Abstract
Introduction
Irritant contact dermatitis (ICD) is characterized by direct injury to the epidermal cells, activating the innate immune response. Allergic contact dermatitis (ACD), in contrast, is delineated by a delayed hypersensitivity reaction of type IV. Despite the distinct etiopathogenic mechanisms under-pinning each condition, the differentiation between them presents a significant diagnostic challenge.
Objective
This study aimed to determine whether a combination of clinical evaluation and noninvasive measurements—encompassing oxidative stress, erythema, hydration, melanin content, transepidermal water loss (TEWL), hemoglobin concentration, and skin texture and volume—could distinguish ICD from ACD.
Methods
Two cohorts, each comprising 21 patients, were evaluated: one diagnosed with ICD and the other with ACD. All participants underwent biophysical and clinical assessments, along with Antera® 3D evaluations. Tape strips were utilized for skin sampling, and oxidative stress levels were measured via fluorescence assessments.
Results
ICD prompted an almost immediate inflammatory reaction (peaking at 24 hours), whereas ACD incited a delayed response (72 hours). Noninvasive evaluated parameters such as hemoglobin concentration, skin texture and volume, melanin content, erythema, and TEWL showed significant differences between the ICD and ACD cohorts (P < 0.05). The allergens amcinonide, nickel sulphate, cobalt chloride, budesonide, PPD, and thiuram mix were found to induce elevated levels of oxidative stress.
Conclusions
The evaluation of patients with noninvasive parameters, including transepidermal water loss (TEWL), hemoglobin concentration, and skin texture and volume, could markedly aid in distinguishing irritant contact dermatitis from allergic contact dermatitis (ACD). Nevertheless, the study was constrained by a limited sample size.
Keywords: Irritant Contact Dermatitis, Allergic Contact Dermatitis, Antera® 3D Camera, Transepidermal Water Loss, Oxidative Stress
Introduction
Contact dermatitis manifests in two primary forms: irritant contact dermatitis (ICD), accounting for 80% of cases, and allergic contact dermatitis (ACD), comprising the remaining 20% [1,2]. ICD is distinguished by direct damage to keratinocytes, initiating the activation of the innate immune system and leading to the release of a cascade of pro-inflammatory cytokines to mediate the damage [3–7]. These cytokines stimulate the activation of epidermal Langerhans cells, dermal dendritic cells, and endothelial cells. Experimentally, sodium lauryl sulfate (SLS) induces damage analogous to that observed in ICD [3,8].
Conversely, ACD is typified by a delayed hypersensitivity reaction of type IV (Gell-Coombs), induced by dermal contact with haptens or non-protein allergens. Acute manifestations include erythema, vesicles, and blisters, while chronic exposure may result in lichenification accompanied by cracks and fissures [9]. The mediation of ACD involves T cells that recognize small chemical molecules or metal ions within the major histocompatibility complex (MHC) [10]. These electrophilic chemicals penetrate the skin and interact with extracellular and intracellular proteins, a property underpinning their capability to activate both innate immune and T cell responses [11–13].
Following contact with a specific allergen, allergen-specific T cells proliferate and differentiate into effector T cells, which enter the circulation [14]. The repeated application of a specific allergen on the skin leads to the recruitment of these T cells back to the dermal layer, inducing apoptosis in keratinocytes and culminating in ACD [15,16]. Notably, skin lesions in ACD manifest upon re-exposure to the allergens, with metals, preservatives, antibiotics, and fragrances being the most common allergens linked to sensitization within the general population [17].
Objectives
This study endeavored to enhance the clinical differential evaluation of ACD and ICD by integrating the analysis of noninvasive parameters. The investigation focused on several key indicators of skin health as response to irritants or allergens, including oxidative stress levels, erythema, skin hydration, melanin content, transepidermal water loss (TEWL), hemoglobin concentration, and the texture and volume of the skin fraction affected. Oxidative stress, for instance, can provide insights into the cellular damage and inflammatory response triggered by contact with irritants or allergens. Similarly, changes in TEWL, erythema, and skin hydration offer quantitative measures of the skin's barrier function and inflammatory status. By comparing these parameters between individuals diagnosed with ACD and those with ICD, the study aimed to identify distinctive patterns that could aid in their differentiation.
Incorporating such noninvasive assessments into the diagnostic process seems promising for improving the accuracy of ACD and ICD diagnosis. By providing a more nuanced understanding of the skin's response to irritants and allergens, healthcare professionals could tailor treatment strategies more effectively. Moreover, this approach aligns with the growing emphasis on minimally invasive techniques in dermatological diagnosis. As the study progresses, the findings could contribute valuable insights into the pathophysiological distinctions between ACD and ICD, ultimately enhancing patient care and therapeutic outcomes.
