights Imaging 2017, 8(2):213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu D, Hippe DS, Underhill HR, et al. : Prediction of high-risk plaque development and plaque progression with the carotid atherosclerosis score. JACC Cardiovasc Imaging 2014, 7(4):366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pugliese G, Iacobini C, Blasetti Fantauzzi C, Menini S: The dark and bright side of atherosclerotic calcification. Atherosclerosis 2015, 238(2):220–230. [DOI] [PubMed] [Google Scholar]
- 66. Pletsch-Borba L, Selwaness M, van der Lugt A, Hofman A, Franco OH, Vernooij MW: Change in Carotid Plaque Components: A 4-Year Follow-Up Study With Serial MR Imaging. JACC Cardiovasc Imaging 2018, 11(2 Pt 1):184–192. * This paper indicated that carotid plaque characteristics can change significantly over a period of 4 years, and the development of plaque components was mainly associated with hypertension and serum cholesterol levels.
- 67.Vickers KC, Castro-Chavez F, Morrisett JD: Lyso-phosphatidylcholine induces osteogenic gene expression and phenotype in vascular smooth muscle cells. Atherosclerosis 2010, 211(1):122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hope MD, Hope TA, Zhu C, et al. : Vascular Imaging With Ferumoxytol as a Contrast Agent. AJR American journal of roentgenology 2015, 205(3):W366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brenner DJ: Slowing the increase in the population dose resulting from CT scans. Radiat Res 2010, 174(6):809–815. [DOI] [PubMed] [Google Scholar]
- 70.Silvennoinen HM, Ikonen S, Soinne L, Railo M, Valanne L: CT angiographic analysis of carotid artery stenosis: comparison of manual assessment, semiautomatic vessel analysis, and digital subtraction angiography. AJNR American journal of neuroradiology 2007, 28(1):97–103. [PMC free article] [PubMed] [Google Scholar]
- 71.Hounsfield GN: Computed medical imaging. Nobel lecture, Decemberr 8, 1979. J Comput Assist Tomogr 1980, 4(5):665–674. [DOI] [PubMed] [Google Scholar]
- 72.Saba L, Mallarini G: Carotid plaque enhancement and symptom correlations: an evaluation by using multidetector row CT angiography. AJNR American journal of neuroradiology 2011, 32(10):1919–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leschka S, Scheffel H, Desbiolles L, et al. : Image quality and reconstruction intervals of dual-source CT coronary angiography: recommendations for ECG-pulsing windowing. Invest Radiol 2007, 42(8):543–549. [DOI] [PubMed] [Google Scholar]
- 74.Das M, Braunschweig T, Muhlenbruch G, et al. : Carotid plaque analysis: comparison of dual-source computed tomography (CT) findings and histopathological correlation. Eur J Vasc Endovasc Surg 2009, 38(1):14–19. [DOI] [PubMed] [Google Scholar]
- 75.de Weert TT, Ouhlous M, Meijering E, et al. : In vivo characterization and quantification of atherosclerotic carotid plaque components with multidetector computed tomography and histopathological correlation. Arteriosclerosis, thrombosis, and vascular biology 2006, 26(10):2366–2372. [DOI] [PubMed] [Google Scholar]
- 76.Chrencik MT, Khan AA, Luther L, et al. : Quantitative assessment of carotid plaque morphology (geometry and tissue composition) using computed tomography angiography. Journal of vascular surgery 2019, 70(3):858–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Anzidei M, Suri JS, Saba L, et al. : Longitudinal assessment of carotid atherosclerosis after Radiation Therapy using Computed Tomography: A case control Study. Eur Radiol 2016, 26(1):72–78. [DOI] [PubMed] [Google Scholar]
- 78.van Gils MJ, Vukadinovic D, van Dijk AC, Dippel DW, Niessen WJ, van der Lugt A: Carotid atherosclerotic plaque progression and change in plaque composition over time: a 5-year follow-up study using serial CT angiography. AJNR American journal of neuroradiology 2012, 33(7):1267–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pelz DM, Lownie SP, Lee DH, Boulton MR: Plaque morphology (the PLAC Scale) on CT angiography: predicting long-term anatomical success of primary carotid stenting. J Neurosurg 2015, 123(4):856–861. [DOI] [PubMed] [Google Scholar]
