Abstract
Objective:
To investigate the longitudinal effects of COVID-19 on major vascular structures and parameters and clinical outcomes.
Design:
Observational prospective trial.
Setting:
Post-COVID-19 research clinic established by University of Louisville Division of Infectious Diseases.
Participants:
The study population consisted of 72 post-COVID-19 individuals and 11 non-COVID-19 infected participants in the control group. The participants were recruited from adult hospitals and from the community. The enrollment started in October 2020 and follow-up periods were at 3, 6, and 12 months from their initial COVID-19 diagnosis.
Interventions:
The participants were interviewed for medical and COVID-19 infection history. Samples of white blood cell (WBC), C-reactive protein (CRP), and D-dimer were taken at each visit. Certified sonographers performed vascular ultrasound on the study participants.
Measurements and Main Results:
Median intima-media thickness (IMT) was increased in mild/asymptomatic (0.80 mm) and severe/critical (0.90 mm) groups when compared with controls (0.60 mm; P < .001 for both groups). In the asymptomatic/mild group, 6-month median IMT (0.88 mm) was increased, compared with the 3-month group (0.75 mm), with P = .026. Increased age was associated with decreased mean arterial blood velocities (cm/s): common carotid (r = −0.236, P = .032), internal carotid (r = −0.208, P = .048), and subclavian artery mean velocity (r = −0.357, P = .003). We did not find any instance of deep vein thrombosis. Median D-dimer, CRP, and WBC in the control group differed from asymptomatic/mild COVID-19 group (P = .026, .011, and .003, respectively). Moreover, WBC in the asymptomatic/mild group and moderate COVID-19 group differed from severe/critical group (P = .025 and P = .027, respectively); CRP also differed between asymptomatic/mild group and severe/critical group (P = .014).
Conclusions:
There were differences in intima-media lumen thickness (IMT), arterial velocities, and inflammatory markers in post-COVID-19 patients. There was no instance of deep vein thrombosis in this post-COVID-19 study cohort. The increased IMT might infer atherosclerosis, which has shown to increase cardiovascular risks. It is not yet known whether the increase in IMT due to COVID should be treated in the same way as non-COVID-19 atherosclerosis—through statins, for example—or whether regular cardiovascular risk reduction would be useful. Clinical trial and mechanistic studies should be performed to further our understanding of COVID-19-related vascular pathologies.
Keywords: intima-media lumen thickness (IMT), endothelial dysfunction, COVID-19 infection, deep vein thrombosis (DVT), arterial blood velocities, vascular ultrasound, vascular dysfunction
Background
As of January 31, 2023, more than 670 million people had been infected from COVID-19, with an estimated 6.8 million fatalities worldwide,1 yet there remains a scarcity of longitudinal data concerning symptom etiology, estimated recovery time, and long-term consequences on multiple organ systems.2,3
For hospitalized COVID-19 patients, severity of infection appears to drive morbidity and mortality. Vascular effects are thought to be exacerbated by preexisting comorbidities, such as hypertension, diabetes, age, and hyperlipidemia due to increased structural, metabolic, and blood pressure derangements.4–7
The duration of the hyperinflammatory state driving disease progression remains unknown.8 A population-wide cohort analysis of 48 million adults in England and Wales found increased hazard ratios in COVID-19-infected individuals compared with non–COVID-19-infected individuals for arterial thrombosis, venous thromboembolism events, and other vascular events, extending to at least 49 weeks postinfection.9
Less is known about long-term, post-COVID-19 vascular structural and functional effects, such as intima-media thickness (IMT) and arterial blood velocities.10–12 Understanding the long-term effects of COVID-19 will be beneficial, as we transition from acute pandemic to long-term endemic state. Given that there are limited data, this study aims to investigate the longitudinal effects of COVID-19 on vascular structures, including arteries and veins, as well as the correlation between vascular parameters and clinical outcomes after COVID-19 infection.
Methods
Study Design
This observational prospective study was undertaken by the University of Louisville Division of Infectious Diseases to help understand the impact of COVID-19 on the vasculature of previously infected patients.
Human Participants Protection
This study was approved by the institutional review board (IRB) at the University of Louisville Human Subjects Research Protection Program Office (IRB number 20.0557) and by research offices at any participating hospital. All study participants gave informed consent. Standard data security procedures were utilized and approved by IRB to safeguard private health care information.
