Abstract
Introduction
Dermoscopy is a noninvasive diagnostic tool that allows the recognition of morphologic structures not visible to the naked eye. Trichoscopy is useful for the diagnosis and follow-up of hair and scalp disorders.
Objective
The aim of the present study was to evaluate the causes of focal non-cicatricial alopecia in Egyptian children and to assess the importance of the trichoscope in the diagnosis of each disease.
Methods
This study was done with 200 Egyptian pediatric patients aged from 2 to 18 years who suffered from focal non-cicatricial alopecia. Clinical and dermoscopic evaluations were performed on all patients, and informed consent was obtained from their parents.
Results
The most prevalent diagnoses were alopecia areata (42%) and tinea capitis (40.5%), followed by trichotillomania (8%) and tractional alopecia (7%). Congenital triangular alopecia (1.5%) and patchy androgenetic alopecia (1%) were less common. Trichoscopy revealed distinct features in alopecia areata cases, such as short vellus hair, exclamation mark hair, black dots, broken hair, pigtail hair, and upright regrowing hair. The most common trichoscopic features of tinea capitis were comma hair, corkscrew hair, broken hair, bent hair, zigzag hair, morse code hair, perifollicular scaling, and diffuse scaling. These findings contribute to understanding the etiology and clinical presentation of childhood alopecia, facilitating accurate diagnosis and appropriate management.
Conclusion
The routine use of trichoscopy in the clinical evaluation of scalp and hair disorders enhances diagnostic capabilities beyond simple clinical inspection. Trichoscopy reveals disease features that contribute to accurate diagnosis and improved management.
Keywords: Trichoscopic, Focal non-cicatricial alopecia, Egyptian children
Introduction
Dermoscopy, a noninvasive diagnostic tool, allows for the identification of morphologic structures not visible to the naked eye. Trichoscopy has proven valuable in diagnosing and monitoring hair and scalp disorders [1]. The predominant form of alopecia in children is patchy alopecia, with alopecia areata being the most common diagnosis. However, patchy hair loss can also result from other causes such as tinea capitis (TC), trichotillomania (TTM), tractional alopecia (TA), androgenetic alopecia (AGA), and temporal triangular alopecia (TTA) [2]. Alopecia areata presents as a distinct bald patch on the scalp [3], while tinea capitis is a fungal infection affecting hair follicles and surrounding skin [4]. Trichotillomania, characterized by repetitive hair pulling, closely resembles alopecia areata [5] and exhibits additional trichoscopic features like flame hair, tulip hair, i hair, V sign, and hair powder [6]. Tractional alopecia, caused by unintentional hair pulling and stretching, primarily results in hair loss at the hairline [7].
Objective
The primary goal of this study was to evaluate different diseases manifesting as focal non-cicatricial alopecia in Egyptian children and to assess the significance of trichoscopy in diagnosing each condition.
Methods
In this observational analytical cross-sectional study, 200 Egyptian children suffering from focal non-cicatricial alopecia were included. All patients were recruited from the Dermatology Outpatient Clinic of our University Hospital between April 2021 and February 2023. Informed consent was obtained from parents, and the study received approval from the Research Ethics Committee of the Faculty of Medicine with IRP 202104789. Patients presented with either solitary or multiple lesions of patchy hair loss on the scalp. A comprehensive history, including systemic or autoimmune diseases, was obtained. General examination excluded associated systemic diseases, and local dermatological examination covered hair, nails, mucous membranes, and hair loss in other body areas.
Trichoscopic pictures were collected with DermLite IV (3Gen) polarized mode with 10-fold magnification (dry and/or wet) to identify trichoscopic findings for hair disorders. Digital photography of patchy hair loss area was conducted with DermLite IV dermoscopy from a 20-cm distance using REDMI NOT10 mobile 4G with a 64-megapixel camera and a magnification power of 10X. Microscopic examination involved skin scraping and plucked hairs from the affected area, mounted in 10% potassium hydroxide solution, and examined under a microscope for the presence of spores and hyphae.