Methods
SLS Gauze
A gauze impregnated with a 6% w/v solution of SLS (Farmalabor, Italy) was applied to the forearm skin for 24 hours. The clinical manifestations were evaluated following the removal of the SLS-impregnated gauze.
Patch Test
Patch testing remains the definitive standard for diagnosing ACD. Following the European Society of Contact Dermatitis guidelines for patch testing, substances were applied to the upper back and removed after 48 hours. The evaluations were conducted 72 hours post-application, utilizing a European baseline and supplemental series of allergens (allergEAZE ® Patch Test Chambers, SmartPractice Canada).
Study Design
All conducted procedures adhered to the Good Clinical Practice (GCP) guidelines as outlined in Directive 2001/20/EC, the US Federal Code of Users (21 CFR Part 312), and the International Conference on Harmonization (ICH). The research was performed in alignment with the Declaration of Helsinki principles (Directive 2001/83/EC; ICH Issue E9 1996; Directive 2001/20/EC; Directive 2002/98/EC; Directive 2003/63/EC; ICH E(6) R1; 21 CFR Part 312; WHO 2008). Approval for the protocol was granted by the Institutional Scientific Review Board of the Andreas Syggros Hospital, NKUA,University Medical School (Protocol Nr. 3612/2021).
Participants
Forty-two participants (21 ACD and 21 ICD diagnosed patients) were recruited from the 1st Department of Dermatology and Venereal Diseases, Andreas Syggros University Hospital, between June 2021 and December 2021. All patients provided informed consent. Exclusion criteria included pregnancy, the use of anti-inflammatory and antihistaminic medications, sweating, and physical activity. Inclusion criteria encompassed a history of acute, subacute, and chronic dermatitis and the manifestation of rashes following exposure to allergens and irritants.
Clinical Assessment
All participants successfully completed the study. Medical history and demographic data such as sex, age, body mass index (BMI), phototype (Fitzpatrick skin type), medical conditions, family history, and smoking habits were collected and found to be homogeneous (Table 1).
Table 1.
Characteristics of Patients at Baseline.
| Characteristics of Patients | Allergic Contact Dermatitis | Irritant Contact Dermatitis |
|---|---|---|
| Demographics | ||
| Median Age | 46 | 50 |
| Sex (%) | ||
| Male | 9.52 | 9.52 |
| Female | 90.48 | 90.48 |
| BMI classification (%) | ||
| Normal weight | 76.19 | 66.67 |
| Overweight | 23.81 | 23.81 |
| Underweight | - | 4.76 |
| Morbid obesity | - | 4.76 |
| Fitzpatrick skin type (%) | ||
| I | 4.76 | 23.81 |
| II | 57.14 | 61.9 |
| III | 33.33 | 14.29 |
| IV | 4.76 | - |
| Current smoker (%) | 42.86 | 38.1 |
| Characteristics of Disease | ||
| Patient atopic dermatitis history (%) | 47.62 | 28.57 |
| Family atopic dermatitis history (%) | 28.57 | 28.57 |
| Disease (%) | ||
| Primary (%) | 9.52 | - |
| Recurrence (%) | 90.48 | - |
| Exposure to contact allergens (%) | 80.95 | - |
Abbreviation: BMI: body mass index.
Contact Dermatitis Evaluation
The International Contact Dermatitis Research Group (ICDRG) criteria were employed to assess the intensity of positive reactions in ACD patients, ranging from weak positive reactions, characterized by erythema, infiltration, and possibly papules (1+), to extreme positive reactions, marked by intense erythema, infiltration, and coalescing vesicles (3+). For ICD patients, the clinical evaluation was based on the reaction within 24 hours and the clinical assessment of irritation.
Oxidative Stress Evaluation
Prior to and at the time of patch evaluation, two tape strips (Standard D100 Squame Discs, USA) were applied to the patients’ skin and stored at −20°C. The samples were then processed with 500 μl of H2O HPLC (Fischer Chemical U.K. Limited), 200 μl of 90% MeOH (Fischer Chemical U.K. Limited, Bishop Meadow) Na2EDTAx2H2O (Lach-Ner, s.r.o., Turkey), 75 μl of 10 mg/ml BHT (Sigma Chemical Co., USA) in Ethanol HPLC (Acros Organics, Germany), and 1.5 μl of 10 mM Desferal (Ciba-Geigy Switzerland) in H2O HPLC. Following vortex mixing (MSI Minishaker IKA, USA) at 2,500 rpm for one minute and centrifugation at 9,000 rpm for eight minutes at 4°C, 50 μl from each vial solution and 100 μl of saline were transferred to a 96-well plate (Costar – Corning, USA), with three wells per solution. Three wells received 150 μl of saline as a control. Subsequently, 10 μl of 30 μM 5-(and-6)-chloromethyl-2',7'-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA) ethanolic solution (Molecular Probes-Thermo Fisher Scientific USA) was added to each well. The plate was then vortexed at 300 rpm for 10 minutes and placed in a Fluostar BMG (Germany) reader with the excitation filter set at 485 nm and emission at 520 nm, with a gain of 85. The plate underwent incubation at 37°C for 50 minutes prior to measurements.
