Abstract
Background
Establishing the effectiveness of high-protein supplementation in reducing cancer-related side effects is crucial.
Objective
The study aimed to assess the effectiveness and safety of high-protein supplementation on clinical outcomes of patients undergoing cancer therapy.
Methods
Systematic searches were conducted on Medline, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase, Cochrane Central Register of Controlled Trials, and Scopus from inception until July 2023. Randomized controlled trials administering supplements with ≥10 g protein/serving, given to 20+ adult patients undergoing cancer therapy were included. Random-effects meta-analyses were used to estimate the effects of high-protein supplementation on the primary outcomes of body weight and health-related quality of life (HRQoL). We employed a vote-counting approach based on effect direction for secondary outcomes (that is, body composition, muscle function, hospitalization, response to cancer therapy/toxicity, survival, and systemic inflammation). Risk-of-bias (ROB) was assessed.
Results
Thirty-five studies involving 3701 patients with diverse cancer types were included. Patients who received high-protein supplementation lost less body weight than controls (mean difference = 1.45 kg; 95% CI: 0.42, 2.48 kg; P = 0.006; I2 = 80%). No differences in HRQoL were observed; all studies assessing HRQoL were rated as high ROB. A beneficial effect on muscle mass was found in 11 of 13 studies, although most had a high ROB due to assessment techniques. When considering higher quality studies, evidence of a beneficial effect was found in 5 of 5 studies for muscle strength, and 3 of 4 for hospitalization rate. Effects on other secondary outcomes were inconsistent or limited. No serious adverse effects were reported.
Conclusions
High-protein supplementation mitigates weight loss, improves muscle strength, and lowers hospitalization rates in patients undergoing cancer therapy. These positive clinical outcomes, along with a favorable safety profile, suggest that high-protein supplementation may be a valuable addition to medical practice. However, given the need for more robust trials and the high ROB observed in the existing studies, these conclusions should be interpreted with caution.
This review was prospectively registered with PROSPERO under the registration number CRD42021237372.
Keywords: nutrition intervention, protein supplement, body weight, quality of life, body composition, cancer
Introduction
Global cancer burden is rising [1], accounting for 1 in 6 deaths [2]. Cancer impacts patient’s lives and strains families and healthcare systems [[3], [4], [5], [6], [7]]. Patients with cancer often experience side effects, regardless of disease type, stage, and prediagnosis health status [8], which include nutrition impact symptoms, fatigue, and depression, among others. These side effects can lead to weight loss, deterioration in health-related quality of life (HRQoL), and unfavorable body composition and muscle function changes, resulting in poor clinical outcomes and overall prognosis [[9], [10], [11], [12], [13]].
Cancer proinflammatory state accelerates muscle catabolism and reduces muscle protein synthesis, leading to muscle loss [[14], [15], [16]]. This increased catabolic state, coupled with decreased food intake and nutrient absorption, underscores the critical need for nutrition interventions, particularly increasing protein consumption. Protein requirements for patients with cancer are set at higher levels compared with the general population [17] as a minimum of 1.0 g protein/kg body weight/d, with a target consumption of 1.2−2.0 g/kg/d [18,19]. However, attaining such protein intake through dietary means can be difficult [17,20]. Hence, protein supplementation, especially when combined with personalized dietary advice, is a viable strategy [21]. This approach ensures adequate protein intake and addresses individual nutritional needs effectively.
A 2018 systematic review explored the effects of protein- and omega-3-enriched oral nutritional supplements (omega-3 ONS) on patients undergoing treatment with or without nutrition counseling [21]. Omega-3 ONS reduced muscle loss (assessed as fat-free mass or lean mass) in half the trials and improved HRQoL domains in 3 out of 4 studies. Although ONS are nutritionally complete and recommended in nutritional oncology guidelines [18,19], research on alternative high-protein supplements [for example, branched-chain amino acids (BCAA), glutamine, arginine] is limited [18].
Considering the emerging literature, an updated and comprehensive review is needed to explore the effects of high-protein supplementation (≥10 g protein/serving) on patients with cancer. We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs), evaluating the effectiveness and safety of high-protein supplementation in patients being actively treated for cancer. High-protein supplementation consisted of single or mixed amino acids, which may contain or was enriched with omega-3 fatty acids, protein precursors, and modulators of protein metabolism. This analysis covered several outcomes, including body weight and HRQoL as primary outcomes and body composition, muscle function, survival, hospitalization, response to cancer therapy/toxicity, and systemic inflammation as secondary outcomes.
Methods
This review followed the PRISMA [22] and the Synthesis Without Meta-analysis (SWiM) reporting guidelines [23]. The research protocol was registered with PROSPERO (CRD42021237372).
Eligibility criteria
We included RCTs comparing high-protein supplementation (≥10 g of protein/serving) to placebo, standard of care, or lower dose protein supplements among patients receiving treatment for any cancer type (Supplemental Material 1). High-protein supplementation in the form of food supplements, ONS, or specialized ONS was included, with or without nutrition counseling or dietary advice. Studies on tube feeding alone or parenteral nutrition were excluded. Studies including exercise intervention were ineligible. For patients undergoing surgery, high-protein supplementation had to be administered perioperatively. We only included studies that reported ≥1 of our primary or secondary outcomes or explored intervention safety.
Outcomes
Primary outcomes were body weight (or BMI, when weight was unavailable) and HRQoL. Body weight assessed physical health, whereas HRQoL evaluated the broader impact of protein supplementation on the patient's overall well-being. Secondary outcomes included muscle and fat masses, muscle function, survival, hospitalization, response to cancer therapy/toxicity, and systemic inflammation. Although therapy administration outcomes, including chemotherapy modifications and treatment delays, are often regarded as toxicity-related outcomes [24], we presented our findings on these outcomes separately from cancer therapy-induced toxicity. Systemic inflammation was evaluated as an outcome in this review; however, studies that assessed inflammatory markers to report patient profiling (characteristics) and/or as an outcome of the intervention were included. Specifically, the markers and proxies evaluated included albumin, lymphocyte count, c-reactive protein, prealbumin, IL-6, transferrin, LPS-binding protein, leukocytes, eosinophils, cortisol, hemoglobin, tumor necrosis factor-alpha, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, white blood cells, and red blood cells.
Search strategy and data extraction
We searched Medline (via Ovid), Cumulative Index to Nursing and Allied Health Literature (CINAHL), Embase (via Elsevier), Cochrane Central Register of Controlled Trials, and Scopus from inception to 5 July, 2023 (last search date). Terms related to “food and oral nutritional supplements,” “oncology,” and “RCTs” were included, limited to English language, humans, and adult studies (Supplemental Material 2). ClinicalTrials.gov, Google, and reference lists of retrieved reports were also searched. Records were imported to Covidence (Veritas Health Innovation Ltd) for automated deduplication and study selection by 2 independent reviewers.
One reviewer extracted data on study characteristics and primary outcomes using Covidence Extraction 2 or an online spreadsheet in Google Sheets for secondary and safety outcomes. Effect sizes and corresponding P values were collected for primary and secondary outcomes, along with baseline and postintervention data. We used data from the closest to the conclusion of the intervention for multiple follow-up evaluations and extracted graphical data using Plot Digitizer (V.2.6.9; http://plotdigitizer.sourceforge.net) [25]. For primary outcomes, we collected the mean and SD of absolute or relative changes, if available, or the mean and SD of postintervention values. When required, median and interquartile data were transformed to mean and SD, or SD was estimated from P values of differences between groups [26]. Similar interventions were combined for multiple intervention arms studies to form a single pair-wise comparison [27]. We attempted to contact study authors for missing data and sought information on the nutritional composition of the supplement from pertinent websites or manufacturing companies. Data were cross-checked for accuracy by 2 reviewers, and discrepancies were resolved through consensus. Note that the number of studies reported may not reflect the number of citations because some studies may have been documented in multiple publications.
Risk-of-bias assessment
Two independent reviewers evaluated risk-of-bias (ROB) for each outcome domain using the Revised Cochrane ROB tool for randomized trials (RoB2) [28], which examines bias due to randomization, deviations from intended interventions, missing outcome data, outcome methods, and selective reporting of results. Results were represented graphically using ROB VISualization (robvis) [29].
Statistical analysis
Meta-analysis
We conducted random-effects meta-analysis to evaluate the weighted average intervention effect on our primary outcomes, along with 95% confidence intervals (CIs) in Review Manager 5 version 5.4.1 (The Cochrane Collaboration). Effect estimates of body weight in kilograms were determined using mean difference (MD). Sensitivity analysis combined studies assessing body weight, percentage change, and BMI using the standardized mean difference (SMD). We estimated the effect size of HRQoL using SMD, owing to using different assessment tools. We assessed heterogeneity using I2 statistics and set statistical significance at P = 0.033 for multiple outcomes. When data for ≥2 studies were available, subgroup analyses were conducted to explore the influence of tumor type, cancer therapy, supplement type, protein dose, duration of high-protein supplementation, preintervention weight loss and (risk of) malnutrition, systemic inflammation changes, adherence, and ROB on the primary outcomes. Studies not included in statistical pooling were summarized narratively.
Synthesis without meta-analysis
Intervention effects on secondary outcomes were synthesized using vote-counting, focusing (solely) on the direction of effect [23,30]. We categorized the direction of effect as “beneficial effect” when the intervention positively influenced health or resulted in an unchanged outcome. Conversely, “no beneficial effect” was attributed to the negatively influenced health. For studies with multiple related outcomes, “beneficial effect” or “no beneficial effect” was defined if ≥70% of outcomes showed a consistent direction; those with <70% had a “mixed effect” [31]. Results were represented graphically using Harvest plots [[31], [32], [33]]; effect estimates and P values for each individual study were provided. Sensitivity analysis, using combined data from studies with low and moderate ROB, was also conducted [34].