Study Setting and Participants
The University of Louisville Division of Infectious Diseases set up a multidisciplinary COVID-19 outpatient research clinic to examine the short-term and long-term effects of COVID-19. Participants were recruited primarily from 8 adult hospitals as well as from the general community through e-mails and phone calls in Louisville, KY, USA. Enrollment started in October 2020. Study participants came to this clinic at 3-, 6-, and 12-month intervals from their initial COVID-19 diagnosis. Inclusion criteria: (1) more than 18 years of age, (2) consent to participate in this research, and (3) confirmed SARS-CoV-2 infection by RT-PCR or serological tests. Exclusion criteria: refusal to participate in this research.
Classification of COVID-19 Infection Severity
The COVID-19 patients were classified as “asymptomatic/mild,” “moderate,” and “severe/critical” based on the severity of the initial COVID-19 infection. Asymptomatic/mild participants did not require hospitalization at the time of the initial infection. Moderate participants required hospitalization but did not require mechanical ventilation nor admission to the intensive care unit. Severe/critical participants were hospitalized at the time of the initial infection and required invasive mechanical ventilation and/or admission to the intensive care unit.
Data Collection
Participants completed questionnaires and surveys that included their COVID-19 test results, history of COVID-19 infection, course of disease, demographics, socioeconomic status, past medical and social history, current medications, medication history, prior vaccination history, allergy history, and review of systems/symptoms relevant to initial and postacute COVID-19 infection. We obtained blood specimens at each visit to examine white blood cell (WBC), C-reactive protein (CRP), and D-dimer. The remaining specimens were retained in the Infectious Diseases’ biorepository at the University of Louisville.
Post-COVID-19 Assessments
Vascular ultrasound.
Certified sonographers used high-resolution grayscale imaging, color Doppler ultrasound, and spectral analysis with pulse wave Doppler to examine bilateral upper and lower extremity venous and arterial systems, and carotid arteries for thrombosis, atheroma, and stenosis. Study participants had blood samples taken and underwent full echocardiographic, vascular, and lung ultrasound examinations at each visit. The sonographer was blinded to COVID-19 status and was instructed to not discuss COVID-19 status with the study participant.
Board certified physicians who passed the Examination of Special Competence in Critical Care Echocardiography (CCeXAM), performed readings on vascular ultrasound studies that included scans for deep vein thromboses in bilateral internal jugular, subclavian, axillary, femoral, and deep profunda veins. The physicians also measured bilateral common carotid artery IMT. Finally, bilateral systolic and diastolic blood velocities were calculated in common and internal carotid, subclavian, and femoral arteries. Data were entered into a spreadsheet with a coding system and forwarded to statisticians for analysis.
For IMT measurements, regions of interest were zoomed in. Using calipers, the distance between the leading edges of the lumen-intima interface and the media-adventitia interface were measured. Three different points were measured and the thickest measurement was recorded unless it was a clear outlier. All the zoomed images and measured caliper distances have been stored and referenced.
Quality of life assessments: EuroQol 5 dimension.
Participants also completed the EuroQol 5 dimension (EQ-5D-5L) questionnaire consisting of the EQ-5D descriptive system and EQ visual analog scale (VAS). Mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, are measured on a 5-point scale: no problems, slight, moderate, severe, and extreme problems. EQ-VAS reports subjective health on a vertical scale from 0 to 100, with the endpoints labeled, “The best health you can imagine” and “The worst health you can imagine.”13
Data Analysis
The data were collected at 3-, 6-, and 12-month intervals from hospital discharge or symptom resolution. Continuous measurements were summarized by median and interquartile range (IQR; Q1, Q3), and categorical variables were summarized by counts and percentages. Comparison among the severity of infection groups was performed using Kruskal-Wallis test for continuous variables and Fisher Exact Test for categorical variables. Comparison over different time intervals was performed using generalized mixed effect models, where time points were treated as fixed effects and each participant was treated as a random effect. Pearson’s correlation coefficients were used to evaluate the correlation between 2 different variables. A statistical test was claimed significant if P < .05. The analyses were carried out in Statistical software R (https://www.r-project.org/)
Results
Our study focused on assessing vascular ultrasound parameters stratified by infection severity, inflammatory marker levels (WBC, CRP, and D-Dimer), and quality of life metrics.