Skin Analysis
Skin assessments were conducted using an Antera® 3D camera (Miravex, Dublin, Ireland) at 0 hours and 72 hours in ACD patients and at 0 hours and 24 hours in ICD patients. Hemoglobin concentration and skin texture were evaluated using Antera® 3D software (Miravex, Dublin, Ireland).
Measurements of Skin Biophysical Parameters
In both ACD and ICD patients, skin parameters, including hydration, TEWL, erythema, and melanin, were measured using noninvasive biophysical methods at 0 hours and 72 hours and at 0 hours and 24 hours, respectively. Hydration levels were gauged using a Corneometer CM 820 (Courage + Khazaka electronic GmbH, Cologne, Germany), based on changes in the dielectric constant, with data recorded in arbitrary units. The skin barrier function (TEWL) was evaluated using a Tewameter TM 210 (Courage + Khazaka electronic GmbH, Cologne, Germany) by measuring the density gradient of water evaporation from the skin, with estimations based on the mean value of the flux density of water (in g/m2/h). Erythema and melanin levels were quantified using a Mexameter MX 18 (Courage + Khazaka electronic GmbH, Cologne, Germany), with data also recorded in arbitrary units. Before each measurement, the treated area was cleansed with 0.9% sodium chloride solution and dried with sterile gauze.
Statistical Analysis
The statistical analysis commenced with the determination of descriptive criteria and estimation of scatter metrics. Normality testing was performed prior to statistical comparisons among two or more groups. The Shapiro-Wilk test was utilized to assess the normality of data distribution. Upon rejection of the nominal normality hypothesis, the data were deemed to deviate from a normal distribution. When data appeared normally distributed, parametric procedures were employed. However, in all instances within this study, as the data were found not to be normally distributed, non-parametric methods were applied. The Mann-Whitney U test was employed for comparisons between two independent groups, for instance, to evaluate the effect of sex. For pairwise comparisons between groups, such as the comparison of skin biophysical parameters across two different time points, the Wilcoxon signed-rank test was used. The type I error (significance level) was set at 5% for all analyses within this study. A result was deemed significant if the estimated p-value (P) was less than the significance level, with p-values of < 0.05 denoted as statistically significant and indicated by the symbols (*) for P < 0.05, (**) for P < 0.01, (***) for P < 0.001, and (****) for P < 0.0001. All statistical analyses were conducted using SPSS® (v.25, IBM, Chicago, IL, USA).
Results
Clinical Assessment
ICD Patients
All patients exhibited erythema at the time of evaluation (24 hours).
ACD Patients
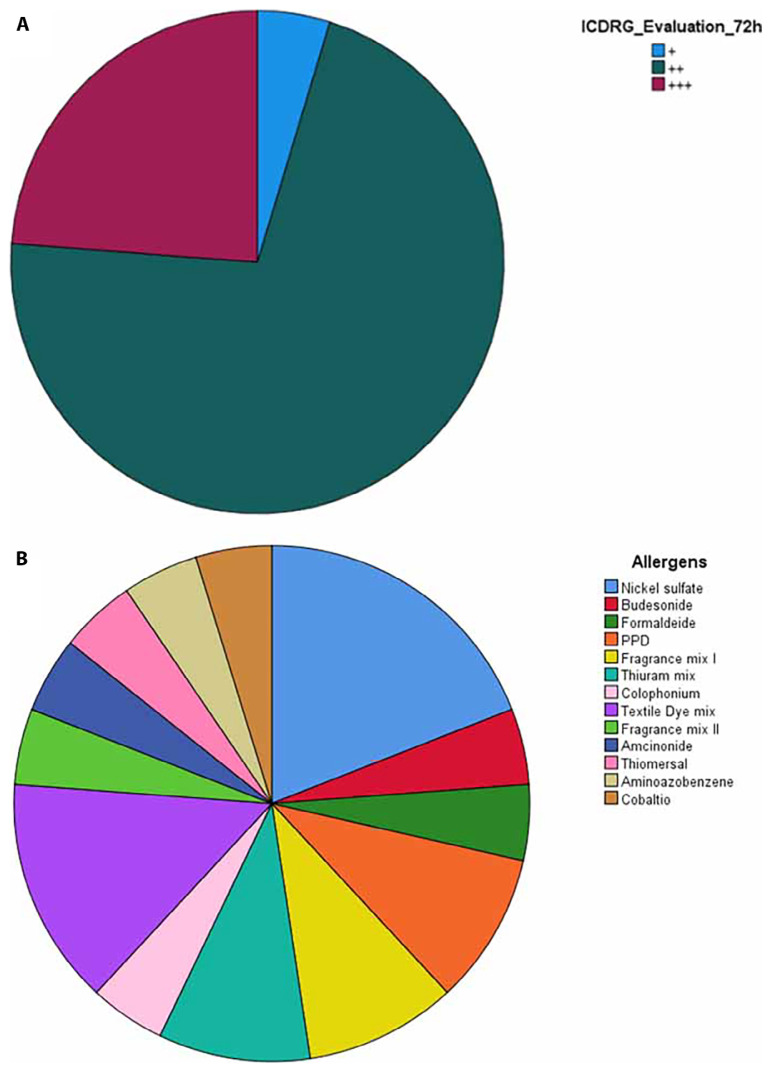
The clinical assessment of ACD patients at 72 hours, based on the ICDRG criteria, is illustrated in Figure 1. Strong reaction (+ + +) according to the ICDRG scale was presented by PPD 1%, textile dye mix 6.6%, and 4 - aminoazobenzene 1% (Figure 1A). Nickel sulfate 5% w/w solution was responsible for 19.05% of reactions and textile dye mix 6.6% for 14.29%; thiuram mix 1%, fragrance mix I 8%, and PPD 1% each accounted for 9.52%. Cobalt chloride 1%, 4-aminoazobenzene 1%, thiomersal 0.1%, amcinonide 0.1%, fragrance mix II 14%, colophonium 20%, budesonide 0.01%, and formaldehyde 2% each contributed to 4.76% of reactions (Figure 1B).