Results
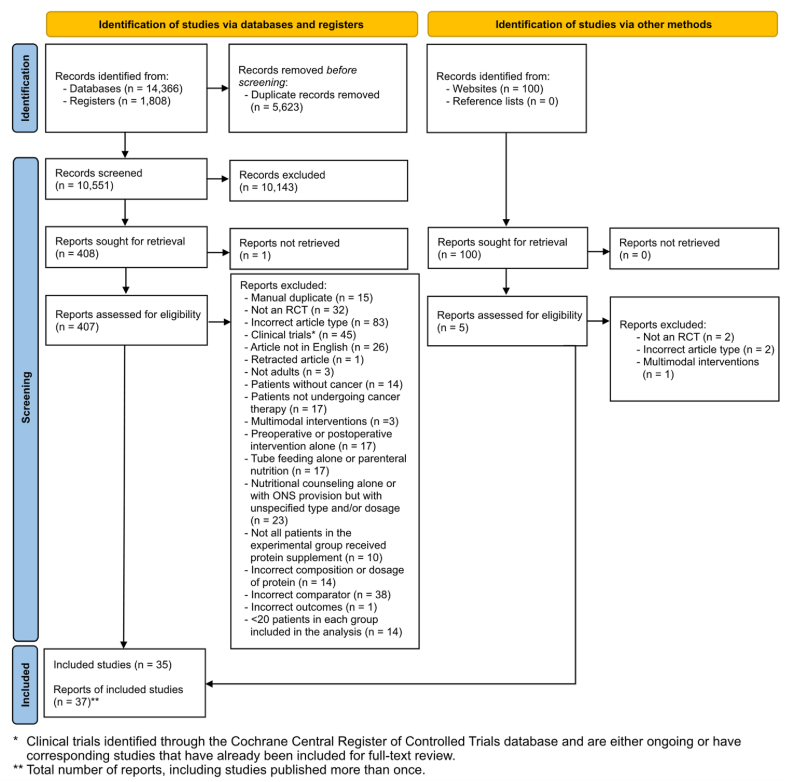
A total of 16,274 records were originally identified and 412 full-text reports were reviewed, with 37 meeting eligibility criteria; these yielded findings from 35 unique studies (Figure 1). This discrepancy occurred because 4 studies reported different outcomes for the same study [[35], [36], [37], [38]]. Studies were published between 1998 and 2023.
FIGURE 1.
PRISMA 2020 flow diagram for study selection.
Study characteristics
Study characteristics are summarized in Supplemental Table 1. Included studies were conducted across 22 different countries, with most from Japan (7 studies; 20%) [[37], [38], [39], [40], [41], [42], [43], [44]], China (4 studies; 11%) [[45], [46], [47], [48]], and the United Kingdom (3 studies; 9%) [[49], [50], [51]]. Among these, 24 (69%) studies had an open-label design [[35], [36], [37], [38], [39], [40], [41], [42], [43],[45], [46], [47],49,[52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64]], whereas 7 (20%) were double-blinded [44,50,51,[65], [66], [67], [68]], and 3 (9%) were single-blinded [48,69,70]. Additionally, 25 studies (71%) were conducted at a single center [35,36,[40], [41], [42], [43], [44], [45], [46],48,[51], [52], [53], [54], [55],57,[59], [60], [61], [62],64,65,[67], [68], [69], [70]], whereas 8 (23%) were multicenter trials involving 2–16 centers [[37], [38], [39],47,49,50,56,58,66].
Studies included 3701 patients, with experimental group sizes ranging from 22 to 171 individuals and the control group from 23 to 166. The mean or median age of patients in the experimental group ranged from 44.1 to 69.1 y, and in the control group from 44.4 to 70.6 y. At the time of nutrition intervention, patients were diagnosed with gastrointestinal tract (25 studies; 71%) [[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50],54,56,[58], [59], [60], [61],63,65,67,71], lung (6 studies; 17%) [49,53,56,66,68,69], breast (4 studies; 11%) [51,52,56,70], gynecological (4 studies; 11%) [55,56,58,59], head and neck (4 studies; 11%) [57,58,63,64], mesothelioma (1 study; 3%) [49], and bladder cancer (1 study; 3%) [62] (Supplemental Figure 1). Cancer stages ranged from I to III (2 studies; 6%) [37,38,52], II (1 study; 3%) [43], I–IV (10 studies; 29%) [35,36,40,45,46,53,55,57,62,64,66], and III–IV (3 studies; 9%) [39,68,69]. Studies included patients undergoing chemotherapy (14 studies; 40%) [39,40,45,46,49,51,52,54,56,59,63,66,69,70], surgery (12 studies; 34%) [37,38,42,44,47,50,53,55,[60], [61], [62],65,71], concurrent chemoradiotherapy (7 studies; 20%) [35,36,43,56,58,59,67,68], radiotherapy (3 studies; 9%) [57,59,64], or transarterial chemoembolization (2 studies; 6%) [41,48].
Patients in both the experimental and control groups exhibited nutritional vulnerabilities, including risk of malnutrition or malnutrition (8 studies; 23%) [35,36,45,46,54,55,64,65,67] or pre-cachexia (1 study; 3%) [54]. Preintervention weight loss was documented for all patients or in a proportion of them in 10 (29%) studies and categorized as <5% weight loss [59,61], ≥5% weight loss [50,59], <10% weight loss [54,55,66,69], or > 10% weight loss [48,50,69,71] over 1–12 mo before intervention in most studies; no significant preintervention weight loss was reported in 1 study [57]. Preintervention protein intake was reported in 9 studies [35,36,50,52,56,57,59,62,64,69], with values ranging from 0.83 to 1.16 g/kg/d in the experimental group and 0.83 to 1.11 g/kg/d in the control group. Total energy intake at baseline was reported in 10 studies [35,36,43,48,52,56,57,59,62,64,69], with values ranging from 23.3 to 28.8 kcal/kg/d in the experimental group and 22.3 to 30.9 kcal/kg/d in the control group.
Studies used various types of high-protein supplementation, including high-protein ONS (11 studies) [35,36,40,[45], [46], [47],49,54,55,57,64,65]; omega-3 ONS (9 studies) [[37], [38], [39],43,50,52,56,59,66,69]; glutamine (4 studies) [63,67,68,70]; ONS containing arginine and omega-3 or omega-6 (Arg/omega-3, -6 ONS; 4 studies) [60,61,65,71]; BCAA (3 studies) [41,42,48]; β-hydroxy β-methylbutyrate (HMB) combined with arginine and glutamine (HMB/Arg/Gln; 2 studies) [44,53]; arginine (1 study) [51]; ONS containing arginine, glutamine, and omega-3 (Arg/Gln/omega-3 ONS; 1 study) [58]; and ONS containing HMB and omega-3 (HMB/omega-3 ONS; 1 study) [62] (Supplemental Figure 2). The highest protein content per supplement serving was 25 g [47], with most studies (20 in total) administering 2 daily servings [[35], [36], [37], [38], [39], [40],42,43,45,46,48,[53], [54], [55],58,60,62,[64], [65], [66],69,70]. Sixteen studies combined high-protein supplementation with other nutrition interventions, such as nutrition counseling or dietary advice [43,45,46,49,52,[56], [57], [58], [59], [60]], standard/routine nutrition care [44,47,48,53,62], and standardized menus [69]. The standard/routine nutrition care varied across studies. It included the following: isocaloric juice plus a regular hospital diet [44], regular hospital diet with a target energy requirement of 20–30 kcal/kg/d [47], a usual hospital diet [48], a usual hospital diet with target energy calculated by Harris–Benedict formula and protein according to the dietitian's prescription [53], and twice daily multivitamins [62].The goals of nutrition counseling or dietary advice varied and aimed at achieving a high-protein, plant-based diet [52], increasing the intake of fat and protein-rich foods [45,46], attaining an isocaloric diet [56], meeting protein and energy requirements [59], or prevent under-nutrition [58]. Length of intervention for patients undergoing surgery ranged from 3 to 4 wk [62] preoperatively to 6 mo [42] postoperatively; for those receiving other cancer therapies, the intervention lasted between 5 d [70] and 1 y [48]. In most studies, controls received standard/routine nutrition care (8 studies) [37,38,47,48,53,55,62,64,68] and neither supplementation nor intervention (8 studies) [35,36,[39], [40], [41],49,54,64,71].
Meta-analyses of primary outcomes
Body weight
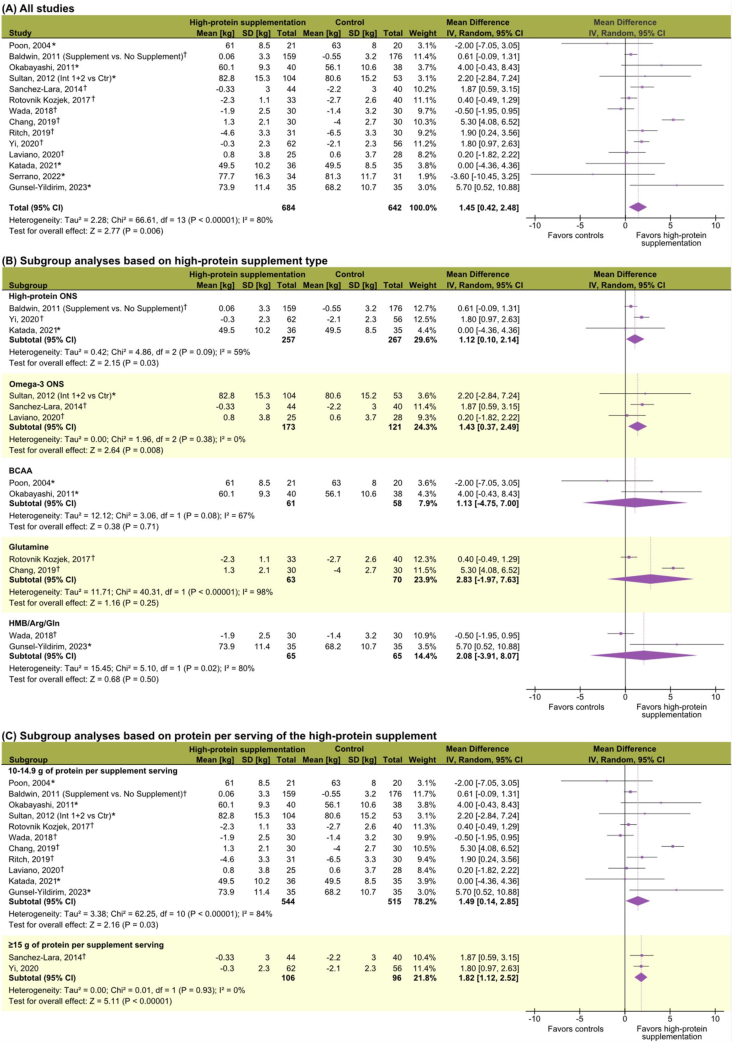
Twenty of 23 (87%) studies examining body weight (or BMI) were included [37,[40], [41], [42], [43], [44],[48], [49], [50],[52], [53], [54], [55],57,62,[65], [66], [67], [68], [69]]. Patients receiving high-protein supplementation lost less body weight than controls, with a pooled MD of 1.45 kg (95% CI: 0.42, 2.48 kg; P = 0.006; I2 = 80%) (Figure 2). Mean absolute changes in body weight ranged from −4.6 to 1.3 kg in the experimental group, and −6.5 to 0.6 kg in the control group. Combining body weight data of different units of measurement resulted in a significant SMD of 0.22 (95% CI: 0.03, 0.41; P = 0.02; I2 = 74%) (Supplemental Figure 3). A significant effect was observed with high-protein ONS and omega-3 ONS, whereas no significant effects were detected for BCAA, glutamine, and HMB/Arg/Gln (Figure 2). Studies using supplements containing either 10–14.9 g of protein/serving or ≥15 g of protein/serving (Figure 2), or providing a total daily protein intake of ≥40 g (MD = 1.81 kg; 95% CI: 0.99, 2.63 kg; P < 0.001; I2 = 0%) (Supplemental Figure 4) also had a significant effect on body weight. Furthermore, a significant effect was observed in studies that administered high-protein supplementation for 3–12 wk (MD = 0.82 kg; 95% CI: 0.24, 1.40; P = 0.006; I2 = 20%) and for those that continued beyond 13 wk (MD = 5.21 kg; 95% CI: 4.03, 6.39) (Supplemental Figure 4). Subgroup analyses revealed a positive intervention effect for patients with lung cancer, chemotherapy and surgery recipients, preintervention weight losers, and patients with lower systemic inflammation following intervention (Supplemental Figures 5 and 6).