Statistical analysis utilized Excel and SPSS software version 22. Quantitative data are presented as mean ± SD and range while qualitative data are expressed as events and percentages.
Inferential statistics, including ANOVA and chi-square tests, facilitated multiple-arm comparison of quantitative and qualitative data, respectively. A confidence level of 95% was set, determining significance based on the p-value: significant if P ≤ 0.05, and highly significant if ≤ 0.001.
Results
This observational analytical cross-sectional study involved 200 patients with focal non-scarring alopecia in childhood from ages 2 to 18 years; 113 patients (56.5%) were males, and 87 patients (43.5%) were females. The most common diagnosis in this study was alopecia areata (AA) (n=84, 42%), followed by tinea capitis (TC) (n=81, 40.5%), trichotillomania (TTM) (n=16, 8%), tractional alopecia (TA) (n=14, 7%), congenital triangular alopecia (n=3, 1.5%), and patchy androgenetic alopecia (AGA) (n=2, 1%) (Table 1). Hair pull test was positive in patients with tinea capitis and alopecia areata. Hair fall in other sites and nail changes were found only in alopecia areata patients (Table 2). As regards the site of lesion, the vertical region was the most common site in our study in AA, TC, TTM, and AGA (65.47%, 61.72%, 50%, and 100%, respectively). KOH test was positive in 92.65% of patients with tinea capitis (Table 3).
Table 1.
Sex Distribution of Patients in Each Disease.
| Female | Male | p-value | Total Number | Percentage | |||
|---|---|---|---|---|---|---|---|
| Alopecia areata | 52 | 61.90% | 32 | 38.09% | 0.06 | 84 | 42 |
| Patchy androgenetic alopecia | 2 | 100% | 0 | 0% | 0.07 | 2 | 1 |
| Congenital triangular alopecia | 2 | 66.67% | 1 | 33.33% | 0.06 | 3 | 1.5 |
| Tractional alopecia | 14 | 100% | 0 | 0 | 0.2 | 14 | 7 |
| Tinea capitis | 10 | 12.34% | 71 | 87.65% | 0.13 | 81 | 40.5 |
| Trichotillomania | 7 | 43.75% | 9 | 56.25% | 0.02 | 16 | 8 |
| Total | 87 | 43.50% | 113 | 56.50% | 200 | 100 | |
p-value > 0.05: Non-significant; p-value ≤ 0.05: Significant; p-value ≤ 0.001: Highly significant.
Table 2.
Demographic Data and Characteristics of Studied Patients (n=200).
| Risk Factors | AA | TC | TTM | TA | CTA | Patchy AGA | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Contact with infected persons or tools | 0 | 0 | 10 | 12.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dealing with animals | 0 | 0 | 71 | 87.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Infection | 4 | 4.76 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stress | 20 | 23.8 | 0 | 0 | 16 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hair traction | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 100 | 0 | 0 | 0 | 0 |
| Medical history | ||||||||||||
| Atopy | 7 | 8.33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Down syndrome | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 7.14 | 0 | 0 | 0 | 0 |
| Familial Mediterranean fever | 1 | 1.19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Thyroid disease | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Family history of similar condition | 2 | 2.38 | 8 | 9.87 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 100 |
| Past history | 6 | 7.14 | 1 | 1.23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: AA: alopecia areata; AGA: androgenetic alopecia; CTA: congenital triangular alopecia; TA: traction alopecia; TC: tinea capitis; TTM: trichotillomania.
Table 3.