Figure 1.
(A) Clinical assessment of ACD patients after 72 hours. Incidents presented (+) 4.76%, (++) 71.43 % and (+++) 23.81%. (B) The most common allergens were, in order, nickel sulfate solution 5 % w/w and textile dye mix 6.6% w/w.
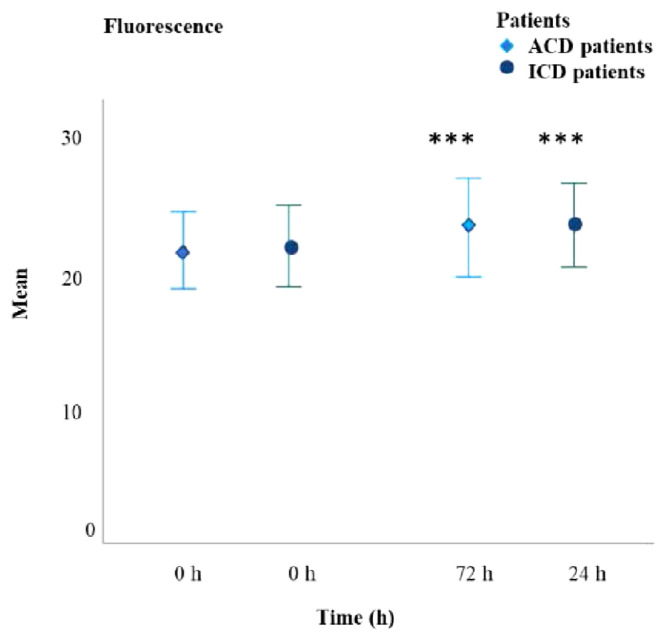
Oxidative Stress
Oxidative stress, indicated by increased fluorescence intensity, was significantly elevated in both ICD (P < 0.001) and ACD (P < 0.001) patients (Figure 2). The mean fluorescence increase in ICD patients was 6.586%, while in ACD patients, it was 5.515%. There were no significant differences between the two groups at the time of evaluation. All allergens led to a significant rise in oxidative stress, with the most substantial increases observed for amcinonide, nickel sulfate, cobalt chloride, budesonide, PPD, and thiuram mix allergens (Table 2).
Figure 2.
Mean value of fluorescence for ICD and ACD patients. Fluorescence values were significantly increased for both groups at the time of evaluation (***P < 0.001).
Table 2.
Increase in Allergen Oxidative Stress at 72 Hours Expressed in Fluorescence Units.