FIGURE 2.
Meta-analyses of the effects ofhigh-proteinsupplementation on body weight (all units of measurement are in kilograms). (A) All included studies. (B) Subgroup analyses based on supplement type. (C) Subgroup analyses based on protein content per serving per day. The summary statistic table displays postintervention values (∗) or absolute changes (†) based on available data, resulting in varying magnitudes. The study by Baldwin et al. [49] combined study groups when reporting body weight changes; thus, the “Nutrition supplement” group included those patients who received supplements alone or concurrently with nutritional counseling, and the “No nutrition supplement” group included those who did not receive any intervention nor nutritional counseling. CI, confidence interval.
Studies excluded from meta-analyses failed to report variance data [56] or only reported weight loss frequency [39,59]. In these studies, concurrent omega-3 ONS supplementation and nutrition counseling resulted in increased body weight [56] or in a lower frequency of weight loss compared with controls [59]; however, when omega-3 ONS was administered alone, more patients lost weight in the experimental group [39].
Health-related quality of life
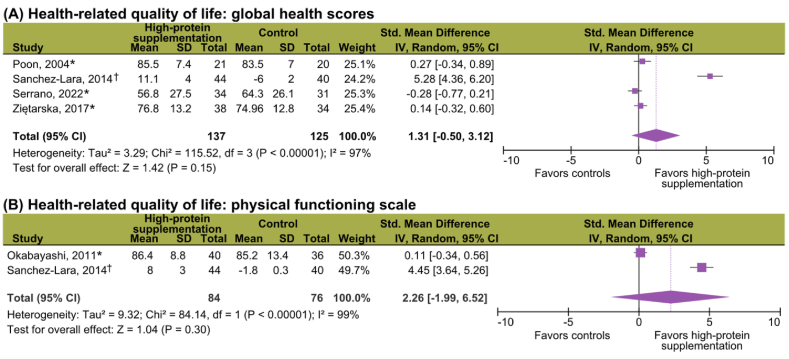
Five of 10 (50%) studies exploring HRQoL were included [42,48,54,65,69]. High-protein supplementation had no significant effect on global health scores (SMD = 1.31; 95% CI: −0.50, 3.12; P = 0.15; I2 = 97%) and the physical functioning domain (SMD = 2.26; 95% CI: −1.99, 6.52; P = 0.30, I2 = 99%) (Figure 3); heterogeneity was high in both analyses. Excluded studies failed to report variance data [35,36,64] or only reported on significant differences [49,56,59]. Of these, Ravasco et al. [35,36,64] reported improved global and function scores with high-protein ONS, with no significant intervention effect for the remaining studies [49,56,59].
FIGURE 3.
Meta-analyses of the effects ofhigh-proteinsupplementation onhealth-relatedquality of life. (A) Global health scores (all studies included in the analysis). (B) Physical functioning scale (all studies included in the analysis). The summary statistic table displays postintervention values (∗) or absolute changes (†) based on available data, resulting in varying magnitudes. HRQoL was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) [65,69], the Functional Assessment of Cancer Therapy - General (FACT-G) [48,65], the 36-Item Short Form Survey (SF-36) [42], and the Functional Assessment of Anorexia/Cachexia Treatment (FAACT) [54]. CI, confidence interval.
Synthesis without meta-analyses of secondary outcomes
Muscle mass
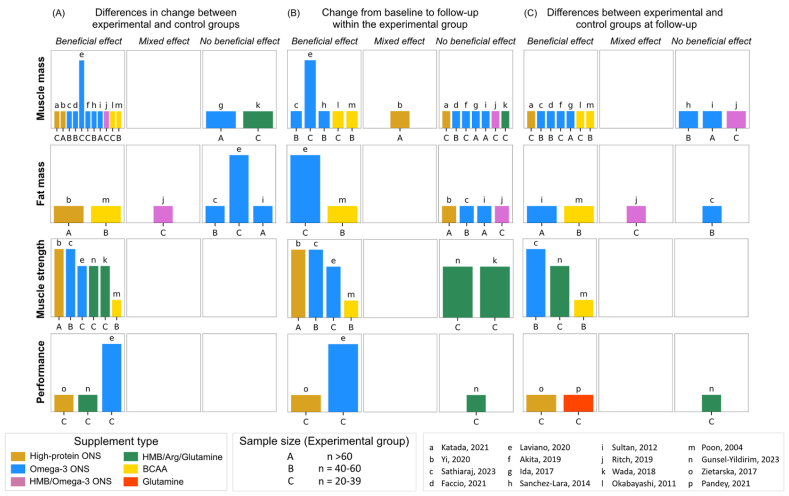
Muscle mass (or its related compartments) was evaluated in 13 studies [38,40,42,43,44,48,50,52,55,56,62,66,69]. Various body composition techniques were used, such as bioelectrical impedance analysis [38,40,43,44,52,55,56,69], computed tomography [43,62], and dual-energy X-ray absorptiometry [66] (Supplemental Figure 7). Mid-arm muscle circumference was used as a surrogate anthropometric measure of muscle mass in 4 studies [42,48,50,55]. Skeletal muscle radiodensity was also evaluated in 1 study [62]. Overall, 11 of 13 (85%) studies showed that high-protein supplementation had a beneficial effect on muscle mass and skeletal muscle radiodensity compared with controls [40,42,43,48,50,52,55,56,62,66,69] (Figure 4, Supplemental Figure 8). Although a decline in muscle mass was experienced in both groups, the experimental group lost less muscle mass than controls.
FIGURE 4.
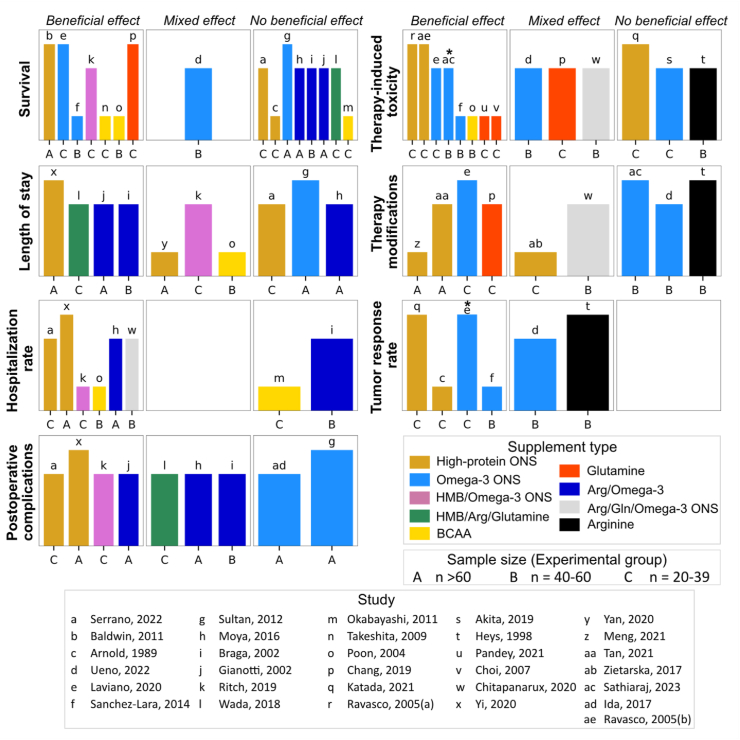
Harvest plots summarizing the effects ofhigh-proteinsupplementation on muscle mass, fat mass, and muscle function (muscle strength and performance). (A) Describes the effects by comparing changes in outcomes between experimental and control groups. (B) Indicates the effects within experimental group considering changes from baseline to follow-up. (C) Represents the effects based on differences between experimental and control groups at follow-up. The height of each bar served as an indicator of study quality, where taller bars signify a lower risk-of-bias, medium-height bars denote a moderate risk-of-bias, and shorter bars signify a high risk-of-bias. Each lowercase letter corresponds to a unique study, while uppercase letters indicate the sample size of experimental groups. Different supplement types are represented by varying colors. Arg, arginine; BCAA, branched-chain amino acids; Gln, glutamine; HMB, β-hydroxy β-methylbutyrate; ONS, oral nutritional supplement.
A diverse array of protein supplements exhibited this beneficial effect, encompassing high-protein ONS [40,55], omega-3 ONS [43,50,52,56,66,69], BCAA [42,48], and ONS enriched with HMB and omega-3 [62]. HMB, administered with amino acids arginine and glutamine, had no beneficial effects on muscle mass [44]. To assess how inflammation influences the effectiveness of high-protein supplementation on muscle mass, we conducted a subgroup analysis on 10 studies [37,38,40,42,48,50,55,56,62,66,69] assessing changes in systemic inflammation and muscle mass; 60% of the studies found that patients with worsened inflammation also experienced muscle loss [37,38,40,50,55,56,62]. Conversely, 20% of the studies observed muscle gains associated with reduced systemic inflammation [42,69].