Our sample population consisted of 72 post-COVID-19 patients in the study group and 11 COVID-19 noninfected participants in the control group. Among the 72 post-COVID-19 patients, there were 52 (72%) asymptomatic/mild cases, 10 (14%) moderate cases, and 10 (14%) severe/critical cases. (Table 1)
Table 1.
Participant Characteristics and Control Group.
| Study group (count, %) | Control group (count, %) | |||||
|---|---|---|---|---|---|---|
| Men | Women | Total | Men | Women | Total | |
| Asymptomatic/mild | 16 (73%) | 36 (72%) | 52 (72%) | — | — | — |
| Moderate | 2 (9%) | 8 (16%) | 10 (14%) | — | — | — |
| Severe/critical | 4 (18%) | 6 (12%) | 10 (14%) | — | — | — |
| Total | 22 | 50 | 72 | 4 | 7 | 11 |
| Hospitalized | 6 (27.3%) | 14 (28%) | 20 (27.8%) | — | — | — |
| Age, years | ||||||
| <40 | 3 (14%) | 11 (22%) | 14 (19%) | 3 (75%) | 4 (57%) | 7 (64%) |
| 40–49 | 1 (5%) | 3 (6%) | 4 (6%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 50–59 | 7 (32%) | 12 (24%) | 19 (26%) | 1 (25%) | 1 (14%) | 2 (18%) |
| 60–69 | 8 (36%) | 15 (30%) | 23 (32%) | 0 (0%) | 2 (29%) | 2 (18%) |
| ≥70 | 3 (14%) | 9 (18%) | 12 (17%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Total | 22 | 50 | 72 | 4 | 7 | 11 |
| Comorbidities | ||||||
| Vascular comorbidities | ||||||
| Age >70 years | 3 (14%) | 8 (16%) | 11 (15%) | — | — | — |
| BMI >35 | 5 (23%) | 9 (18%) | 14 (19%) | 0 (0%) | 1 (14%) | 1 (9%) |
| HTN | 6 (27%) | 18 (36%) | 24 (33%) | 0 (0%) | 1 (14%) | 1 (9%) |
| Hyperlipidemia | 8 (38%) | 9 (18%) | 17 (24%) | 2 (50%) | 2 (29%) | 4 (36%) |
| Diabetes | 4 (18%) | 5 (10%) | 9 (13%) | — | — | — |
| Coronary artery disease | 4 (19%) | 2 (4%) | 6 (8%) | — | — | — |
| Stroke | 0 (0%) | 1 (2%) | 1 (1%) | — | — | — |
| Renal disease | 2 (9%) | 1 (2%) | 3 (4%) | 0 (0%) | 1 (14%) | 1 (9%) |
| Other comorbidities | ||||||
| Deep vein thrombosis | 0 (0%) | 4 (9%) | 4 (6%) | — | — | — |
| Pulmonary embolism | 1 (5%) | 1 (2%) | 2 (3%) | — | — | — |
| Anticoagulation | ||||||
| Lung disease | 9 (41%) | 17 (34%) | 26 (36%) | 0 (0%) | 1 (14%) | 1 (9%) |
| Home O2 therapy | 2 (9%) | 4 (8%) | 6 (8%) | — | — | — |
| Liver disease | 0 (0%) | 2 (4%) | 2 (3%) | 0 (0%) | 1 (14%) | 1 (9%) |
Note. BMI = body mass index; HTN = hypertension.
Inflammatory markers D-Dimer (mg/L), CRP (mg/L), and WBC (10−3/uL) were different between control and asymptomatic/mild study groups (P = .026, P = .011, and P = .003, respectively). Severe/critical infection participants had significantly higher CRP (median 2.52; IQR [1.31–4.84]), and higher WBC (median 7.65; IQR [6.37–9.35]) when compared with asymptomatic/mild participants with CRP (median 0.97; IQR [0.53–2.2]) and WBC (median 5.8; IQR [5–7.1]), respectively, with P values of .014, and .027, respectively. (Table 2).
Table 2.