| Allergens | Mean Increase in Fluorescence Units |
|---|---|
| Formaldehyde 2% | 420 |
| Thiuram mix 1% | 1537 |
| Nickel sulfate 5% | 0 |
| Budesonide 0.01% | 2936 |
| Amcinonide 0.1% | 2677 |
| Colophonium 20% | 0 |
| Thiomersal 0.1% | 2155 |
| PPD 1% | 2046 |
| Textile Dye Mix 6.6% | 1323 |
| Textile Dye Mix 6.6% | 1528 |
| Fragrance Mix II 14% | 1195 |
| Fragrance Mix I 8% | 576 |
| Nickel Sulfate 5% | 3049 |
| Cobalt chloride 1% | 3703 |
| PPD 1% | 1576 |
| Textile Dye Mix 6.6% | 463 |
| 4-aminoazobenzene 1% | 598 |
| Nickel sulfate 5% | 2965 |
| Thiuram mix 1% | 4990 |
| Nickel sulfate 5% | 3936 |
| Fragrance mix I 8% | 0 |
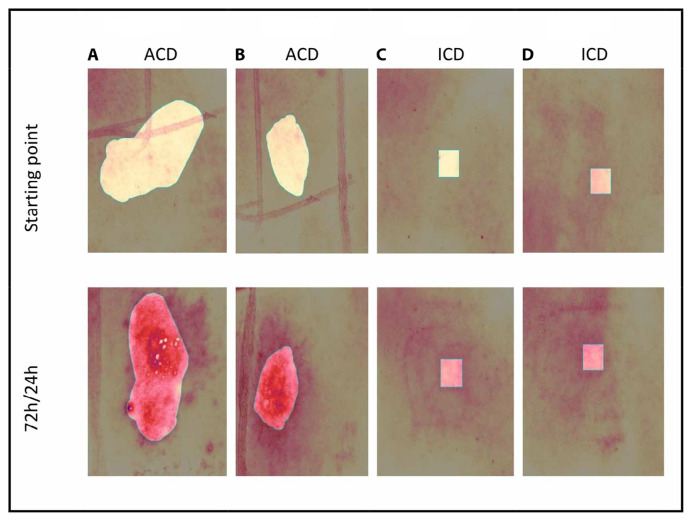
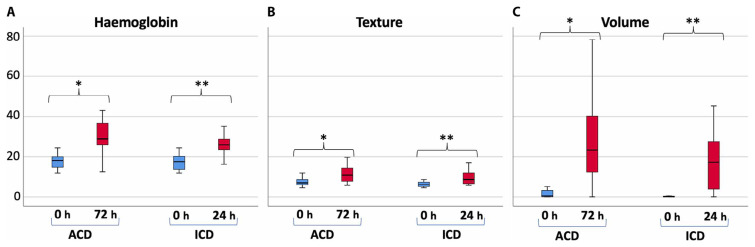
Antera® Measurements
Analysis using Antera® 3D software (Figure 3) revealed pronounced inflammatory activity in both ICD and ACD patients. All Antera® 3D parameters, including hemoglobin concentration, skin texture, and volume values, were significantly higher in ICD patients within 24 hours (Figure 4, P < 0.001). Likewise, these parameters were significantly elevated at 72 hours in ACD patients (Figure 4, P < 0.001). The median hemoglobin concentration, skin texture, and volume increased in both groups as the intensity of inflammation escalated. The percentage difference in median hemoglobin concentration was 94.95% in ACD and 47.14% in ICD patients. Notably, significant differences were observed between ACD and ICD patients at 72 hours and 24 hours, respectively (Figure 4A, P < 0.001). The percentage difference in the median texture value was 66.39% in ACD compared to 37.73% in ICD patients. The distinction between ACD and ICD patients at the time of evaluation was statistically significant (Figure 4B, P < 0.05). Similarly, significant differences were noted between ACD and ICD patients in terms of volume (Figure 4C, P < 0.05).
Figure 3.
Antera® 3D images of ACD and ICD patients. (A) Textile dye mixture: a 21-year-old female patient with allergic contact dermatitis. (B) PPD: a 21-year-old female patient with allergic contact dermatitis. (C) A 45-year-old female patient irritated with SLS 6% in the forearm. (D) A 55-year-old female patient irritated with SLS 6% in the forearm.
Figure 4.
(A) Hemoglobin concentration, (B) skin texture, and (C) mean volume values at the time of evaluation for ICD and ACD patients. Significant differences were observed at 0 h compared to at the time of evaluation. (***P < 0.001).
Biophysical Measurements
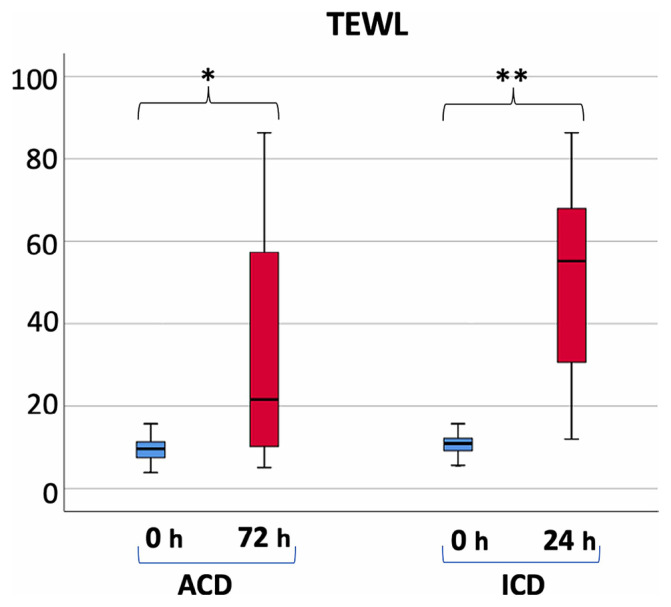
Transepidermal water loss (TEWL) is directly associated with skin barrier functionality and indirectly with the intensity of inflammation. Significantly higher TEWL levels were noted in ICD patients at 24 hours and in ACD patients at 72 hours (Figure 5, P < 0.001). The median TEWL showed a 20% increase in ACD and a 406.42% increase in ICD patients. Noteworthy differences were identified between ACD and ICD patients at the time of analysis (Figure 5, P < 0.05).