Fat mass
Three studies evaluated fat mass using either bioelectrical impedance analysis or dual-energy X-ray absorptiometry [52,55,66], whereas 2 studies assessed triceps skinfolds as a surrogate of fat mass [48,50] (Supplemental Figure 7). Additionally, 1 study used computed tomography scans to quantify visceral and subcutaneous adipose tissues [62]. Overall, 3 of 6 studies (50%) found no beneficial effect of high-protein supplementation on fat mass, compared with control groups [50,52,66] (Figure 4, Supplemental Figure 9).
Results varied across supplement types and analyses. Three of 3 studies (100%) reported that omega-3 ONS resulted in reduced fat mass (that is, no beneficial effect) in patients undergoing chemotherapy [52,66] or triceps skinfolds in surgical patients [50], as compared with control groups. BCAA supplementation consistently effected fat mass across analyses during transarterial chemoembolization in 1 study [48]. However, the effects of perioperative supplementation with high-protein ONS and HMB/omega-3 ONS on fat mass [55] and visceral and subcutaneous adipose tissues [62], respectively, varied across analyses.
Muscle function
Compared with controls, high-protein supplementation was associated with a beneficial effect on handgrip strength in 6 of 6 studies (100%) [44,48,52,53,55,66] (Figure 4, Supplemental Figure 10). Supplements included high-protein ONS [55], omega-3 ONS [52,66], HMB/Arg/glutamine [44,53], and BCAA [48]. A beneficial effect of high-protein supplementation compared with controls was also observed on physical performance outcomes in 3 of 3 studies (100%) [53,54,66]. Among these studies, high-protein ONS demonstrated a beneficial effect on Karnofsky Performance Scale [54], and omega-3 ONS showed a beneficial effect on walking distance [66] in patients receiving neoadjuvant chemotherapy. Patients undergoing surgery who received HMB/Arg/Gln supplementation showed less increase in the Eastern Cooperative Oncology Group Performance Status scale scores than controls, indicating a beneficial effect [53]. Additionally, glutamine supplementation during neoadjuvant or adjuvant chemotherapy was also positively associated with Karnofsky Performance Scale scores at follow-up [70].
Survival
In 7 of 16 (44%) studies, high-protein supplementation demonstrated a beneficial effect on survival outcomes, including overall survival, progression-free survival, and survival rates compared with controls at follow-up [41,48,49,62,66,68,69] (Figure 5, Supplemental Figure 11). Conversely, 8 of 16 studies reported no beneficial effect, and 1 of 16 found mixed effects regarding the impact of high-protein supplementation on survival. In an analysis stratified by supplement type, findings pertaining to high-protein ONS [49,57,65], omega-3 ONS [39,50,66,69], and BCCA [41,42,48] exhibited inconsistent directions of effects. No beneficial effects were found for Arg/omega-3 supplementation on postoperative survival rates among patients with gastrointestinal cancers in 3 studies [60,61,71]. Furthermore, 1 study reported a longer 30-d survival rate with perioperative HMB/omega-3 ONS supplementation [62]. Another study observed a longer progression-free survival with glutamine supplementation during concurrent chemoradiotherapy [68].
FIGURE 5.
Harvest plots summarizing the effects ofhigh-proteinsupplementation on survival, length of stay, hospitalization rate, postoperative complications, cancertherapy-inducedtoxicity, therapy modifications, and tumor response rate. Plots represent the direction of effects based on the differences between experimental and control groups at follow-up, otherwise specified by the symbol (∗) indicating differences in change between experimental and control groups. The height of each bar served as an indicator of study quality, where taller bars signify a lower risk-of-bias, medium-height bars denote a moderate risk-of-bias, and shorter bars signify a high risk-of-bias. Each lowercase letter corresponds to a unique study, while uppercase letters indicate the sample size of experimental groups. Different supplement types are represented by varying colors. Arg, arginine; BCAA, branched-chain amino acids; Gln, glutamine; HMB, β-hydroxy β-methylbutyrate; ONS, oral nutritional supplement.
Hospitalization
High-protein supplementation conferred no beneficial or mixed effects on postoperative complications in 5 of 9 (56%) studies [37,38,44,50,60,71] and on length of stay in 6 of 10 (60%) [47,48,50,60,62,65], compared with controls at follow-up (Figure 5, Supplemental Figures 12 and 13). In contrast, 6 of 8 (75%) studies reported a beneficial effect of high-protein supplementation on hospital admission rates [48,55,58,60,62,65] (Figure 5, Supplemental Figure 14). Notably, a consistent beneficial effect on postoperative complications and hospitalization rates was demonstrated with high-protein ONS in 2 studies [55,65] and perioperative HMB/omega-3 ONS supplementation in another study [62].
Response to cancer therapy/toxicity
High-protein supplementation showed a beneficial effect on cancer therapy-induced toxicity in 8 out of 14 (57%) studies [35,36,48,52,63,64,66,69,70] (Figure 5, Supplemental Figure 15). Nevertheless, results were inconclusive or limited when evaluating the effect based on supplement type. Findings also varied within the context of therapy administration outcomes: 4 of 9 (44%) studies reported a beneficial effect of high-protein ONS [45,46] and omega-3 ONS [66] on chemotherapy modifications, and glutamine supplement on treatment delay [68]; 2 of 9 showed mixed effects of high-protein ONS [54] and Arg/Gln/omega-3 ONS [58]; and 3 of 9 reported no beneficial effect for omega-3 ONS [39,52] and arginine supplementation [51] (Figure 5, Supplemental Figure 16). Additionally, 2 of 2 studies demonstrated a beneficial effect of high-protein ONS on rates of partial and complete response to chemotherapy [40,57] (Figure 5, Supplemental Figure 17).
Systemic inflammation
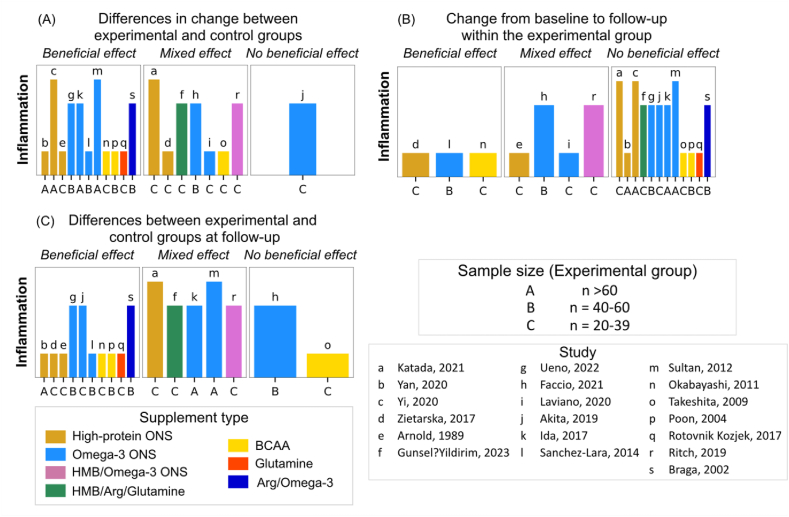
Eleven of nineteen (58%) revealed a beneficial effect of high-protein supplementation on systemic inflammation compared with controls, despite variations in cancer types, disease stages, nutritional statuses, and treatment modalities [37,39,42,47,48,50,55,57,67,69,71] (Figure 6, Supplemental Figure 18). Nevertheless, inconsistencies emerged when examining different supplement types, inflammatory markers, and analytical approaches.
FIGURE 6.
Harvest plots summarizing the effects of high-protein supplementation on systemic inflammation. (A) Describes the effects by comparing changes in outcomes between experimental and control groups. (B) Indicates the effects within experimental group considering changes from baseline to follow-up. (C) Represents direction of effects based on the differences between experimental and control groups at follow-up. The height of each bar served as an indicator of study quality, where taller bars signify a lower risk-of-bias, medium-height bars denote a moderate risk-of-bias, and shorter bars signify a high risk-of-bias. Each lowercase letter corresponds to a unique study, while uppercase letters indicate the sample size of experimental groups. Different supplement types are represented by varying colors. Studies examined several markers of systemic inflammation, including albumin, prealbumin, transferrin, white blood cells, lymphocytes, c-reactive protein, IL-6, LPS-binding protein, tumor necrosis factor-alpha, and the neutrophil:lymphocyte ratio. Additionally, systemic inflammation and prognostic nutrition indexes were calculated using inflammatory markers in 1 study [53]. Arg, arginine; BCAA, branched-chain amino acids; HMB, β-hydroxy β-methylbutyrate; ONS, oral nutritional supplement.
Safety and tolerability of high-protein supplementation
Sixteen studies evaluated the potential adverse events linked to high-protein supplementation (Supplemental Table 2). Of these, 10 (63%) reported positive safety outcomes, including good tolerance of the high-protein supplementation [42,54,66,68], no occurrence of adverse [67] or serious adverse events [39,55], and comparable rates of adverse outcomes between experimental and control groups [39,54,59,61,69]. Incidence of gastrointestinal adverse events – such as diarrhea, abdominal pain, constipation, and nausea – varied across studies, primarily attributed to differences in supplement types, cancer types, and treatment modalities [51,56,59,61,65,66]. Supplements assessed included omega-3 ONS [56,59,66], high-protein ONS [65], Arg/omega-3 ONS [61], and arginine [51]. Additionally, 2 studies suggested that gastrointestinal symptoms and suboptimal supplement tolerability were associated with low adherence to the prescribed supplement regimen [50,65].
Potential confounding effects
Adherence
Adherence to high-protein supplementation was reported in 23 (66%) studies (Supplemental Table 1) [[37], [38], [39], [40],43,44,[48], [49], [50],52,[54], [55], [56], [57], [58], [59], [60], [61], [62],[65], [66], [67],69,71]. In 9 studies, ≥80% of the patients adhered to ≥70% of the prescribed high-protein supplementation regimen [44,52,54,55,[60], [61], [62],65,71]. Conversely, in 8 studies, fewer than 80% of patients had adherence rates below 70% [37,38,43,49,56,57,59,66,69]. Five studies provided information on adherence, but their data could not be categorized into specific groups [39,40,50,58,67]. Lower adherence did not significantly affect the impact of high-protein supplementation on body weight (Supplemental Figure 19). Adherence to the recommended supplementation range was broad, from 53% to 100% [37,38,44,[55], [56], [57],62]. Four studies observed a decline in adherence rates over time [37,38,49,60,71]. Additionally, 2 studies reported lower adherence attributed to taste-related issues, specifically in omega-3 ONS supplementation in pancreatic cancer chemoradiotherapy [43] and esophageal or gastric cancer surgery [50].
