Abstract
Nine hybridomas secreting monoclonal antibody to proteins of bovine milk-fat-globule membrane were isolated. All nine cell lines continued to secrete monoclonal antibody after serial transfer in culture and after passage as solid tumours in Balb/cJ mice. Four of the cell lines secreted monoclonal antibody specific for xanthine oxidase, one of the major proteins of milk-fat-globule membrane.
Full text
PDF
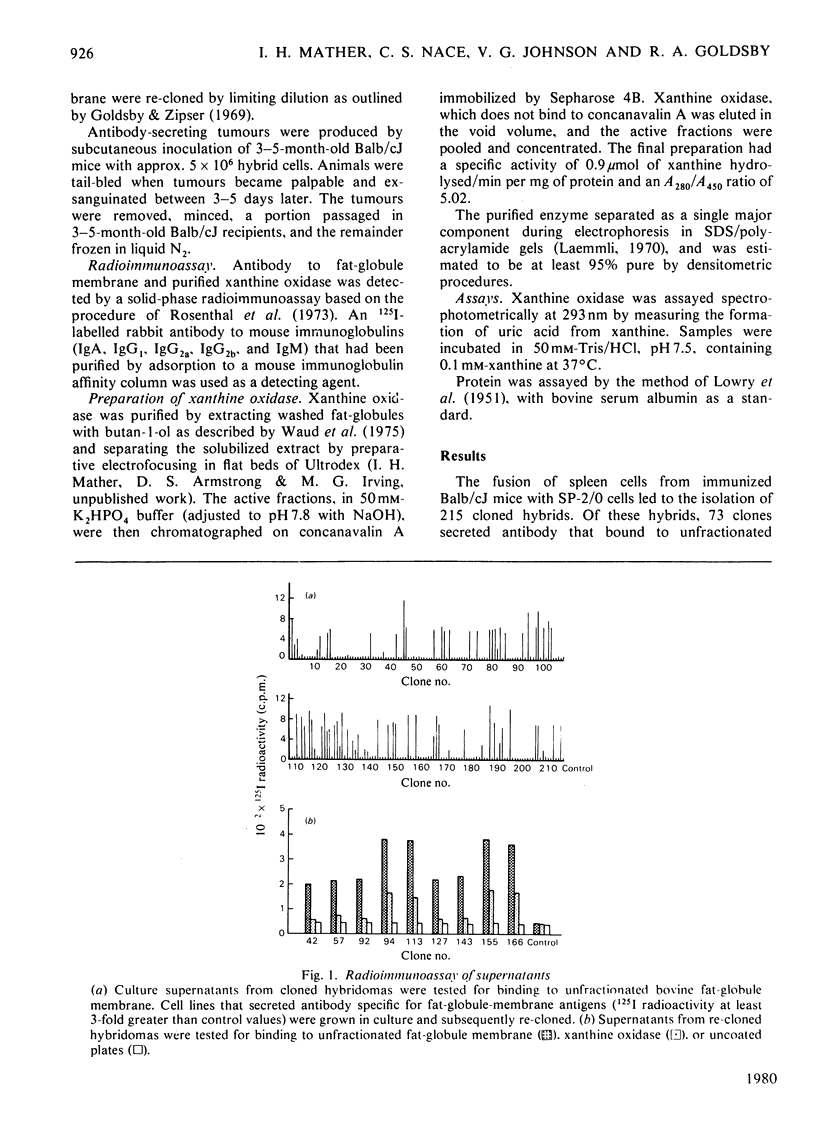
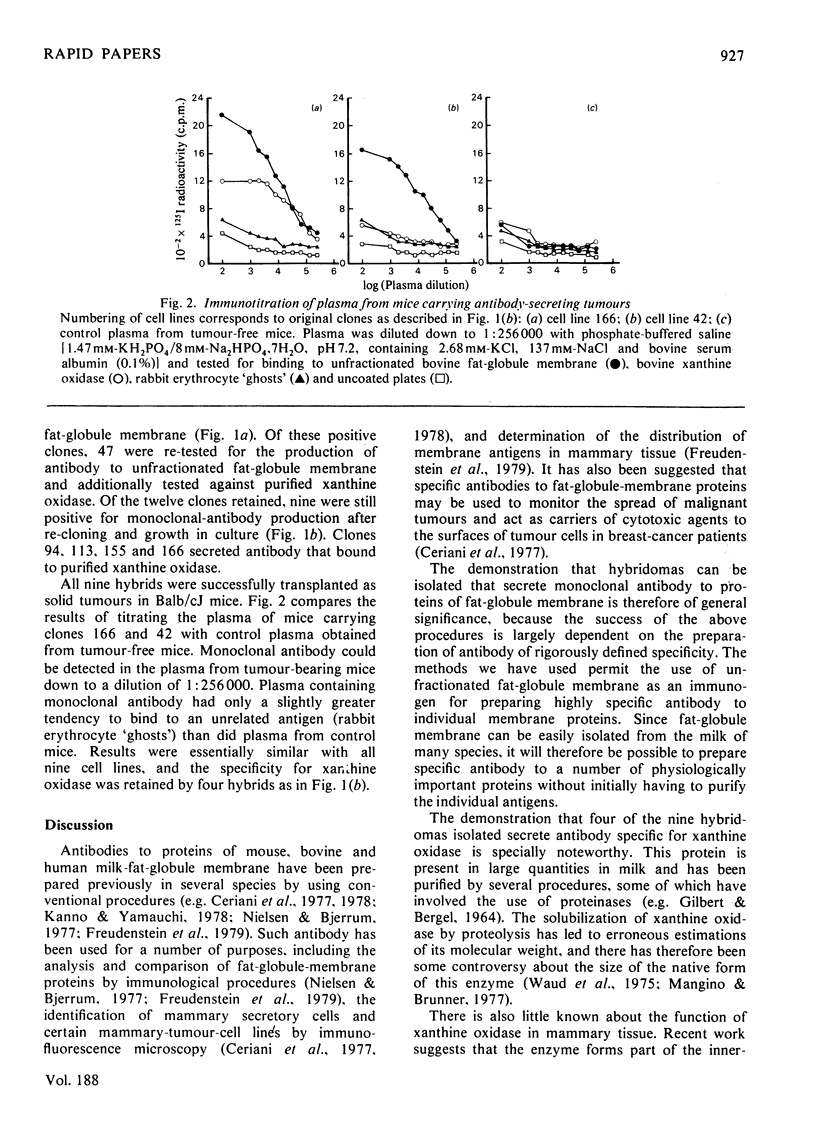

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ceriani R. L., Peterson J. A., Abraham S. Immunologic methods for the identification of cell types. II. Expression of normal mouse mammary epithelial cell antigens in mammary neoplasia. J Natl Cancer Inst. 1978 Sep;61(3):747–751. [PubMed] [Google Scholar]
- Ceriani R. L., Thompson K., Peterson J. A., Abraham S. Surface differentiation antigens of human mammary epithelial cells carried on the human milk fat globule. Proc Natl Acad Sci U S A. 1977 Feb;74(2):582–586. doi: 10.1073/pnas.74.2.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenstein C., Keenan T. W., Eigel W. N., Sasaki M., Stadler J., Franke W. W. Preparation and characterization of the inner coat material associated with fat globule membranes from bovine and human milk. Exp Cell Res. 1979 Feb;118(2):277–294. doi: 10.1016/0014-4827(79)90153-8. [DOI] [PubMed] [Google Scholar]
- Gilbert D. A., Bergel F. The chemistry of xanthine oxidase. 9. An improved method of preparing the bovine milk enzyme. Biochem J. 1964 Feb;90(2):350–353. doi: 10.1042/bj0900350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsby R. A., Mandell N. The isolation and replica plating of cell clones. Methods Cell Biol. 1973;7:261–268. doi: 10.1016/s0091-679x(08)61781-1. [DOI] [PubMed] [Google Scholar]