Clinical Examination of the Studied Patients (n=200).
| AA | TC | TTM | TA | CTA | Patchy AGA | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | N | % | ||
| Erythema | 17 | 20.2 | 39 | 48.1 | 0 | 0 | 10 | 71.4 | 0 | 0 | 0 | 0 | 0.02 |
| Pustules | 3 | 3.57 | 28 | 34.5 | 0 | 0 | 2 | 14.2 | 0 | 0 | 0 | 0 | 0.025 |
| Scaling | 1 | 1.19 | 65 | 80.2 | 0 | 0 | 4 | 28.5 | 0 | 0 | 0 | 0 | 0.7 |
| Pruritus | 9 | 10.7 | 58 | 71.6 | 0 | 0 | 6 | 42.8 | 0 | 0 | 0 | 0 | |
| Nail changes | 2 | 2.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.034 |
| Hair fall in other sites | 4 | 4.76 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.02 |
| Hair pull test (positive) | 21 | 25 | 69 | 85.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.012 |
| KOH test | 0 | 0 | 75 | 92.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.002 |
p-value > 0.05: Non-significant; p-value ≤ 0.05: Significant; p-value ≤ 0.001: Highly significant. Abbreviations: AA: alopecia areata; AGA: androgenetic alopecia; CTA: congenital triangular alopecia; TA: traction alopecia; TC: tinea capitis; TTM: trichotillomania.
Dermoscopic Findings of the Studied Patients (Table 4).
Table 4.
Dermoscopic Findings.
| Dermoscopic Findings in Alopecia Areata | ||
| Exclamation mark | 31 | 36.9% |
| Tapered hair | 12 | 14.28% |
| Pigtail hair | 12 | 14.28% |
| Regrowing hair | 21 | 25% |
| Vellus hair | 33 | 39.28% |
| Pohl-Pinkus constriction | 3 | 3.57% |
| Broken hair | 14 | 16.66% |
| Black dots | 25 | 29.76% |
| Yellow dots | 2 | 2.38% |
| Empty hair follicle | 23 | 27.38% |
| Erythema | 13 | 15.5% |
| Dermoscopic Findings in Tinea Capitis | ||
| Broken hair | 81 | 100% |
| Comma-shaped | 38 | 46.91% |
| Corkscrew hair | 21 | 25.9% |
| Bent hair | 47 | 58% |
| Zigzag hair | 28 | 34.5% |
| Morse code hair | 22 | 27.16% |
| I hair | 4 | 4.93% |
| Black dots | 16 | 19.70% |
| Empty hair follicle | 1 | 1.23% |
| Hair cast | 2 | 2.46% |
| Diffuse scaling | 61 | 75.30% |
| Perifollicular scaling | 59 | 72.8% |
| Perifollicular erythema | 44 | 54.32% |
| Perifollicular pustules | 28 | 34.56% |
| Dermoscopic Findings in Trichotillomania | ||
| Exclamation mark | 3 | 18.75% |
| Broken hair at different level | 15 | 93.75% |
| V-shaped hair | 8 | 50% |
| Tulip hair | 4 | 25% |
| Trichoptilosis | 7 | 43.75% |
| Flame hair | 7 | 43.75% |
| Hair powder | 8 | 50% |
| Mace sign | 3 | 18.75% |
| Burnt matchstick sign | 7 | 43.75% |
| I hair | 5 | 31.25% |
| Coiled hair | 1 | 6.25% |
| Hook hair | 3 | 18.75% |
| Black dots | 12 | 75% |
| Empty hair follicle | 1 | 6.25% |
| Dermoscopic Findings in Tractional Alopecia | ||
| Coiled hair | 2 | 14.2% |
| Hair diameter diversity | 8 | 57.14% |
| Vellus hair | 14 | 100% |
| Black dots | 1 | 7.14% |
| Empty hair follicle | 3 | 21.4% |
| Hair cast | 4 | 28.57% |
| Diffuse scaling | 1 | 7.14% |
| Perifollicular pustules | 2 | 14.2% |
| Perifollicular erythema | 14 | 100% |
| Coiled hair | 2 | 14.2% |
| Dermoscopic Findings in Congenital Triangular Alopecia | ||
| Vellus hair | 3 | 100% |
| White hair | 1 | 33.3% |
| Dermoscopic Findings in Patchy Androgenetic Alopecia | ||
| Peripheral terminal hair | 3 | 100% |
| Hair diameter diversity | 2 | 100% |
| Vellus hair >20% | 2 | 100% |
| Single hair pilosebaceous unit | 2 | 100% |
In alopecia areata, the most frequent trichoscopic dermoscopic findings were vellus hair and exclamation mark hair, in 39.28% and 36.9%, respectively (Figure 1). In TC, the most reported finding was broken hair, which was found in all cases (Figure 2). In trichotillomania, the most common trichoscopic dermoscopic finding was broken hair at different levels, which appeared in 93.75% (Figure 3). In tractional alopecia, the most common findings were interfollicular erythema and vellus hair appeared in 100% of cases (Figure 3). In congenital triangular alopecia, clusters of vellus hair and peripheral terminal hair appeared in all patients (Figure 4). In patchy androgenetic alopecia, hair diameter diversity, single hair pilosebaceous unit, and vellus hair were reported in 100% of patients (Figure 4).