ROB
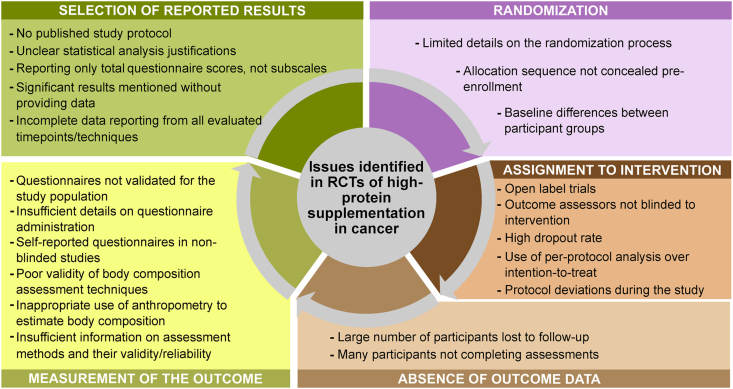
ROB assessment revealed various issues across multiple domains (Figure 7, Supplemental Material 3), including a lack of an intention-to-treat statistical analysis approach and reporting bias. Except for postoperative complications, almost every evaluated outcome had ≥1 study rated as having a high ROB. Outcomes with the highest frequency of high ROB studies were muscle mass (92% of studies), fat mass (83% of studies), physical performance (75% of studies), and HRQoL (60% of studies).
FIGURE 7.
Issues identified in randomized controlled trials (RCTs) of high-protein supplementation in patients with cancer undergoing therapy as part of the risk-of-bias evaluation.
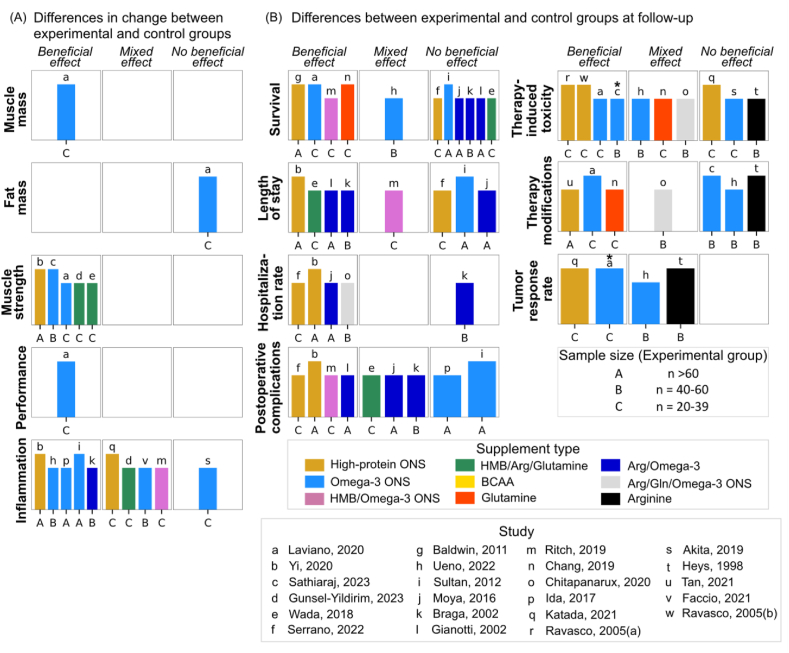
Sensitivity analysis revealed an effect of high-protein supplementation on body weight in studies with low to intermediate ROB (MD = 1.87 kg; 95% CI: 0.41, 3.34 kg; P = 0.01; I2 = 84%) but not in those with high ROB (Supplemental Figure 19). Sensitivity analysis on HRQoL was not possible due to the prevalent high ROB in studies. Among secondary outcomes assessed in ≥3 higher quality studies, improvement in muscle strength was found in all 5 studies, and a decrease in hospitalization rate was observed in 4 of 5 studies (Figure 8). However, the effects of high-protein supplementation on mortality, length of stay, postoperative complications, therapy-induced toxicity, therapy modifications, and tumor response rate varied, indicating a degree of heterogeneity.
FIGURE 8.
Evidence overview from higher quality studies on the effects ofhigh-proteinsupplementation on health outcomes of patients with cancer undergoing therapy. (A) The effects by comparing changes in outcomes between experimental and control groups. (B) The effects based on differences between experimental and control groups at follow-up, otherwise specified by the symbol. Asterisk (∗) indicates differences in change between experimental and control groups. The height of each bar served as an indicator of study quality, where taller bars signify a lower risk-of-bias, and medium-height bars denote a moderate risk-of-bias. Each lowercase letter corresponds to a unique study, while uppercase letters indicate the sample size of experimental groups. Different supplement types are represented by varying colors. Arg, arginine; BCAA, branched-chain amino acids; Gln, glutamine; HMB, β-hydroxy β-methylbutyrate; ONS, oral nutritional supplement.
Discussion
This systematic review and meta-analysis examined the effects of high-protein supplementation on patients undergoing cancer therapy. Our findings suggest that high-protein supplementation is safe and leads to less body weight loss, increased handgrip strength, and decreased hospitalization rates (Supplemental Figure 20). These results are primarily supported by studies with low to moderate ROB, ensuring the robustness of these outcomes.
Body weight, often used as an outcome in nutrition research, is a key indicator for diagnosing malnutrition and is clinical/prognostic important for these patients [72,73]. Unintentional weight loss in cancer is associated with postoperative complications, poorer quality of life, and lower overall survival, among other adverse outcomes at all stages of the disease [13,[74], [75], [76]]. Subgroup meta-analyses revealed a significant positive impact of high-protein supplementation on body weight in patients with prior weight loss. However, although this effect was not observed in those at risk of or currently experiencing malnutrition, it remains challenging to isolate weight loss alone from malnutrition. This finding supports the notion that weight gain can be more challenging without prior weight loss [77,78]. It suggests that malnutrition-related factors, such as decreased food intake or absorption, increased disease burden, and inflammation [79,80], can counteract the benefits of high-protein supplementation. Therefore, our findings highlight the potential effectiveness of early and targeted high-protein supplementation in improving treatment outcomes for patients with cancer, especially those who have experienced preintervention weight loss.
HRQoL is an important patient-centered outcome, directly impacted by cancer and its treatment [81]. Although many factors influence HRQoL, previous research links weight loss to lower quality of life [13,82,83]. Managing weight loss with high-protein supplementation may have long-term HRQoL benefits. Although direct HRQoL benefits were not observed, our review consistently showed improved muscle strength with high-protein supplementation. However, evidence for its effects on physical performance was limited.
Our systematic review identified a beneficial effect of high-protein supplementation on muscle mass, a finding supported by biological mechanisms suggesting high protein consumption to promote muscle anabolism by stimulating muscle protein synthesis [79,80]. However, inflammation can inhibit muscle protein synthesis, as reflected in our findings, where 60% of studies showing increased systemic inflammation from baseline to follow-up also reported no gains in muscle mass. Elevated systemic inflammation in cancer accelerates muscle protein breakdown for producing hepatic proteins required in the inflammatory response, reducing responsiveness to nutritional interventions [80,84]. Notably, 2 studies [42,69] using BCAA and omega-3 ONS, known for their anti-inflammatory properties [85,86], reported simultaneous muscle mass increase and systemic inflammation reduction. This concomitant stimulation of muscle protein synthesis and reduction of muscle protein breakdown are ideal for promoting muscle anabolism. As 6 of 7 studies using omega-3 ONS showed a beneficial effect on muscle mass [43,50,52,56,66,69], incorporating immune-modulating nutrients into high-protein supplements may further enhance this process, supporting more effective muscle growth and maintenance. Additionally, high-protein supplementation provides the necessary amino acids for muscle synthesis and contributes to overall energy intake, impacting body weight.
The reviewed supplements contained a minimum of 10 g of protein/serving. Our analysis showed that high-protein supplements, whether in the range of 10−14.9 g or ≥15 g of protein/serving, had a positive effect on body weight. Furthermore, higher daily protein intake (≥40 g) from supplements was more effective than lower doses. Although the precise protein threshold needed to stimulate muscle protein synthesis remains uncertain, our findings suggest that protein requirements likely exceed the minimum recommendation of 1 g/kg/d [17]. This raises the question of whether future guidelines should specify a minimal protein supplement dose per meal or recommend a minimum daily protein intake, a shift from current practice of adjusting total protein intake based on body weight (g/kg/d).
The high-protein supplements reviewed in this study varied, including single and mixed amino acids and ONS. Interestingly, the effects on each outcome differed based on the type of supplement. Concerning ONS, most are recognized for their nutritional completeness and higher energy content. Subgroup meta-analyses revealed that high-protein ONS and omega-3 ONS had beneficial effects on body weight. Additionally, supplements such as BCAA and ONS enriched with HMB and omega-3 also benefited muscle mass. Notably, HMB operates through increased muscle protein synthesis and decreased muscle protein breakdown [87]. Although protein quality plays a role, with essential amino acids and proteins like whey and casein promoting greater muscle protein synthesis [88,89], our analysis was limited due to insufficient information or heterogeneity regarding the protein composition of the supplements. This underscores the importance of understanding supplement composition when planning and prescribing interventions.
Our review found that higher-quality studies suggest that high-protein supplementation reduces hospitalization rates, potentially due to improvements in body weight or muscle mass. Despite the positive impact observed, the overall effects of high-protein supplementation on other outcomes were inconsistent. Contributing factors to this heterogeneity include variations in patient populations, such as cancer type, stage, and nutritional status, as well as differences in supplement composition, including protein content, and methods used for outcome assessment.
High-protein supplementation proves safe for these patients, dispelling concerns that it might fuel tumor growth. These (erroneous) fears, stemming from concerns regarding protein potentially activating the mTOR pathway [17,90] are unfounded, as exercise, widely considered beneficial, also activates mTOR to promote muscle growth [91]. Animal studies showed that protein intake does not affect tumor response to chemotherapy or immune responses [92]. Gastrointestinal events in reviewed studies varied due to cancer, treatments, and supplement types used.