- 80.Joshi FR, Rajani NK, Abt M, et al. : Does Vascular Calcification Accelerate Inflammation?: A Substudy of the dal-PLAQUE Trial. Journal of the American College of Cardiology 2016, 67(1):69–78. [DOI] [PubMed] [Google Scholar]
- 81.Shanahan CM: Inflammation ushers in calcification: a cycle of damage and protection? Circulation 2007, 116(24):2782–2785. [DOI] [PubMed] [Google Scholar]
- 82.Wolf RL, Wehrli SL, Popescu AM, et al. : Mineral volume and morphology in carotid plaque specimens using high-resolution MRI and CT. Arteriosclerosis, thrombosis, and vascular biology 2005, 25(8):1729–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr., Detrano R: Quantification of coronary artery calcium using ultrafast computed tomography. Journal of the American College of Cardiology 1990, 15(4):827–832. [DOI] [PubMed] [Google Scholar]
- 84.Shaw LJ, Raggi P, Schisterman E, Berman DS, Callister TQ: Prognostic value of cardiac risk factors and coronary artery calcium screening for all-cause mortality. Radiology 2003, 228(3):826–833. [DOI] [PubMed] [Google Scholar]
- 85.Budoff MJ, Nasir K, McClelland RL, et al. : Coronary calcium predicts events better with absolute calcium scores than age-sex-race/ethnicity percentiles: MESA (Multi-Ethnic Study of Atherosclerosis). Journal of the American College of Cardiology 2009, 53(4):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Criqui MH, Denenberg JO, Ix JH, et al. : Calcium density of coronary artery plaque and risk of incident cardiovascular events. Jama 2014, 311(3):271–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Farb A, Burke AP, Tang AL, et al. : Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation 1996, 93(7):1354–1363. [DOI] [PubMed] [Google Scholar]
- 88.Virmani R, Burke AP, Kolodgie FD, Farb A: Vulnerable plaque: the pathology of unstable coronary lesions. JIntervCardiol 2002, 15(6):439–446. [DOI] [PubMed] [Google Scholar]
- 89.Falk E, Shah PK, Fuster V: Coronary plaque disruption. Circulation 1995, 92(3):657–671. [DOI] [PubMed] [Google Scholar]
- 90.Vengrenyuk Y, Carlier S, Xanthos S, et al. : A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proceedings of the National Academy of Sciences of the United States of America 2006, 103(40):14678–14683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cox AJ, Hsu FC, Agarwal S, et al. : Prediction of mortality using a multi-bed vascular calcification score in the Diabetes Heart Study. Cardiovascular diabetology 2014, 13:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McKinney AM, Casey SO, Teksam M, et al. : Carotid bifurcation calcium and correlation with percent stenosis of the internal carotid artery on CT angiography. Neuroradiology 2005, 47(1):1–9. [DOI] [PubMed] [Google Scholar]
- 93.Sarikaya B, Lohman B, McKinney AM, Gadani S, Irfan M, Lucato L: Correlation between carotid bifurcation calcium burden on non-enhanced CT and percentage stenosis, as confirmed by digital subtraction angiography. The British journal of radiology 2012, 85(1015):e284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nandalur KR, Baskurt E, Hagspiel KD, et al. : Carotid artery calcification on CT may independently predict stroke risk. AJR American journal of roentgenology 2006, 186(2):547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ababneh B, Rejjal L, Pokharel Y, et al. : Distribution of Calcification in Carotid Endarterectomy Tissues: Comparison of micro-Computed Tomography Imaging to Histology. Vascular Medicine 2014, 19(5):343–350. [DOI] [PubMed] [Google Scholar]
- 96.Jeziorska M, McCollum C, Woolley DE: Calcification in atherosclerotic plaque of human carotid arteries: associations with mast cells and macrophages. The Journal of pathology 1998, 185(1):10–17. [DOI] [PubMed] [Google Scholar]