Figure 1.
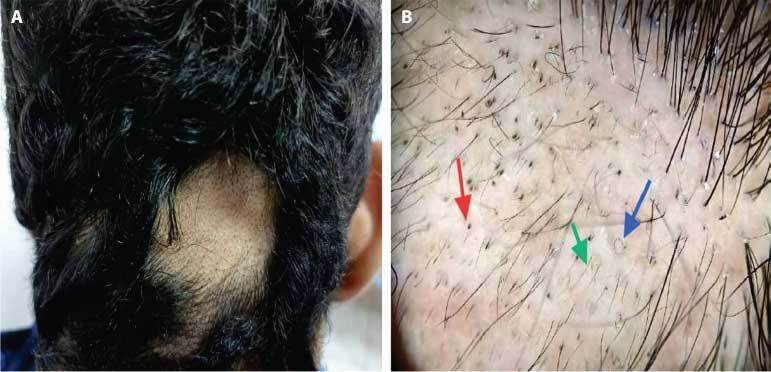
(A) A 16-year-old male patient complained of focal alopecia. (B) Trichoscopic view shows black dots (red arrow), pigtail hair (blue arrow), and short vellus hair (green arrow). Diagnosis: Alopecia areata.
Figure 2.
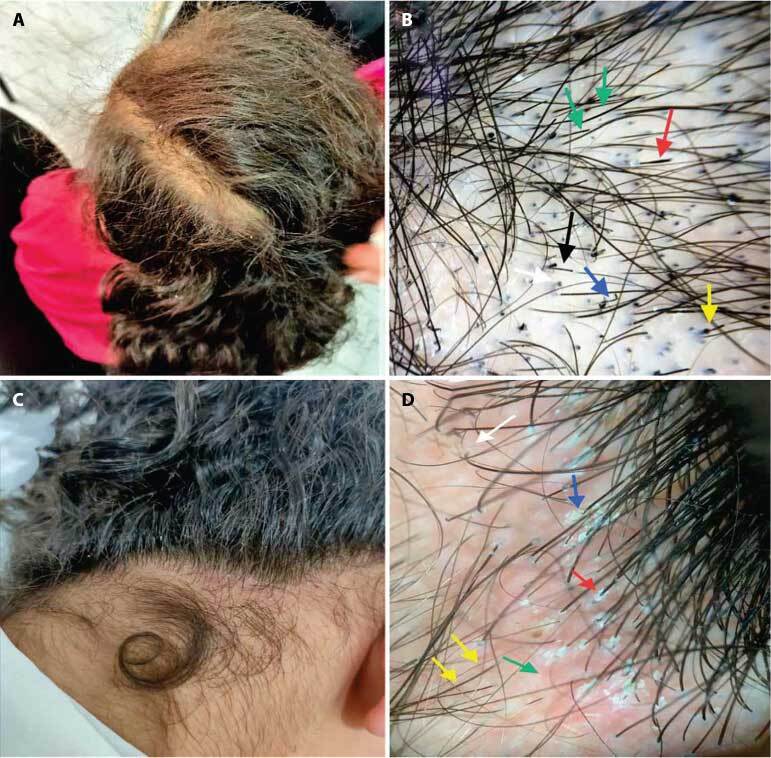
(A) A 9-year-old male patient presented with a single rounded patch of hair loss of 3 weeks’ duration, measuring about 8 cm in diameter. (B) Trichoscopic view shows diffuse scaling (yellow arrow) and perifollicular scaling (red arrow). (C) The trichoscopic view shows zigzag-shaped hair (black arrow), morse code hair (green arrow), and short broken hair (white arrow). Diagnosis: Tinea capitis.