This systematic review assessed the effectiveness of high-protein supplementation with or without nutrition counseling or dietary advice. Although nutrition counseling is a key strategy recommended by nutritional oncology guidelines [18,19], our review excluded studies where high-protein supplementation was provided only after failed nutrition interventions or as a preventive measure in control groups. This exclusion was necessary to avoid issues like inconsistent supplement distribution among participants or cross-contamination. Another limitation was the inclusion of supplements containing additional nutrients or ingredients, such as HMB and/or omega-3, alongside protein. Although these components could not be separated from the effects of protein, they exist in certain commercially available supplements and may provide added benefits. Our results may differ from previous reviews focusing on these specific nutrients/ingredients among patients with cancer [87,93] due to our distinct eligibility criteria and a smaller number of included studies on these supplements. Additionally, our review could not determine if changes in body weight were due to alterations in fat mass and/or muscle mass. Limited higher-quality studies, particularly those addressing HRQoL and secondary outcomes, such as muscle mass, also pose limitations that require cautious interpretation of findings. The high ROB in these outcomes not only constrained our analysis but also underscored the need for higher quality clinical nutrition studies. Researchers should provide clear methodological descriptions, particularly when evaluating body composition, to enhance study quality and advance the field. Additionally, the high variability in body composition techniques presented a challenge in comparing findings. Quality of life, often assessed by questionnaires, also requires more clarity, as it was often unclear whether these assessments were self-reported or administered by researchers, making evaluation difficult. Furthermore, the included studies were not sufficiently powered to detect outcome changes, which limited our analysis.
Finally, integrating these findings into clinical practice is feasible considering the importance of early and continuing nutrition therapy during cancer treatment for optimal benefits. Healthcare professionals should consider using high-protein supplements, especially for patients experiencing weight loss. Regularly monitoring patient responses to supplementation is crucial to assess its effectiveness. Additionally, it is important to recognize that while high-protein supplementation can have a positive impact on specific health outcomes, its full effectiveness may be limited without adequate energy and nutrient intakes.
Author contributions
The authors’ responsibilities were as follows – CEO, JA, MAEdvdS, NK, AL, and CMP: designed the research; CEO: searched the literature; CEO, AC, and TSP: evaluated the quality of the data and composed the tables; CEO: analyzed data; and all authors: contributed to the interpretation of the findings, contributed to writing the final article, and read and approved the final manuscript.
Funding
The work of CMP was partially supported by the Canada Research Chairs Program and the Campus Alberta Innovation Program.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending approval from the corresponding author.
Conflict of interest
CEO has received honoraria from Abbott Nutrition. JA reports receiving speaking and lecture fees from Baxter and Nutricia. AL reports receiving honoraria and/or paid consultancy from Abbott, Baxter, B. Braun, Fresenius Kabi, Nestlé Health Science, Nutricia, and Smartfish, and research grant from Fresenius Kabi. CMP has previously received honoraria and/or paid consultancy from Abbott Nutrition, Nutricia, Almased, Nestlé Health Science, Pfizer, and AMRA Medical. NK reports speaking and lecture fees and paid consultancy from Abbott Nutrition. The other authors report no conflicts of interest.
Acknowledgments
We thank Dr Bruna Ramos da Silva for her assistance in reviewing this manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2024.08.016.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Global Burden of Disease 2019 Cancer Collaboration. Kocarnik J.M., Compton K., Dean F.E., Fu W., Gaw B.L., et al. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022;8(3):420–444. doi: 10.1001/jamaoncol.2021.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Global cancer observatory – Cancer Today [Internet] 2020 https://gco.iarc.fr/today/home [cited 10 October, 2023]. Available from: [Google Scholar]
- 3.Kim A., Chung K.C., Keir C., Patrick D.L. Patient-reported outcomes associated with cancer screening: a systematic review. BMC Cancer. 2022;22(1):223. doi: 10.1186/s12885-022-09261-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright P., Smith A., Booth L., Winterbottom A., Kiely M., Velikova G., et al. Psychosocial difficulties, deprivation and cancer: three questionnaire studies involving 609 cancer patients. Br. J. Cancer. 2005;93(6):622–626. doi: 10.1038/sj.bjc.6602777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schouten B., Avau B., Bekkering G.T.E., Vankrunkelsven P., Mebis J., Hellings J., et al. Systematic screening and assessment of psychosocial well-being and care needs of people with cancer. Cochrane Database Syst. Rev. 2019;3(3) doi: 10.1002/14651858.CD012387.pub2. CD012387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donkor A., Atuwo-Ampoh V.D., Yakanu F., Torgbenu E., Ameyaw E.K., Kitson-Mills D., et al. Financial toxicity of cancer care in low- and middle-income countries: a systematic review and meta-analysis. Support. Care Cancer. 2022;30(9):7159–7190. doi: 10.1007/s00520-022-07044-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitch M.I., Longo C.J., Chan R.J. Cancer patients’ perspectives on financial burden in a universal healthcare system: analysis of qualitative data from participants from 20 provincial cancer centers in Canada, Patient. Educ. Couns. 2021;104(4):903–910. doi: 10.1016/j.pec.2020.08.013. [DOI] [PubMed] [Google Scholar]
- 8.Kuderer N.M., Desai A., Lustberg M.B., Lyman G.H. Mitigating acute chemotherapy-associated adverse events in patients with cancer. Nat. Rev. Clin. Oncol. 2022;19(11):681–697. doi: 10.1038/s41571-022-00685-3. [DOI] [PubMed] [Google Scholar]
- 9.Patel V.R., Hussaini S.M.Q., Blaes A.H., Morgans A.K., Haynes A.B., Adamson A.S., et al. Trends in the prevalence of functional limitations among US cancer survivors, 1999–2018. JAMA Oncol. 2023;9(7):1001–1003. doi: 10.1001/jamaoncol.2023.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prado C.M., Purcell S.A., Alish C., Pereira S.L., Deutz N.E., Heyland D.K., et al. Implications of low muscle mass across the continuum of care: a narrative review. Ann. Med. 2018;50(8):675–693. doi: 10.1080/07853890.2018.1511918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kiss N., Prado C.M., Daly R.M., Denehy L., Edbrooke L., Baguley B.J., et al. Low muscle mass, malnutrition, sarcopenia, and associations with survival in adults with cancer in the UK Biobank cohort. J. Cachexia Sarcopenia Muscle. 2023;14(4):1775–1788. doi: 10.1002/jcsm.13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown D., Loeliger J., Stewart J., Graham K.L., Goradia S., Gerges C., et al. Relationship between global leadership initiative on malnutrition (GLIM) defined malnutrition and survival, length of stay and post-operative complications in people with cancer: a systematic review. Clin. Nutr. 2023;42(3):255–268. doi: 10.1016/j.clnu.2023.01.012. [DOI] [PubMed] [Google Scholar]
- 13.Ryan A.M., Prado C.M., Sullivan E.S., Power D.G., Daly L.E. Effects of weight loss and sarcopenia on response to chemotherapy, quality of life, and survival. Nutrition. 2019;67–68 doi: 10.1016/j.nut.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Acharyya S., Guttridge D.C. Cancer cachexia signaling pathways continue to emerge yet much still points to the proteasome. Clin. Cancer Res. 2007;13(5):1356–1361. doi: 10.1158/1078-0432.CCR-06-2307. [DOI] [PubMed] [Google Scholar]
- 15.Hardee J.P., Montalvo R.N., Carson J.A. Linking cancer cachexia-induced anabolic resistance to skeletal muscle oxidative metabolism, Oxid. Med. Cell Longev. 2017;2017 doi: 10.1155/2017/8018197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lecker S.H., Goldberg A.L., Mitch W.E. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J. Am. Soc. Nephrol. 2006;17(7):1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- 17.Ford K.L., Arends J., Atherton P.J., Engelen M.P.K.J., Gonçalves T.J.M., Laviano A., et al. The importance of protein sources to support muscle anabolism in cancer: an expert group opinion. Clin. Nutr. 2022;41(1):192–201. doi: 10.1016/j.clnu.2021.11.032. [DOI] [PubMed] [Google Scholar]
- 18.Arends J., Bachmann P., Baracos V., Barthelemy N., Bertz H., Bozzetti F., et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017;36(1):11–48. doi: 10.1016/j.clnu.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Muscaritoli M., Arends J., Bachmann P., Baracos V., Barthelemy N., Bertz H., et al. ESPEN practical guideline: clinical nutrition in cancer. Clin. Nutr. 2021;40(5):2898–2913. doi: 10.1016/j.clnu.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Ford K.L., Orsso C.E., Kiss N., Johnson S.B., Purcell S.A., Gagnon A., et al. Dietary choices after a cancer diagnosis: a narrative review. Nutrition. 2022:103–104. doi: 10.1016/j.nut.2022.111838. 111838. [DOI] [PubMed] [Google Scholar]
- 21.de van der Schueren M.A.E., Laviano A., Blanchard H., Jourdan M., Arends J., Baracos V.E. Systematic review and meta-analysis of the evidence for oral nutritional intervention on nutritional and clinical outcomes during chemo(radio)therapy: current evidence and guidance for design of future trials. Ann. Oncol. 2018;29(5):1141–1153. doi: 10.1093/annonc/mdy114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Page M.J., Mckenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell M., McKenzie J.E., Sowden A., Katikireddi S.V., Brennan S.E., Ellis S., et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:l6890. doi: 10.1136/bmj.l6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kok D.E., Winkels R.M., van Herpen C.M., Kampman E. Toxicity-induced modification of treatment: what is in a name? Eur. J. Cancer. 2018;104:145–150. doi: 10.1016/j.ejca.2018.09.018. [DOI] [PubMed] [Google Scholar]
- 25.Jelicic Kadic A., Vucic K., Dosenovic S., Sapunar D., Puljak L. Extracting data from figures with software was faster, with higher interrater reliability than manual extraction. J. Clin. Epidemiol. 2016;74:119–123. doi: 10.1016/j.jclinepi.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 26.J. Higgins, T. Li, J. Deeks, Choosing effect measures and computing estimates of effect, in: J.P.T. Higgins, J. Thomas, J. Chandler, M. Cumpston, T. Li, M.J. Page, (Eds.), Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023), Cochrane. [Internet]. 2023 [cited October 17, 2023]. Available from: www.training.cochrane.org/handbook.
- 27.J. Higgins, S. Eldridge, T. Li, Including variants on randomized trials, in: J. Higgins, J. Thomas, J. Chandler, M. Cumpston, T. Li, M. Page, (Eds.), Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023), Cochrane [Internet]. 2023 [cited November 22, 2023]. Available from: www.training.cochrane.org/handbook.
- 28.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 29.McGuinness L.A., Higgins J.P.T. Risk-of-bias VISualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55–61. doi: 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 30.J. McKenzie, S. Brennan, Synthesizing and presenting findings using other methods, in: J.P.T. Higgins, J. Thomas, J. Chandler, M. Cumpston, T. Li, M.J. Page, (Eds.), Cochrane Handbook for Systematic Reviews of Interventions version 62 (updated February 2021) [Internet]. Cochrane 2021 [cited October 13, 2023]. Available from: www.training.cochrane.org/handbook.