Labs by Time and Severity.
| Study group | ||||
| Lab | Control group | Follow-up period (months) | ||
| 3 | 6 | >12 | ||
| WBC | 6.5 (4.5–7.4) | 6.3 (6.0–6.8) | 5.8 (5.0–7.5) | 7.0 (5.2–8.0) |
| Lab | Control group | Severity | ||
| Asymp/mild | Mod | Severe/critical | ||
| WBC | 6.5 (4.5–7.4) | 5.8 (5–7.1) | 7.3 (7.1–8.2) | 7.7 (6.4–9.4) |
| P values | ||||
| Lab | Control versus | Asympt/mild versus | ||
| All severity | Asympt/mild | Moderate | Severe/critical | |
| WBC | .384 | .003 | .025 | .027 |
Note. Data presented are median (interquartile range: low 25%, high 25%). CRP = C-reactive protein; WBC = white blood cell; Asympt/mild = asymptomatic/mild: not hospitalized; Mod = moderate: hospitalized but not requiring ICU; severe/critical = hospitalized, requiring mechanical intubation, +/− ICU admission.
D-dimer and age were positively correlated with higher IMT (Pearson’s correlation coefficient, r = 0.292, P = .011 and r = 0.600, P < .001, respectively). Increasing age was also associated with decreased mean arterial blood velocities (cm/s): common carotid (r = −0.236, P = .032), internal carotid (r = −0.208, P = .048), and subclavian artery mean velocity (r = −0.357, P = .003). All showed significant negative Pearson’s correlation coefficients (Table 3).
Table 3.
Labs and Age Correlation to Arterial Blood Velocities and IMT.
| Correlation coefficient | P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Common carotid artery mean velocity | Internal carotid artery mean velocity | Subclavian artery mean velocity | Femoral artery mean velocity | Mean IMT | Common carotid artery mean velocity | Internal carotid artery mean velocity | Subclavian artery mean velocity | Femoral artery mean velocity | Mean IMT | |
| D-Dimer | −0.046 | 0.045 | −0.020 | −0.053 | 0.292 | .700 | .710 | .886 | .686 | .011 |
| CRP | −0.042 | −0.018 | −0.098 | 0.082 | 0.011 | .721 | .882 | .472 | .530 | .927 |
| WBC | 0.044 | 0.058 | 0.047 | 0.182 | −0.037 | .721 | .638 | .735 | .164 | .761 |
| Age | −0.236 | −0.208 | −0.357 | −0.222 | 0.600 | .032 | .048 | .003 | .055 | <.001 |
Note. IMT = intima media lumen thickness; CRP = C-reactive protein; WBC = white blood cell.
Control group median IMT (0.60 mm, range = 0.45–0.80 mm) differed significantly from asymptomatic/mild group (0.80 mm, range = 0.60–1.25 mm) and severe/critical group (0.9 mm, range = 0.70–1.25 mm), with P < .001 for both comparisons (Table 4). Asymptomatic/mild median IMT was higher at 6 months versus 3 months post infection (0.75 mm vs 0.88 mm, P = .026); however, no other significant differences were noted in comparing median longitudinal IMT postinfection, neither in aggregate nor by severity.
Table 4.
Median Intima-Media Lumen Thickness (mm) by Severity.
| Severity | P value | ||||
|---|---|---|---|---|---|
| Control | Asympt/mild | Mod | Severe/critical | ||
| Median | 0.60 | 0.80 | 0.75 | 0.90 | <.001 |
| High | 0.80 | 1.25 | 0.85 | 1.25 | |
| Low | 0.45 | 0.60 | 0.45 | 0.70 | |
| P value vs control | <0.001 | 0.87 | <0.001 | ||
As arterial velocities could be significantly affected by variable cardiac physiologic factors, we performed correlation analysis with echocardiographic parameters. Lower right ventricular internal diameter in diastole (RVIDd)/BSA (r = −0.25, P = .016), higher left ventricular (LV) volume in diastole/BSA (r = 0.259, P = .019), and a lower left atrial (LA) volume/BSA (r = −0.282, P = .01) were associated with the higher common carotid artery mean velocity. A shorter pulmonary artery (PA) acceleration time (indicating higher PA pressure) was associated with lower internal carotid artery mean velocity (r = 0.228, P = .026) and a lower subclavian artery mean velocity (r = 0.296, P = .012). Higher LV ejection fraction (LVEF) was associated with higher femoral artery mean velocity (r = 0.223, P = .043) and higher right atrial (RA) volume/BSA was associated with higher IMT (r = 0.252, P = .014) in post-COVID-19 patients (Figure 1).