Figure 3.
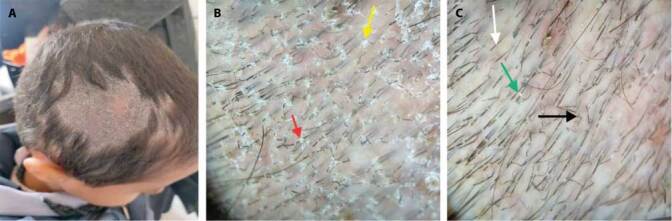
(A) A 7-year-old female patient complained of bizarre-shaped patchy hair loss. (B) Trichoscopic view shows broken hair at different lengths (green arrow), black dots (white arrow), V sign (black arrow), mace sign (red arrow), hook hair (blue arrow), and burnt matchstick sign (yellow arrow). Diagnosis: Trichotillomania. (C) A 13-year-old female patient complained of patchy alopecia. (D) Trichoscopic view shows perifollicular casts (white arrow), hair diameter diversity (yellow arrow), vellus hair (white arrow), perifollicular scales (blue arrow), and interfollicular erythema (green arrow). Diagnosis: Tractional alopecia.
Figure 4.
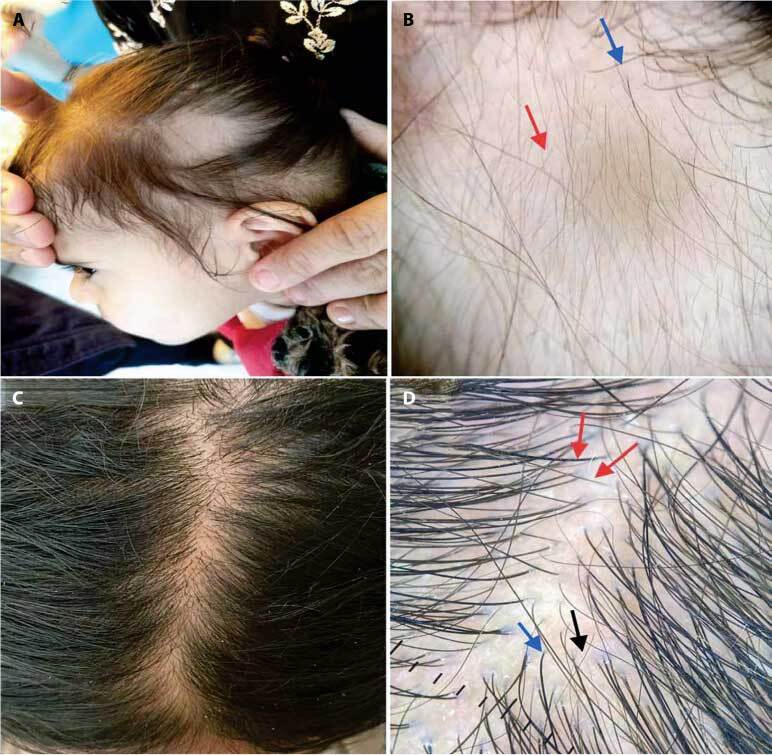
(A) A 5-year-old female patient complained of patchy hair loss since birth. (B) Trichoscopic view shows vellus hair (red arrow) and peripheral terminal hair (blue arrow). Diagnosis: Congenital triangular alopecia. (C) A 16-year-old female patient complained of patchy hair loss. (D) Trichoscopic view shows vellus hair (black arrow) and hair diameter diversity (red arrow) and single hair pilosebaceous unit (blue arrow). Diagnosis: Patchy androgenetic alopecia.