- 31.Boon M.H., Thomson H. The effect direction plot revisited: application of the 2019 Cochrane Handbook guidance on alternative synthesis methods. Res. Synth. Methods. 2021;12(1):29–33. doi: 10.1002/jrsm.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crowther M., Avenell A., MacLennan G., Mowatt G. A further use for the Harvest plot: a novel method for the presentation of data synthesis. Res. Synth. Methods. 2011;2(2):79–83. doi: 10.1002/jrsm.37. [DOI] [PubMed] [Google Scholar]
- 33.Ogilvie D., Fayter D., Petticrew M., Sowden A., Thomas S., Whitehead M., et al. The harvest plot: a method for synthesising evidence about the differential effects of interventions. BMC Med. Res. Methodol. 2008;8:8. doi: 10.1186/1471-2288-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sterne J.A., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravasco P., Monteiro-Grillo I., Vidal P.M., Camilo M.E. Dietary counseling improves patient outcomes: a prospective, randomized, controlled trial in colorectal cancer patients undergoing radiotherapy. J. Clin. Oncol. 2005;23(7):1431–1438. doi: 10.1200/JCO.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 36.Ravasco P., Monteiro-Grillo I., Camilo M. Individualized nutrition intervention is of major benefit to colorectal cancer patients: long-term follow-up of a randomized controlled trial of nutritional therapy. Am. J. Clin. Nutr. 2012;96(6):1346–1353. doi: 10.3945/ajcn.111.018838. [DOI] [PubMed] [Google Scholar]
- 37.Ida S., Hiki N., Cho H., Sakamaki K., Ito S., Fujitani K., et al. Randomized clinical trial comparing standard diet with perioperative oral immunonutrition in total gastrectomy for gastric cancer. Br. J. Surg. 2017;104(4):377–383. doi: 10.1002/bjs.10417. [DOI] [PubMed] [Google Scholar]
- 38.Aoyama T., Yoshikawa T., Ida S., Cho H., Sakamaki K., Ito Y., et al. Effects of perioperative eicosapentaenoic acid-enriched oral nutritional supplement on lean body mass after total gastrectomy for gastric cancer. J. Cancer. 2019;10(5):1070–1076. doi: 10.7150/jca.29632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ueno M., Sugimori K., Taguri M., Ohkawa S., Kobayashi S., Miwa H., et al. Randomized phase II study of gemcitabine monotherapy vs. gemcitabine with an EPA-enriched oral supplement in advanced pancreatic cancer. Nutr. Cancer. 2022;74(1):122–130. doi: 10.1080/01635581.2020.1871495. [DOI] [PubMed] [Google Scholar]
- 40.Katada C., Fukazawa S., Sugawara M., Sakamoto Y., Takahashi K., Takahashi A., et al. Randomized study of prevention of gastrointestinal toxicities by nutritional support using an amino acid-rich elemental diet during chemotherapy in patients with esophageal cancer (KDOG 1101) Esophagus. 2021;18(2):296–305. doi: 10.1007/s10388-020-00787-w. [DOI] [PubMed] [Google Scholar]
- 41.Takeshita S., Ichikawa T., Nakao K., Miyaaki H., Shibata H., Matsuzaki T., et al. A snack enriched with oral branched-chain amino acids prevents a fall in albumin in patients with liver cirrhosis undergoing chemoembolization for hepatocellular carcinoma. Nutr. Res. 2009;29(2):89–93. doi: 10.1016/j.nutres.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 42.Okabayashi T., Iyoki M., Sugimoto T., Kobayashi M., Hanazaki K. Oral supplementation with carbohydrate- and branched-chain amino acid-enriched nutrients improves postoperative quality of life in patients undergoing hepatic resection, Amino. Acids. 2011;40(4):1213–1220. doi: 10.1007/s00726-010-0748-3. [DOI] [PubMed] [Google Scholar]
- 43.Akita H., Takahashi H., Asukai K., Tomokuni A., Wada H., Marukawa S., et al. The utility of nutritional supportive care with an eicosapentaenoic acid (EPA)-enriched nutrition agent during pre-operative chemoradiotherapy for pancreatic cancer: prospective randomized control study. Clin. Nutr. ESPEN. 2019;33:148–153. doi: 10.1016/j.clnesp.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Wada N., Kurokawa Y., Tanaka K., Miyazaki Y., Makino T., Takahashi T., et al. Perioperative nutritional support with beta-hydroxy-beta-methylbutyrate, arginine, and glutamine in surgery for abdominal malignancies. Wounds. 2018;30(9):251–256. [PubMed] [Google Scholar]
- 45.Tan S., Meng Q., Jiang Y., Zhuang Q., Xi Q., Xu J., et al. Impact of oral nutritional supplements in post-discharge patients at nutritional risk following colorectal cancer surgery: a randomised clinical trial. Clin. Nutr. 2021;40(1):47–53. doi: 10.1016/j.clnu.2020.05.038. [DOI] [PubMed] [Google Scholar]
- 46.Meng Q., Tan S., Jiang Y., Han J., Xi Q., Zhuang Q., et al. Post-discharge oral nutritional supplements with dietary advice in patients at nutritional risk after surgery for gastric cancer: a randomized clinical trial. Clin. Nutr. 2021;40(1):40–46. doi: 10.1016/j.clnu.2020.04.043. [DOI] [PubMed] [Google Scholar]
- 47.Yan X., Liu L., Zhang Y., Song T., Liang Y., Liu Z., et al. Perioperative enteral nutrition improves postoperative recovery for patients with primary liver cancer: a randomized controlled clinical trial. Nutr. Cancer. 2021;73(10):1924–1932. doi: 10.1080/01635581.2020.1814824. [DOI] [PubMed] [Google Scholar]
- 48.Poon R.T.P., Yu W.C., Fan S.T., Wong J. Long-term oral branched chain amino acids in patients undergoing chemoembolization for hepatocellular carcinoma: a randomized trial, Aliment. Pharmacol. Ther. 2004;19(7):779–788. doi: 10.1111/j.1365-2036.2004.01920.x. [DOI] [PubMed] [Google Scholar]
- 49.Baldwin C., Spiro A., McGough C., Norman A.R., Gillbanks A., Thomas K., et al. Simple nutritional intervention in patients with advanced cancers of the gastrointestinal tract, non-small cell lung cancers or mesothelioma and weight loss receiving chemotherapy: a randomised controlled trial. J. Hum. Nutr. Diet. 2011;24(5):431–440. doi: 10.1111/j.1365-277X.2011.01189.x. [DOI] [PubMed] [Google Scholar]
- 50.Sultan J., Griffin S.M., Di Franco F., Kirby J.A., Shenton B.K., Seal C.J., et al. Randomized clinical trial of omega-3 fatty acid-supplemented enteral nutrition versus standard enteral nutrition in patients undergoing oesophagogastric cancer surgery. Br. J. Surg. 2012;99(3):346–355. doi: 10.1002/bjs.7799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heys S.D., Ogston K., Miller I., Hutcheon A.W., Walker L.G., Sarker T.K., et al. Potentiation of the response to chemotherapy in patients with breast cancer by dietary supplementation with L-arginine: results of a randomised controlled trial. Int. J. Oncol. 1998;12(1):221–225. doi: 10.3892/ijo.12.1.221. [DOI] [PubMed] [Google Scholar]
- 52.Sathiaraj E., Afshan K., Sruthi R., Jadoni A., Murugan K., Patil S., et al. Effects of a plant-based high-protein diet on fatigue in breast cancer patients undergoing adjuvant chemotherapy–a randomized controlled trial. Nutr. Cancer. 2023;75(3):846–856. doi: 10.1080/01635581.2022.2159044. [DOI] [PubMed] [Google Scholar]
- 53.Gunsel-Yildirim G., Ceylan K.C., Dikmen D. The effect of perioperative immunonutritional support on nutritional and inflammatory status in patients undergoing lung cancer surgery: a prospective, randomized controlled study. Support Care Cancer. 2023;31(6):365. doi: 10.1007/s00520-023-07838-9. [DOI] [PubMed] [Google Scholar]
- 54.Ziętarska M., Krawczyk-Lipiec J., Kraj L., Zaucha R., Małgorzewicz S. Chemotherapy-related toxicity, nutritional status and quality of life in precachectic oncologic patients with, or without, high protein nutritional support. a prospective, randomized study. Nutrients. 2017;9(10):1108. doi: 10.3390/nu9101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yi H.C., Ibrahim Z., Abu Zaid Z., Mat Daud Z'., Md Yusop N.B., Omar J., et al. Impact of enhanced recovery after surgery with preoperative whey protein-infused carbohydrate loading and postoperative early oral feeding among surgical gynecologic cancer patients: an open-labelled randomized controlled trial. Nutrients. 2020;12(1):264. doi: 10.3390/nu12010264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faccio A.A., Mattos C.H.P.S., Santos E.A.S.D., Neto N.R.M., Moreira R.P., Tonelo Batella Luciara, et al. Oral nutritional supplementation in cancer patients who were receiving chemo/chemoradiation therapy: a multicenter, randomized phase II study. Nutr. Cancer. 2021;73(3):442–449. doi: 10.1080/01635581.2020.1758170. [DOI] [PubMed] [Google Scholar]
- 57.Arnold C., Richter M.P. The effect of oral nutritional supplements on head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 1989;16(6):1595–1599. doi: 10.1016/0360-3016(89)90968-1. [DOI] [PubMed] [Google Scholar]
- 58.Chitapanarux I., Traisathit P., Chitapanarux T., Jiratrachu R., Chottaweesak P., Chakrabandhu S., et al. Arginine, glutamine, and fish oil supplementation in cancer patients treated with concurrent chemoradiotherapy: a randomized control study. Curr. Probl. Cancer. 2020;44(1) doi: 10.1016/j.currproblcancer.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 59.Poulsen G.M., Pedersen L.L., Østerlind K., Bæksgaard L., Andersen J.R. Randomized trial of the effects of individual nutritional counseling in cancer patients. Clin. Nutr. 2014;33(5):749–753. doi: 10.1016/j.clnu.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 60.Moya P., Miranda E., Soriano-Irigaray L., Arroyo A., Aguilar M.D.M., Bellón M., et al. Perioperative immunonutrition in normo-nourished patients undergoing laparoscopic colorectal resection. Surg. Endosc. 2016;30(11):4946–4953. doi: 10.1007/s00464-016-4836-7. [DOI] [PubMed] [Google Scholar]