Figure 1.
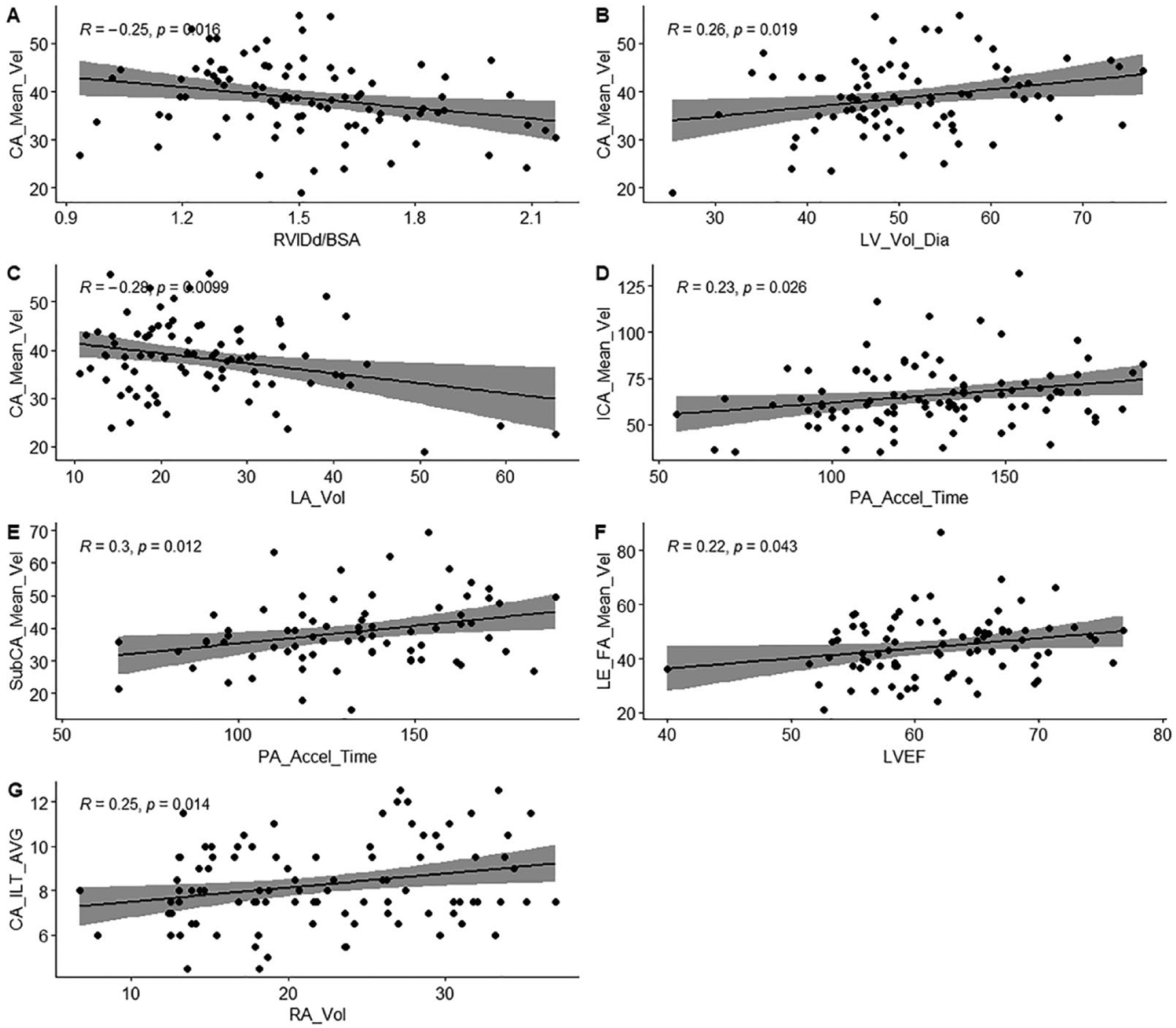
(A-G): Correlations between blood velocities and cardiac function.