Relation Between Trichoscopic Findings and Diagnosis
Regarding the relation between trichoscopic findings and diagnosis, there was a high statistically significant presentation of pigtail hair, regrowing hair, and Pohl-Pinkus constriction with alopecia areata only, with percentages of 14.28%, 25%, and 3.57%, respectively (P=0.001). Tapered hair showed a significant difference with a percentage of 14.28% (P=0.01).
Exclamation marks showed highly statistically significant presentation with alopecia areata and trichotillomania, with percentages of 36.90% and 18.75%, respectively (P=0.001).
Comma-shaped, corkscrew, bent hair, zigzag hair, and morse code hair showed highly statistically significant presentation with tinea capitis only, with percentages of 46.90%, 25.90%, 58%, 34.50%, and 27.10%, respectively (P=0.001).
Trichoptilosis and flame hair showed a highly statistically significant presentation in trichotillomania (43.75%) for both; tulip hair and mace sign were 25% and 18.75%, respectively (P=0.001). Broken hair at different levels, V-shaped hair, hair powder, burnt matchstick sign, and hook hair showed statistically significant presentation with trichotillomania, with percentages of 93.75%, 50%, 50%, 43.75%, and 18.75%, respectively (P=0.01).
The i-shaped hair showed statistically significant presentation with tinea capitis and trichotillomania, with percentages of 94% and 31.25%, respectively. while coiled hair showed a statistically significant presentation in TTM and tractional alopecia, with percentages of 6.25% and 14.28%, respectively (P=0.01).
Hair diameter diversity and single hair pilosebaceous unit showed highly statistically significant presentation with patchy AGA, with a percentage of 100% (P=0.001).
Yellow dots showed a statistically significant presentation in AA, with a percentage of 27.38% (P=0.05).
Black dots showed statistically non-significant presentation in AA, TC, TTM, and TA, with percentages of 29.76%, 19.70%, (75%, and 7.10%, respectively (P=0.054). Empty hair follicles also showed a statistically non-significant presentation in AA, with a percentage of 2.38% (P=0.06). Broken hair showed non-significant presentation in AA, TC, and TTM, with percentages of 16.60%, 100%, 93.75%, respectively (P=0.06), while vellus hair showed a non-significant presentation in all patients with TA, CTA, and AGA, with a percentage of 100%, and in AA with a percentage of 39.28% (P=0.08).
White hair showed significant presentation with CTA, with a percentage of 33.3% (P=0.01).
Hair cast showed statistically significant presentation with TC and TA, with percentages of 2.47% and 28.57%, respectively (P=0.01). Perifollicular scaling and diffuse scaling showed high statistically significant presentation with TC, with percentages of 72.84% and 75.30%, respectively (P=0.001).
Perifollicular erythema showed highly statistically significant presentation with AA, TC, and TTM, with percentages of 15.40%, 54.30%, and 100%, respectively (P=0.001), while perifollicular pustule showed statistically significant presentation with AA, TC, and TTM, with percentages of 3.57%, 34.50%, and 14.20%, respectively (P=0.025) (Table 5).
Table 5.