Figure 5.
Mean values of TEWL for ICD and ACD patients. Significant differences were observed at 0 hours compared to at the time of evaluation (***P < 0.001).
Discussion
All participants in this study exhibited signs of skin inflammation. In patients with ICD, the inflammatory response significantly subsided 48 hours following the application of SLS, indicative of ICD’s characteristic direct harm to keratinocytes, which promptly triggers the innate immune response [18]. Conversely, the reaction to allergens in ACD is typically not instantaneous. Despite ACD’s more severe clinical presentation compared to ICD, the response is delayed, often taking up to 72 hours to manifest [18].
According to the ICDRG criteria, allergens are categorized based on the degree of reaction within 72 hours (Figure 1A). PPD, textile dye mixtures, and 4-aminoazobenzene elicited the most potent reactions, corroborating the ICDRG scale [13]. The findings highlight nickel sulfate (figure 1B) as the most prevalent sensitizer, aligning with observations made by Dekoven et al. [17,19].
Fluorescence-based oxidative stress assessment revealed no significant differences between ACD and ICD patients (Figure 2). However, oxidative stress levels increased in ICD patients after 24 hours and in ACD patients after 72 hours (P < 0.05), with significant elevations observed in response to moderate sensitizers such as amcinonide and budesonide and to strong sensitizers like cobalt chloride, nickel sulfate,
PPD, and thiuram mix allergens (Table 2) [20–23]. PPD, commonly found in hair dyes and black henna tattoos, enhances the formation of reactive oxygen species (Table 2), thereby elevating oxidative stress in human keratinocytes, a finding consistent with our results [24,25]. Our observation regarding the increase in oxidative stress caused by nickel sulfate (Table 2) also supports the notion of enhanced lipid peroxidation, as evidenced in previous studies [27–29].
The Antera® 3D camera facilitated the evaluation of inflammation intensity in ICD and ACD, demonstrating that mean hemoglobin concentration and skin texture values rise in tandem with inflammation severity (Figures 4A, 4B, P < 0.001), a fact previously established [30]. Additionally, volume, as measured by the Antera® 3D camera, augmented with inflammation (Figure 4C, P < 0.001). Significantly higher values of all Antera® 3D parameters were observed in both ICD and ACD patients (Figure 4, P < 0.001), with ACD patients showing a more pronounced increase in Antera® 3D values compared to ICD patients (Figure 4, P < 0.05). Thus, Antera® 3D camera measurements appear to be a valuable tool for distinguishing between ICD and ACD inflammation.
TEWL in ICD patients increased by an average of 406.42%, while in ACD patients it rose by 20% (Figure 5, P < 0.05), indicating a roughly twentyfold greater decrease in skin barrier function in ICD patients compared to ACD. TEWL, a direct indicator of skin barrier integrity and thus inflammation [30,31], could significantly aid in differentiating between ICD and ACD inflammatory responses.
Limitations
Given the limited sample size of 21 patients each for ACD and ICD, further research is necessary to definitively ascertain whether biophysical measurements, Antera® 3D camera imaging, and oxidative stress analysis can distinguish between ICD and ACD inflammation effectively.
Conclusions
Numerous distinctions were identified between ACD and ICD responses. ICD prompts an almost immediate inflammatory reaction (peaking at 24 hours), whereas ACD incites a delayed response (72 hours). The reduction in skin barrier function was substantially greater in ICD than in ACD. All Antera® 3D parameters (hemoglobin concentration, skin texture, and volume) were significantly elevated in ACD compared to ICD patients. No statistically significant differences in oxidative stress were noted between ACD and ICD patients. Nickel sulfate emerged as the most common sensitizer, while PPD, textile dye mix, and 4-aminoazobenzene triggered the most severe inflammatory responses. Allergens such as amcinonide, nickel sulfate, cobalt chloride, budesonide, PPD, and thiuram mix were found to induce significant oxidative stress.