There was no deep vein thrombosis (DVT) nor venous abnormalities identified in any of the COVID-19 patients in our cohort.
Finally, there was no significant correlation between vascular parameters and quality of life metrics (ie, mobility, activities, pain, anxiety, depression, and PTSD [post traumatic stress disorder]).
Discussion
It appears that SARS-CoV-2 preferentially infects organ systems with high concentrations of the ACE-2 receptor including all vasculature structures. Although specific mechanisms of action are debated, subsequent damage to endothelial cells exposes the underlying basement membrane and triggers an inflammatory/cytokine reaction. Such fundamental mechanisms may drive increased risk of acute myocardial infarction, ischemic/hemorrhagic stroke, DVT, pulmonary embolism, angina, and heart failure.9,14
Data on long-term consequences of COVID-19 on vascular function are sparse. Ratchford et al15 and Szeghy et al10 examined vascular function using Doppler ultrasound measuring flow-mediated dilation (FMD) in the arm and passive leg movement in small samples of COVID-19-infected individuals versus healthy controls. In symptomatic young adults, they found impaired peripheral macrovascular and microvascular vasodilation. Large-scale studies such as the COVID-19 Effects on Arterial Stiffness and Vascular Aging (CARTESIAN) trial will provide insight into the relationship between the severity of COVID-19 and early vascular aging; unfortunately, CARTESIAN is not due to be completed until March 2033.16
This study of post-COVID-19 patients demonstrates pertinent findings regarding IMT, arterial blood velocities, and inflammatory markers and DVT.
Common Carotid IMT
With respect to COVID-19, IMT was positively correlated with increased age, D-dimer levels, and disease severity. The IMT is an indicator of potential premature atherosclerosis and future cardiovascular diseases17,18 and could thus denote significant public health impacts, given the number of COVID-19-infected individuals worldwide.
To our knowledge, there are 2 other studies that investigate COVID-19 and IMT: Szeghy et al10 compared 15 young healthy adults with 15 young adults 3 to 4 weeks after contracting COVID-19. The IMT was similar between groups (0.42 mm ± 0.06 vs 0.44 mm ± 0.08; P > .05). Jud et al19 found higher common carotid, axillary, and superficial femoral IMT in COVID-19 patients (0.59 mm, 0.58mm, and 0.54 mm, respectively) compared with healthy controls (0.44 mm, 0.40 mm, and 0.40 mm, respectively) with P < .0001; however, this study also had a very small sample size (14 post COVID-19 patients, 14 controls with atherosclerotic cardiovascular disease, and 14 healthy controls).
COVID-19 disease severity and IMT.
This study found increased IMT in all COVID-19 patients, most notably between our asymptomatic/mild and severe/critical groups, when compared with controls. It is unclear whether participants studied have higher IMTs because they were predisposed to more severe COVID symptoms or whether participants had more severe comorbidities (high comorbid states typically exhibit diabetes, obesity, or heart problems with enhanced vascular diseases). Although there was no statistically significant correlation with follow-up time, IMT appears to trend toward normal over time. Even with this tren d, it is unclear whether IMT ever normalizes to preinfection levels.
D-dimer, age, and IMT in post-COVID-19 patients.
These associations are not surprising and are not exclusive to COVID-19. Hayashi et al20 demonstrated these associations and found that D-dimer was significantly associated with thrombosis in highly atherosclerotic patients.
Blood Velocities
This study found a statistically significant relationship between age and reduced common carotid, internal carotid, subclavian, and femoral artery blood velocities in post-COVID-19 patients. Although increased age generally imputes higher blood velocities, increased age also increases the likelihood of vascular defects; moreover, COVID-19 likely affects the vascular endothelium,21–24 which could reduce compensatory mechanisms that maintain blood velocities. Whether these decreased velocities cause permanent vascular defects is unknown and deserve a longer follow-up study.
Blood Velocities Correlated With Echocardiographic Parameters
This study found multiple statistically significant correlations between echocardiographic parameters and various blood velocities (see “Results” section and Figure 1). However, it is unclear whether these correlations, or lack thereof, are due to COVID-19-associated vascular injury or some other confounder. We evaluated the correlation between cardiac functions and blood velocities. Specifically, we studied LVEF and velocity time integral (left heart function), and right heart systolic function by TAPSE. There were no statistically significant correlations between peripheral vessel velocities and cardiac parameters.