Relationship Between Trichoscopic Findings and Diagnosis.
| Total Number | AA | TC | TTM | TA | CTA | AGA | test | p-value | S | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 84 | 81 | 16 | 14 | 3 | 2 | ||||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | ||||
| Tapered hair | 12 | 14.28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 12 | 0.01 | S |
| Pigtail hair | 12 | 14.28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 0.001 | HS |
| Regrowing hair | 21 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 0.001 | HS |
| Pohl-Pinkus constriction | 3 | 3.57 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 0.001 | HS |
| Exclamation mark | 31 | 36.90 | 0 | 0 | 3 | 18.75 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 0.001 | HS |
| Comma shaped | 0 | 0 | 38 | 46.90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 11 | 0.001 | HS |
| Corkscrew hair | 0 | 0 | 21 | 25.90 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 33 | 0.001 | HS |
| Bent hair | 0 | 0 | 47 | 58.00 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 45 | 0.001 | HS |
| Zigzag hair | 0 | 0 | 28 | 34.50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 35 | 0.001 | HS |
| Morse code hair | 0 | 0 | 22 | 27.10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 63 | 0.001 | HS |
| Vellus hair | 33 | 39.28 | 0 | 0 | 0 | 0 | 14 | 100 | 3 | 100 | 2 | 100 | 8 | 0.08 | NS |
| White hair | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 33.3 | 0 | 0 | 0 | 0.01 | S | |
| Broken hair | 14 | 16.60 | 81 | 100 | 15 | 93.75 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0.06 | NS |
| Broken hair at different | 0 | 0 | 0 | 0 | 15 | 93.75 | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 0.01 | S |
| levels | |||||||||||||||
| V-shaped hair | 0 | 0 | 0 | 0 | 8 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 18 | 0.01 | S |
| Tulip hair | 0 | 0 | 0 | 0 | 4 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 0.001 | HS |
| Trichoptilosis | 0 | 0 | 0 | 0 | 7 | 43.75 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 0.001 | HS |
| Flame hair | 0 | 0 | 0 | 0 | 7 | 43.75 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 0.001 | HS |
| Hair powder | 0 | 0 | 0 | 0 | 8 | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 15 | 0.01 | S |
| Mace sign | 0 | 0 | 0 | 0 | 3 | 18.75 | 0 | 0 | 0 | 0 | 0 | 0 | 20 | 0.001 | HS |
| Burnt matchstick sign | 0 | 0 | 0 | 0 | 7 | 43.75 | 0 | 0 | 0 | 0 | 0 | 0 | 7 | 0.01 | S |
| I hair | 0 | 0 | 4 | 4.90 | 5 | 31.25 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0.01 | S |
| Coiled hair | 0 | 0 | 0 | 0 | 1 | 6.25 | 2 | 14.28 | 0 | 0 | 0 | 0 | 12 | 0.01 | S |
| Hook hair | 0 | 0 | 0 | 0 | 3 | 18.75 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 0.01 | S |
| Hair diameter diversity | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 57.14 | 0 | 0 | 2 | 100 | 19 | 0.001 | HS |
| Single hair pilosebaceous | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.00 | 0 | 0 | 2 | 100 | 19 | 0.001 | HS |
| unit | |||||||||||||||
| Black dots | 25 | 29.76 | 16 | 19.70 | 12 | 75 | 1 | 7.10 | 0 | 0 | 0 | 0 | 7 | 0.054 | NS |
| Yellow dots | 2 | 2.38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 0.05 | S |
| Empty hair follicle | 23 | 27.38 | 1 | 1.23 | 1 | 6.25 | 3 | 21.40 | 0 | 0 | 0 | 0 | 8 | 0.06 | NS |
| Hair cast | 0 | 0.00 | 2 | 2.47 | 0 | 0 | 4 | 28.57 | 0 | 0 | 0 | 0 | 10 | 0.01 | S |
| Perifollicular scaling | 0 | 0.00 | 59 | 72.8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 0.001 | HS |
| Diffuse scaling | 0 | 0.00 | 61 | 75.3 | 0 | 0 | 1 | 7.10 | 0 | 0 | 0 | 0 | 17 | 0.001 | HS |
| Perifollicular erythema | 13 | 15.40 | 44 | 54.30 | 0 | 0 | 14 | 100 | 0 | 0 | 0 | 0 | 20 | 0.001 | HS |
| Perifollicular pustule | 3 | 3.57 | 28 | 34.50 | 0 | 0 | 2 | 14.20 | 0 | 0 | 0 | 0 | 22 | 0.025 | S |
p-value > 0.05: Non-significant; p-value ≤ 0.05: Significant; p-value ≤ 0.001: Highly significant.