- 97.Bertazzo S, Gentleman E, Cloyd KL, Chester AH, Yacoub MH, Stevens MM: Nano-analytical electron microscopy reveals fundamental insights into human cardiovascular tissue calcification. Nature materials 2013, 12(6):576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miller JD: Cardiovascular calcification: Orbicular origins. Nature materials 2013, 12(6):476–478. [DOI] [PubMed] [Google Scholar]
- 99.Han RI, Wheeler TM, Lumsden AB, et al. : Morphometric analysis of calcification and fibrous layer thickness in carotid endarterectomy tissues. Computers in Biology and Medicine 2016, 70:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Castro-Chavez F, Vickers KC, Lee JS, Tung CH, Morrisett JD: Effect of lyso-phosphatidylcholine and Schnurri-3 on osteogenic transdifferentiation of vascular smooth muscle cells to calcifying vascular cells in 3D culture. Biochimica et biophysica acta 2013, 1830(6):3828–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang H, Virmani R, Younis H, Burke AP, Kamm RD, Lee RT: The impact of calcification on the biomechanical stability of atherosclerotic plaques. Circulation 2001, 103(8):1051–1056. [DOI] [PubMed] [Google Scholar]
- 102.Shaalan WE, Cheng H, Gewertz B, et al. : Degree of carotid plaque calcification in relation to symptomatic outcome and plaque inflammation. Journal of vascular surgery 2004, 40(2):262–269. [DOI] [PubMed] [Google Scholar]
- 103.Kelly-Arnold A, Maldonado N, Laudier D, Aikawa E, Cardoso L, Weinbaum S: Revised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proceedings of the National Academy of Sciences of the United States of America 2013, 110(26):10741–10746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mauriello A, Servadei F, Sangiorgi G, et al. : Asymptomatic carotid plaque rupture with unexpected thrombosis over a non-canonical vulnerable lesion. Atherosclerosis 2011, 218(2):356–362. [DOI] [PubMed] [Google Scholar]
- 105.JM UK-I, Fox AJ, Aviv RI, et al. : Characterization of carotid plaque hemorrhage: a CT angiography and MR intraplaque hemorrhage study. Stroke; a journal of cerebral circulation 2010, 41(8):1623–1629. [DOI] [PubMed] [Google Scholar]
- 106.Eisenmenger LB, Aldred BW, Kim SE, et al. : Prediction of Carotid Intraplaque Hemorrhage Using Adventitial Calcification and Plaque Thickness on CTA. AJNR American journal of neuroradiology 2016, 37(8):1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ajduk M, Bulimbasic S, Pavic L, et al. : Comparison of multidetector-row computed tomography and duplex Doppler ultrasonography in detecting atherosclerotic carotid plaques complicated with intraplaque hemorrhage. Coll Antropol 2013, 37(1):213–219. [PubMed] [Google Scholar]
- 108.Saba L, Francone M, Bassareo PP, et al. : CT Attenuation Analysis of Carotid Intraplaque Hemorrhage. AJNR American journal of neuroradiology 2018, 39(1):131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wintermark M, Jawadi SS, Rapp JH, et al. : High-resolution CT imaging of carotid artery atherosclerotic plaques. AJNR American journal of neuroradiology 2008, 29(5):875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Saba L, Mallarin G: Window settings for the study of calcified carotid plaques with multidetector CT angiography. AJNR American journal of neuroradiology 2009, 30(7):1445–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Saba L, Tamponi E, Raz E, et al. : Correlation between fissured fibrous cap and contrast enhancement: preliminary results with the use of CTA and histologic validation. AJNR American journal of neuroradiology 2014, 35(4):754–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Romero JM, Babiarz LS, Forero NP, et al. : Arterial wall enhancement overlying carotid plaque on CT angiography correlates with symptoms in patients with high grade stenosis. Stroke; a journal of cerebral circulation 2009, 40(5):1894–1896. [DOI] [PubMed] [Google Scholar]