Inflammatory Markers
It was found that D-dimer, CRP, and WBC were significantly correlated with COVID-19 disease severity. This is consistent with previous research associating these inflammatory markers with COVID-19 disease severity.25–27
Deep Vein Thrombosis
No sonographic evidence of DVT was identified in the study group across all ranges of COVID-19 severity. It should be noted that 4 out of 5 study participants reported having DVT prior to contracting COVID-19. The remaining participant did not report a timing associated with COVID-19 infection. Therefore, this negative finding is likely due to the lack of initial pathology in the study group correlated with COVID-19 infection.
Quality of Life Parameters
Several studies have shown that quality of life is associated with post-COVID-19 cases,28 with improvement in quality of life from 3 months to 12 months postinfection.29 However, this study found no correlation between vascular parameters and quality of life metrics (mobility, self-care, activities, pain/discomfort, and anxiety/depression).
Limitations
There were several limitations for this study. Our small sample size inherently increased the likelihood for Type II error. Moreover, small sample sizing cannot risk adjust for confounding factors; for example, it is not clear whether IMT, WBC, D-dimer, and/or CRP increase due to COVID-19 infection or due to other confounders such as age. Small sample size reduces the generalizability to the larger populations. Second, control and study groups differ in age and comorbidities; the smaller control group was essentially younger and healthier individuals recruited through the research department and so direct comparisons must be interpreted with caution. Specifically, IMT increases with aging30 and age may confound study findings. Third, medical history was obtained from interviewing our participants; however, we were not able to obtain specific information, such as specific prior IMT and blood velocity data, which would make helpful before/after COVID-19 infection comparisons. Finally, data on the SARS-CoV-2 variant responsible for infection were not available and different variants may produce altered impacts on the vasculature.
Strengths
Our study adds to a very limited data set regarding IMT and blood velocities in post-COVID-19 patients. To our knowledge, there were only 2 studies available that investigated IMT, and both studies were smaller in sample size. There are multiple studies that investigate cardiovascular hazard ratios through observational cohort analysis, but the information specifically on ultrasound evaluation for DVTs in post-COVID-19 is limited. In addition, we are not aware of studies evaluating blood velocities in relation to COVID-19 severity or in a post infection longitudinal time frame.
Conclusions
There was no instance of DVT in this post-COVID-19 study cohort. There were significant differences in IMT, arterial velocities, and inflammatory markers in post-COVID-19 patients. Increased IMT might infer atherosclerosis, which has shown to increase cardiovascular risks. But it is not yet known whether the increase in IMT due to COVID-19 should be treated in the same way as non-COVID-19 atherosclerosis—through statins, for example—or whether regular cardiovascular risk reduction would be useful. Clinical trial and mechanistic studies should be performed to further our understanding of COVID-19-related vascular pathologies.
Acknowledgments
The authors are grateful to their study participants who volunteered their time to help advance the authors’ understanding of how COVID-19 affects vasculature.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Center for Advancing Translational Sciences [grant no. U18TR003787], the National Institute of Environmental Health Sciences (grant no. P30 [P30ES030283]), and Gilead Sciences COMMIT COVID-19 RFP Program [grant no. IN-US-983-6063]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Gilead Sciences.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Johns Hopkins University. COVID-19 dashboard. http://corona-virus.jhu.edu/map.html. Accessed January 31, 2023.