- 61.Gianotti L., Braga M., Nespoli L., Radaelli G., Beneduce A., Di Carlo V. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology. 2002;122(7):1763–1770. doi: 10.1053/gast.2002.33587. [DOI] [PubMed] [Google Scholar]
- 62.Ritch C.R., Cookson M.S., Clark P.E., Chang S.S., Fakhoury K., Ralls V., et al. Perioperative oral nutrition supplementation reduces prevalence of sarcopenia following radical cystectomy: results of a prospective randomized controlled trial. J. Urol. 2019;201(3):470–477. doi: 10.1016/j.juro.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 63.Choi K., Lee S.S., Oh S.J., Lim S.Y., Lim S.Y., Jeon W.K., et al. The effect of oral glutamine on 5-fluorouracil/leucovorin-induced mucositis/stomatitis assessed by intestinal permeability test. Clin. Nutr. 2007;26(1):57–62. doi: 10.1016/j.clnu.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 64.Ravasco P., Monteiro-Grillo I., Marques Vidal P., Camilo M.E. Impact of nutrition on outcome: a prospective randomized controlled trial in patients with head and neck cancer undergoing radiotherapy. Head Neck. 2005;27(8):659–668. doi: 10.1002/hed.20221. [DOI] [PubMed] [Google Scholar]
- 65.Serrano P.E., Parpia S., Simunovic M., Duceppe E., Pinto-Sanchez M.I., Bhandari M., et al. Perioperative optimization with nutritional supplements in patients undergoing gastrointestinal surgery for cancer: a randomized, placebo-controlled feasibility clinical trial. Surgery. 2022;172(2):670–676. doi: 10.1016/j.surg.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 66.Laviano A., Calder P.C., Schols A.M.W.J., Lonnqvist F., Bech M., Muscaritoli M. Safety and tolerability of targeted medical nutrition for cachexia in non-small-cell lung cancer: a randomized, double-blind, controlled pilot trial. Nutr. Cancer. 2020;72(3):439–450. doi: 10.1080/01635581.2019.1634746. [DOI] [PubMed] [Google Scholar]
- 67.Rotovnik Kozjek N., Kompan L., Žagar T., Mrevlje Ž. Influence of enteral glutamine on inflammatory and hormonal response in patients with rectal cancer during preoperative radiochemotherapy. Eur. J. Clin. Nutr. 2017;71(5):671–673. doi: 10.1038/ejcn.2017.11. [DOI] [PubMed] [Google Scholar]
- 68.Chang SC, Lai YC., Hung JC., Chang CY. Oral glutamine supplements reduce concurrent chemoradiotherapy-induced esophagitis in patients with advanced non-small cell lung cancer. Medicine. (Baltimore) 2019;98(8) doi: 10.1097/MD.0000000000014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sánchez-Lara K., Turcott J.G., JuárezHernández E., Nuñez-Valencia C., Villanueva G., Guevara P., et al. Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin. Nutr. 2014;33(6):1017–1023. doi: 10.1016/j.clnu.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Pandey M., Gaurav K., Singh S., Goel R.K. Safety and efficacy of oral and parenteral glutamine supplementation in reducing chemotherapy induced toxicities in patients with breast cancer receiving chemotherapy: results of interim analysis of a prospective, randomized, three arm, placebo-controlled. World J. Surg. 2012;1:17–27. [Google Scholar]
- 71.Braga M., Gianotti L., Vignali A., Carlo V.D. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery. 2002;132(5):805–814. doi: 10.1067/msy.2002.128350. [DOI] [PubMed] [Google Scholar]
- 72.Jensen G.L., Cederholm T., Correia M.I.T.D., Gonzalez M.C., Fukushima R., Higashiguchi T., et al. GLIM criteria for the diagnosis of malnutrition: a consensus report from the global clinical nutrition community. JPEN J. Parenter. Enteral. Nutr. 2019;43(1):32–40. doi: 10.1002/jpen.1440. [DOI] [PubMed] [Google Scholar]
- 73.Cederholm T., Jensen G.L., Correia M.I.T.D., Gonzalez M.C., Fukushima R., Higashiguchi T., et al. GLIM criteria for the diagnosis of malnutrition – a consensus report from the global clinical nutrition community. Clin. Nutr. 2019;38(1):1–9. doi: 10.1016/j.clnu.2019.02.033. [DOI] [PubMed] [Google Scholar]
- 74.Gannavarapu B.S., Lau S.K.M., Carter K., Cannon N.A., Gao A., Ahn C., et al. Prevalence and survival impact of pretreatment cancer-associated weight loss: a tool for guiding early palliative care. J. Oncol. Pract. 2018;14(4):e238–e250. doi: 10.1200/JOP.2017.025221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hue J.J., Sugumar K., Kyasaram R.K., Shanahan J., Lyons J., Ocuin L.M., et al. Weight loss as an untapped early detection marker in pancreatic and periampullary cancer. Ann. Surg. Oncol. 2021;28(11):6283–6292. doi: 10.1245/s10434-021-09861-8. [DOI] [PubMed] [Google Scholar]
- 76.Thirunavukarasu P., Sanghera S., Singla S., Attwood K., Nurkin S. Pre-operative unintentional weight loss as a risk factor for surgical outcomes after elective surgery in patients with disseminated cancer. Int. J. Surg. 2015;18:7–13. doi: 10.1016/j.ijsu.2015.03.021. [DOI] [PubMed] [Google Scholar]
- 77.Hall K.D., Guo J. Obesity energetics: body weight regulation and the effects of diet composition. Gastroenterology. 2017;152(7):1718–1727.e3. doi: 10.1053/j.gastro.2017.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Westerterp K.R. Metabolic adaptations to over- and underfeeding – still a matter of debate? Eur. J. Clin. Nutr. 2013;67(5):443–445. doi: 10.1038/ejcn.2012.187. [DOI] [PubMed] [Google Scholar]
- 79.Phillips S.M., Glover E.I., Rennie M.J. Alterations of protein turnover underlying disuse atrophy in human skeletal muscle. J. Appl. Physiol. 2009;107(3):645–654. doi: 10.1152/japplphysiol.00452.2009. 1985. [DOI] [PubMed] [Google Scholar]
- 80.van der Meij B.S., Teleni L., Engelen M.P.K.J., Deutz N.E.P. Amino acid kinetics and the response to nutrition in patients with cancer. Int. J. Radiat. Biol. 2019;95(4):480–492. doi: 10.1080/09553002.2018.1466209. [DOI] [PubMed] [Google Scholar]
- 81.Laviano A., Medicine P. Current guidelines for nutrition therapy in cancer: the arrival of a long journey or the starting point? JPEN J. Parenter. Enteral. Nutr. 2021;45(S2):12–15. doi: 10.1002/jpen.2288. [DOI] [PubMed] [Google Scholar]
- 82.Regueme S.C., Echeverria I., Monéger N., Durrieu J., Becerro-Hallard M., Duc S., et al. Protein intake, weight loss, dietary intervention, and worsening of quality of life in older patients during chemotherapy for cancer, Support. Care Cancer. 2021;29(2):687–696. doi: 10.1007/s00520-020-05528-4. [DOI] [PubMed] [Google Scholar]
- 83.Wheelwright S., Darlington A.S., Hopkinson J.B., Fitzsimmons D., White A., Johnson C.D. A systematic review of health-related quality of life instruments in patients with cancer cachexia. Support. Care Cancer. 2013;21(9):2625–2636. doi: 10.1007/s00520-013-1881-9. [DOI] [PubMed] [Google Scholar]
- 84.Deutz N.E.P. Basics in clinical nutrition: protein and amino acid metabolism. E. Spen. Eur. E. J. Clin. Nutr. Metab. 2008;3:e185–e187. doi: 10.1016/j.eclnm.2008.06.002. [DOI] [Google Scholar]
- 85.Calder P.C. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006;83(Suppl 6):1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 86.Lee J.H., Park E., Jin H.J., Lee Y., Choi S.J., Lee G.W., et al. Anti-inflammatory and anti-genotoxic activity of branched chain amino acids (BCAA) in lipopolysaccharide (LPS) stimulated RAW 264.7 macrophages. Food. Sci. Biotechnol. 2017;26(5):1371–1377. doi: 10.1007/s10068-017-0165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prado C.M., Orsso C.E., Pereira S.L., Atherton P.J., Deutz N.E.P. Effects of β-hydroxy β-methylbutyrate (HMB) supplementation on muscle mass, function, and other outcomes in patients with cancer: a systematic review. J. Cachexia Sarcopenia Muscle. 2022;13(3):1623–1641. doi: 10.1002/jcsm.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Engelen M.P., van der Meij B.S., Deutz N.E. Protein anabolic resistance in cancer: does it really exist? Curr. Opin. Clin. Nutr. Metab. Care. 2016;19(1):39–47. doi: 10.1097/MCO.0000000000000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang J.E., Moore D.R., Kujbida G.W., Tarnopolsky M.A., Phillips S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009;107(3):987–992. doi: 10.1152/japplphysiol.00076.2009. 1985. [DOI] [PubMed] [Google Scholar]
- 90.Bozzetti F., Stanga Z. Does nutrition for cancer patients feed the tumour? A clinical perspective. Crit. Rev. Oncol. Hematol. 2020;153 doi: 10.1016/j.critrevonc.2020.103061. [DOI] [PubMed] [Google Scholar]
- 91.Deldicque L., Theisen D., Francaux M. Regulation of mTOR by amino acids and resistance exercise in skeletal muscle. Eur. J. Appl. Physiol. 2005;94(1–2):1–10. doi: 10.1007/s00421-004-1255-6. [DOI] [PubMed] [Google Scholar]
- 92.Boutière M., Cottet-Rousselle C., Coppard C., Couturier K., Féart C., Couchet M., et al. Protein intake in cancer: does it improve nutritional status and/or modify tumour response to chemotherapy? J. Cachexia Sarcopenia Muscle. 2023;14(5):2003–2015. doi: 10.1002/jcsm.13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lam C.N., Watt A.E., Isenring E.A., de van der Schueren M.A.E., van der Meij B.S. The effect of oral omega-3 polyunsaturated fatty acid supplementation on muscle maintenance and quality of life in patients with cancer: a systematic review and meta-analysis. Clin. Nutr. 2021;40(6):3815–3826. doi: 10.1016/j.clnu.2021.04.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending approval from the corresponding author.