Acknowledgments
The authors would like to acknowledge Mrs Lagiokapa Polyxeni, Pesli Maria and Kostaki Maria for their valuable help.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Thyssen JP, Johansen JD, Linneberg A, Menné T. The epidemiology of hand eczema in the general population—prevalence and main findings. Contact Dermatitis. 2010 Feb;62(2):75–87. doi: 10.1111/j.1600-0536.2009.01669.x. [DOI] [PubMed] [Google Scholar]
- 2.Alinaghi F, Bennike NH, Egeberg A, Thyssen JP, Johansen JD. Prevalence of contact allergy in the general population: A systematic review and meta-analysis. Contact Dermatitis. 2019 Feb;80(2):77–85. doi: 10.1111/cod.13119. Epub 2018 Oct 29. [DOI] [PubMed] [Google Scholar]
- 3.Gittler JK, Krueger JG, Guttman-Yassky E. Atopic dermatitis results in intrinsic barrier and immune abnormalities: implications for contact dermatitis. J Allergy Clin Immunol. 2013 Feb;131(2):300–13. doi: 10.1016/j.jaci.2012.06.048. Epub 2012 Aug 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark SC, Zirwas MJ.Management of occupational dermatitis Dermatol Clin 2009Jul273365–83., vii–viii 10.1016/j.det.2009.05.002 [DOI] [PubMed] [Google Scholar]
- 5.Nosbaum A, Vocanson M, Rozieres A, Hennino A, Nicolas JF. Allergic and irritant contact dermatitis. Eur J Dermatol. 2009 Jul–Aug;19(4):325–32. doi: 10.1684/ejd.2009.0686. [DOI] [PubMed] [Google Scholar]
- 6.Bonneville M, Chavagnac C, Vocanson M, et al. Skin contact irritation conditions the development and severity of allergic contact dermatitis. J Invest Dermatol. 2007 Jun;127(6):1430–5. doi: 10.1038/sj.jid.5700726. Epub 2007 Feb 1. [DOI] [PubMed] [Google Scholar]
- 7.Vocanson M, Hennino A, Rozières A, Poyet G, Nicolas JF. Effector and regulatory mechanisms in allergic contact dermatitis. Allergy. 2009 Dec;64(12):1699–714. doi: 10.1111/j.1398-9995.2009.02082.x. Epub 2009 Oct 12. [DOI] [PubMed] [Google Scholar]
- 8.DaSilva SC, Sahu RP, Konger RL, Perkins SM, Kaplan MH, Travers JB. Increased skin barrier disruption by sodium lauryl sulfate in mice expressing a constitutively active STAT6 in T cells. Arch Dermatol Res. 2012 Jan;304(1):65–71. doi: 10.1007/s00403-011-1168-2. Epub 2011 Sep 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu K, Qu L, Shimada SG, Nie H, LaMotte RH. Enhanced scratching elicited by a pruritogen and an algogen in a mouse model of contact hypersensitivity. Neurosci Lett. 2014 Sep 5;579:190–4. doi: 10.1016/j.neulet.2014.03.062. Epub 2014 Apr 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin SF, Esser PR, Schmucker S, et al. T-cell recognition of chemicals, protein allergens and drugs: towards the development of in vitro assays. Cell Mol Life Sci. 2010 Dec;67(24):4171–84. doi: 10.1007/s00018-010-0495-3. Epub 2010 Aug 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan DH, Igyártó BZ, Gaspari AA. Early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol. 2012 Jan 13;12(2):114–24. doi: 10.1038/nri3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin SF. Contact dermatitis: from pathomechanisms to immunotoxicology. Exp Dermatol. 2012 May;21(5):382–9. doi: 10.1111/j.1600-0625.2012.01471.x. [DOI] [PubMed] [Google Scholar]
- 13.Johansen JD, Aalto-Korte K, Agner T, et al. European Society of Contact Dermatitis guideline for diagnostic patch testing - recommendations on best practice. Contact Dermatitis. 2015 Oct;73(4):195–221. doi: 10.1111/cod.12432. Epub 2015 Jul 14. [DOI] [PubMed] [Google Scholar]
- 14.Rustemeyer T, Van Hoogstraten IMW, Von Blomberg BME, Gibbs S, Scheper RJ. Contact Dermatitis. Fifth Edition. Springer; Berlin Heidelberg: 2011. Mechanisms of irritant and allergic contact dermatitis; pp. 43–90. [DOI] [Google Scholar]
- 15.Nijhawan RI, Matiz C, Jacob SE. Contact dermatitis: from basics to allergodromes. Pediatr Ann. 2009 Feb;38(2):99–108. doi: 10.3928/00904481-20090201-07. [DOI] [PubMed] [Google Scholar]