- 2.Raveendran AV, Jayadevan R, Sashidharan S. Long COVID: an overview. Diabetes Metab Syndr. 2021;15(3):869–875. doi: 10.1016/j.dsx.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis HE, McCorkell L, Vogel JM, Topol EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. 2023;21(3):133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging. 2020;12(7):6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knight R, Walker V, Ip S, et al. Association of COVID-19 with major arterial and venous thrombotic diseases: a population-wide cohort study of 48 million adults in England and Wales. Circulation. 2022;146(12):892–906. doi: 10.1161/CIRCULATIONAHA.122.060785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szeghy RE, Province VM, Stute NL, et al. Carotid stiffness, intima-media thickness and aortic augmentation index among adults with SARS-CoV-2. Exp Physiol. 2022;107(7):694–707. doi: 10.1113/EP089481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeed S, Mancia G. Arterial stiffness and COVID-19: a bidirectional cause-effect relationship. J Clin Hypertens (Greenwich). 2021;23(6):1099–1103. doi: 10.1111/jch.14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cristina-Oliveira M, Meireles K, Gil S, et al. Carotid intima-media thickness and flow-mediated dilation do not predict acute in-hospital outcomes in patients hospitalized with COVID-19. Am J Physiol Heart Circ Physiol. 2022;322(6):H906–H913. doi: 10.1152/ajpheart.00026.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28(3):583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ratchford SM, Stickford JL, Province VM, et al. Vascular alterations among young adults with SARS-CoV-2. Am J Physiol Heart Circ Physiol. 2021;320(1):H404–H410. doi: 10.1152/ajp-heart.00897.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruno RM, Spronck B, Hametner B, et al. Covid-19 effects on arterial stiffness and vascular ageing: Cartesian study rationale and protocol. Artery Res. 2020;27(2):59. doi: 10.2991/artres.k.201124.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polak JF, Pencina MJ, Pencina KM, et al. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365(3):213–221. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Willeit P, Tschiderer L, Allara E, et al. Carotid intima-media thickness progression as surrogate marker for cardiovascular risk: meta-analysis of 119 clinical trials involving 100 667 patients. Circulation. 2020;142(7):621–642. doi: 10.1161/CIRCULATIONAHA.120.046361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jud P, Gressenberger P, Muster V, et al. Evaluation of endothelial dysfunction and inflammatory vasculopathy after SARS-CoV-2 infection–a cross-sectional study. Front Cardiovasc Med. 2021;8:750887. doi: 10.3389/fcvm.2021.750887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayashi S Significance of plasma D-dimer in relation to the severity of atherosclerosis among patients evaluated by non-invasive indices of cardio-ankle vascular index and carotid intima-media thickness. Int J Hematol. 2010;92(1):76–82. doi: 10.1007/s12185-010-0622-9. [DOI] [PubMed] [Google Scholar]
- 21.Schnaubelt S, Oppenauer J, Tihanyi D, et al. Arterial stiffness in acute COVID-19 and potential associations with clinical outcome. J Intern Med. 2021;290(2):437–443. doi: 10.1111/joim.13275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu SW, Ilyas I, Weng JP. Endothelial dysfunction in COVID-19: an overview of evidence, biomarkers, mechanisms and potential therapies. Acta Pharmacol Sin. 2023;44(4):695–709. doi: 10.1038/s41401-022-00998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Libby P, Lüscher T. COVID-19 is, in the end, an endothelial disease. Eur Heart J. 2020;41(32):3038–3044. doi: 10.1093/eur-heartj/ehaa623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Rooij LPMH, Becker LM, Carmeliet P A role for the vascular endothelium in post-acute COVID-19? Circulation. 2022;145(20):1503–1505. doi: 10.1161/CIRCULATIONAHA.122.059231. [DOI] [PubMed] [Google Scholar]
- 25.Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57(6):389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elshazli RM, Toraih EA, Elgaml A, et al. Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: a meta-analysis of 6320 patients. PLoS ONE. 2020;15(8):e0238160. doi: 10.1371/journal.pone.0238160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ali AM, Rostam HM, Fatah MH, Noori CM, Ali KM, Tawfeeq HM. Serum troponin, D-dimer, and CRP level in severe coronavirus (COVID-19) patients. Immun Inflamm Dis. 2022;10(3):e582. doi: 10.1002/iid3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malik P, Patel K, Pinto C, et al. Post-acute COVID-19 syndrome (PCS) and health-related quality of life (HRQoL)–a systematic review and meta-analysis. J Med Virol. 2022;94(1):253–262. doi: 10.1002/jmv.27309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorent N, Vande Weygaerde Y, Claeys E, et al. Prospective longitudinal evaluation of hospitalised COVID-19 survivors 3 and 12 months after discharge. ERJ Open Res. 2022;8(2):00004–2022. doi: 10.1183/23120541.00004-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simova I Intima-media thickness: appropriate evaluation and proper measurement described. E J Cardiol Pract. 2015;13:1 [Google Scholar]