- Goldsby R. A., Osborne B. A., Simpson E., Herzenberg L. A. Hybrid cell lines with T-cell characteristics. Nature. 1977 Jun 23;267(5613):707–708. doi: 10.1038/267707a0. [DOI] [PubMed] [Google Scholar]
- Goldsby R. A., Zipser E. The isolation and replica plating of mammalian cell clones. Exp Cell Res. 1969 Feb;54(2):271–275. doi: 10.1016/0014-4827(69)90250-x. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Löw H., Crane F. L. Redox function in plasma membranes. Biochim Biophys Acta. 1978 Jul 31;515(2):141–161. doi: 10.1016/0304-4157(78)90002-3. [DOI] [PubMed] [Google Scholar]
- Mather I. H., Keenan T. W. Studies on the structure of milk fat globule membrane. J Membr Biol. 1975 Apr 23;21(1-2):65–85. doi: 10.1007/BF01941062. [DOI] [PubMed] [Google Scholar]
- Mather I. H. Separation of the proteins of bovine milk-fat globule membrane by electrofocusing. Biochim Biophys Acta. 1978 Dec 4;514(1):25–36. doi: 10.1016/0005-2736(78)90074-3. [DOI] [PubMed] [Google Scholar]
- Nielsen C. S., Bjerrum O. J. Crossed immunoelectrophoresis of bovine milk fat globule membrane protein solubilized with non-ionic detergent. Biochim Biophys Acta. 1977 May 2;466(3):496–509. doi: 10.1016/0005-2736(77)90342-x. [DOI] [PubMed] [Google Scholar]
- Patton S., Keenan T. W. The milk fat globule membrane. Biochim Biophys Acta. 1975 Oct 31;415(3):273–309. doi: 10.1016/0304-4157(75)90011-8. [DOI] [PubMed] [Google Scholar]
- Rosenthal J. D., Hayashi K., Notkins A. L. Comparison of direct and indirect solid-phase microradioimmunoassays for the detection of viral antigens and antiviral antibody. Appl Microbiol. 1973 Apr;25(4):567–573. doi: 10.1128/am.25.4.567-573.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Waud W. R., Brady F. O., Wiley R. D., Rajagopalan K. V. A new purification procedure for bovine milk xanthine oxidase: effect of proteolysis on the subunit structure. Arch Biochem Biophys. 1975 Aug;169(2):695–701. doi: 10.1016/0003-9861(75)90214-3. [DOI] [PubMed] [Google Scholar]