- 16.Riemann H, Schwarz T, Grabbe S. Pathomechanismen der Auslösephase der allergischen Kontaktdermatitis [Pathomechanisms of the elicitation phase of allergic contact dermatitis] J Dtsch Dermatol Ges. 2003 Aug;1(8):613–9. German. [PubMed] [Google Scholar]
- 17.DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group Patch Test Results: 2015–2016. Dermatitis. 2018 Nov/Dec;29(6):297–309. doi: 10.1097/DER.0000000000000417. [DOI] [PubMed] [Google Scholar]
- 18.Nassau S, Fonacier L. Allergic Contact Dermatitis. Med Clin North Am. 2020 Jan;104(1):61–76. doi: 10.1016/j.mcna.2019.08.012. Epub 2019 Oct 28. [DOI] [PubMed] [Google Scholar]
- 19.Tagka A, Stratigos A, Lambrou GI, et al. Prevalence of contact dermatitis in the Greek population: A retrospective observational study. Contact Dermatitis. 2019 Dec;81(6):460–462. doi: 10.1111/cod.13364. Epub 2019 Aug 8. [DOI] [PubMed] [Google Scholar]
- 20.Uter W, de Pádua CM, Pfahlberg A, Nink K, Schnuch A, Lessmann H.Contact allergy to topical corticosteroids--results from the IVDK and epidemiological risk assessment J Dtsch Dermatol Ges 2009Jan7134–41., 34–42. English, German 10.1111/j.1610-0387.2008.06844.xEpub 2008 Aug 28 [DOI] [PubMed] [Google Scholar]
- 21.Arslan S, Aksan S, Ucar R, Caliskaner AZ. Contact dermatitis to cobalt chloride with an unusual mechanism. Prosthet Orthot Int. 2015 Oct;39(5):419–21. doi: 10.1177/0309364614534293. Epub 2014 May 29. [DOI] [PubMed] [Google Scholar]
- 22.Goldenberg A, Matiz C, Eichenfield LF. Religious Allergic Contact Dermatitis. Pediatr Dermatol. 2015 Jul–Aug;32(4):e191–2. doi: 10.1111/pde.12613. Epub 2015 May 13. [DOI] [PubMed] [Google Scholar]
- 23.Tang B, Williams PL, Xue KS, Wang JS, Tang L. Detoxification mechanisms of nickel sulfate in nematode Caenorhabditis elegans. Chemosphere. 2020 Dec;260:127627. doi: 10.1016/j.chemosphere.2020.127627. Epub 2020 Jul 10. [DOI] [PubMed] [Google Scholar]
- 24.Schnuch A, Lessmann H, Frosch PJ, Uter W. para-Phenylenediamine: the profile of an important allergen. Results of the IVDK. Br J Dermatol. 2008 Aug;159(2):379–86. doi: 10.1111/j.1365-2133.2008.08644.x. Epub 2008 May 28. Erratum in: Br J Dermatol. 2008 Sep;159(3):772. [DOI] [PubMed] [Google Scholar]
- 25.Zapolanski T, Jacob SE. para-Phenylenediamine. Dermatitis. 2008 May-Jun;19(3):E20–1. [PubMed] [Google Scholar]
- 26.Zanoni TB, Hudari F, Munnia A, et al. The oxidation of p-phenylenediamine, an ingredient used for permanent hair dyeing purposes, leads to the formation of hydroxyl radicals: Oxidative stress and DNA damage in human immortalized keratinocytes. Toxicol Lett. 2015 Dec 15;239(3):194–204. doi: 10.1016/j.toxlet.2015.09.026. Epub 2015 Oct 9. [DOI] [PubMed] [Google Scholar]
- 27.Coogan TP, Latta DM, Snow ET, Costa M. Toxicity and carcinogenicity of nickel compounds. Crit Rev Toxicol. 1989;19(4):341–84. doi: 10.3109/10408448909029327. Erratum in: Crit Rev Toxicol 1989;20(2):135. [DOI] [PubMed] [Google Scholar]
- 28.Stinson TJ, Jaw S, Jeffery EH, Plewa MJ. The relationship between nickel chloride-induced peroxidation and DNA strand breakage in rat liver. Toxicol Appl Pharmacol. 1992 Nov;117(1):98–103. doi: 10.1016/0041-008x(92)90222-e. [DOI] [PubMed] [Google Scholar]
- 29.Das KK, Dasgupta S. Effect of nickel on testicular nucleic acid concentrations of rats on protein restriction. Biol Trace Elem Res. 2000 Feb;73(2):175–80. doi: 10.1385/BTER:73:2:175. [DOI] [PubMed] [Google Scholar]
- 30.Kyritsi A, Kikionis S, Tagka A, et al. Management of Acute Radiodermatitis in Non-Melanoma Skin Cancer Patients Using Electrospun Nanofibrous Patches Loaded with Pinus halepensis Bark Extract. Cancers (Basel) 2021 May 26;13(11):2596. doi: 10.3390/cancers13112596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotroni E, Simirioti E, Kikionis S, et al. In Vivo Evaluation of the Anti-Inflammatory Activity of Electrospun Micro/Nanofibrous Patches Loaded with Pinus halepensis Bark Extract on Hairless Mice Skin. Materials (Basel) 2019 Aug 15;12(16):2596. doi: 10.3390/ma12162596. [DOI] [PMC free article] [PubMed] [Google Scholar]