- 113.Mosleh W, Adib K, Natdanai P, et al. : High-risk carotid plaques identified by CT-angiogram can predict acute myocardial infarction. Int J Cardiovasc Imaging 2017, 33(4):561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Magge R, Lau BC, Soares BP, et al. : Clinical risk factors and CT imaging features of carotid atherosclerotic plaques as predictors of new incident carotid ischemic stroke: a retrospective cohort study. AJNR American journal of neuroradiology 2013, 34(2):402–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mehta A, Rigdon J, Tattersall MC, et al. : Association of Carotid Artery Plaque With Cardiovascular Events and Incident Coronary Artery Calcium in Individuals With Absent Coronary Calcification: The MESA. Circ Cardiovasc Imaging 2021, 14(4):e011701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Brenner DJ, Hall EJ: Computed tomography--an increasing source of radiation exposure. The New England journal of medicine 2007, 357(22):2277–2284. [DOI] [PubMed] [Google Scholar]
- 117.Hong J-Y, Han K, Jung J-H, Kim JS: Association of Exposure to Diagnostic Low-Dose Ionizing Radiation With Risk of Cancer Among Youths in South Korea. JAMA Network Open 2019, 2(9):e1910584–e1910584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Andreucci M, Solomon R, Tasanarong A: Side effects of radiographic contrast media: pathogenesis, risk factors, and prevention. Biomed Res Int 2014, 2014:741018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nickoloff EL, Alderson PO: Radiation exposures to patients from CT: reality, public perception, and policy. AJR American journal of roentgenology 2001, 177(2):285–287. [DOI] [PubMed] [Google Scholar]
- 120.Alkhalil M, Biasiolli L, Akbar N, et al. : T2 mapping MRI technique quantifies carotid plaque lipid, and its depletion after statin initiation, following acute myocardial infarction. Atherosclerosis 2018, 279:100–106. [DOI] [PubMed] [Google Scholar]
- 121.Li Y, Zhu G, Ding V, et al. : Assessing the Relationship between Atherosclerotic Cardiovascular Disease Risk Score and Carotid Artery Imaging Findings. J Neuroimaging 2019, 29(1):119–125. [DOI] [PubMed] [Google Scholar]
- 122.Selwaness M, Bos D, van den Bouwhuijsen Q, et al. : Carotid Atherosclerotic Plaque Characteristics on Magnetic Resonance Imaging Relate With History of Stroke and Coronary Heart Disease. Stroke; a journal of cerebral circulation 2016, 47(6):1542–1547. [DOI] [PubMed] [Google Scholar]
- 123.Bartlett ES, Walters TD, Symons SP, Fox AJ: Quantification of carotid stenosis on CT angiography. AJNR American journal of neuroradiology 2006, 27(1):13–19. [PMC free article] [PubMed] [Google Scholar]
- 124.Saba L, Mallarini G: MDCTA of carotid plaque degree of stenosis: evaluation of interobserver agreement. AJR American journal of roentgenology 2008, 190(1):W41–46. [DOI] [PubMed] [Google Scholar]
- 125.Walker LJ, Ismail A, McMeekin W, Lambert D, Mendelow AD, Birchall D: Computed tomography angiography for the evaluation of carotid atherosclerotic plaque: correlation with histopathology of endarterectomy specimens. Stroke; a journal of cerebral circulation 2002, 33(4):977–981. [DOI] [PubMed] [Google Scholar]
- 126.Watanabe Y, Nakazawa T, Higashi M, Itoh T, Naito H: Assessment of calcified carotid plaque volume: comparison of contrast-enhanced dual-energy CT angiography and native single-energy CT. AJR American journal of roentgenology 2011, 196(6):W796–799. [DOI] [PubMed] [Google Scholar]
- 127.Saba L, Caddeo G, Sanfilippo R, Montisci R, Mallarini G: Efficacy and sensitivity of axial scans and different reconstruction methods in the study of the ulcerated carotid plaque using multidetector-row CT angiography: comparison with surgical results. AJNR American journal of neuroradiology 2007, 28(4):716–723. [PMC free article] [PubMed] [Google Scholar]