Chi-square test.
Abbreviations: AA: alopecia areata; AGA: androgenetic alopecia; CTA: congenital triangular alopecia; TA: traction alopecia; TC: tinea capitis; TTM: trichotillomania.
Discussion
Because the causes of focal non-cicatricial alopecia in pediatric patients are diverse, this poses a formidable challenge for dermatologists to accurately diagnose and treat individuals in order to mitigate further hair loss [8]. Among children, common culprits for localized alopecia include alopecia areata, tinea capitis, trichotillomania, and traction alopecia [9].
Within the study cohort, comprising 113 male patients (56.50%) and 87 female patients (43.50%), alopecia areata emerged as the predominant diagnosis (42%), followed closely by tinea capitis (40.5%), trichotillomania (8%), tractional alopecia (7%), congenital triangular alopecia (1.5%), and patchy androgenetic alopecia (1%). In alignment with our findings, Cortés et al. [10] and Conti et al. [11] reported alopecia areata as the most prevalent, succeeded by tinea capitis. Conversely, Sharma et al. [8] and Shetty et al. [12] reported tinea capitis as the primary disorder, followed by alopecia areata. A noteworthy observation was a solitary case (1.19%) of alopecia areata linked with familial Mediterranean fever (FMF). FMF, an inheritable autoinflammatory disease marked by periodic fever and serositis, exhibited a connection with alopecia areata in three cases, as reported by Atış et al. [13]. This suggests a potential convergence of genetic predisposition and immune system involvement in the pathogenesis of both FMF and alopecia areata, both classified as MHC Class I-related diseases. The association between atopic dermatitis and alopecia areata manifested in 8.33% of cases, mirroring the findings of Shetty et al. [12], who reported a 4.7% prevalence of alopecia areata in atopic children. Positive family history was noted in 2.38% of alopecia areata cases, 9.87% of tinea capitis cases, and 100% of cases with patchy androgenetic alopecia, which is supported by previous findings by Sharma et al. [8].
Asymptomatic hair loss emerged as the predominant presentation in our study, aligning with the observations of Shetty et al. [12], Cortés et al. [10], and Nnoruka et al. [14]. Additionally, 4.76% of patchy alopecia areata patients exhibited associated hair loss from eyelashes and eyebrows, a phenomenon also noted by Sharma et al. [8], who reported hair loss in multiple areas, including eyelashes, eyebrows, and pubic hair, in patients with alopecia universalis.
Nail changes were discerned in 2.3% of alopecia areata cases, consistent with findings by Sharma et al. [8] and Shetty et al. [12], who reported nail changes in 26.41% and 16.5% of cases, respectively.
Trichoscopic findings in alopecia areata in our study revealed exclamation mark hairs in 36.9% of cases, in agreement with Waśkiel et al. (39%) [15] and Rakowska et al. (36%) [16]. Although exclamation mark hairs are considered a characteristic feature of active alopecia areata, they can also be present in other conditions such as trichotillomania and chemotherapy-induced alopecia [18].
Broken hairs were identified in 16.66% of the cases in our study, while Inui et al. [17] reported their presence in 45.7% of alopecia areata cases. Likewise, black dots were observed in 26.76% of our cases; Mane et al. (67.7%) [19] and Karadag and Güleç (27.1%) [20] also reported black dots. The concurrent presence of exclamation mark hair, broken hair, and black dots serves as indicators of disease activity and severity [21].
Tapered hairs were evident in 51% of our cases, a figure echoed in the study by Inui et al. [17], where they were reported in 31.7% of alopecia areata cases. Pohl-Pinkus constrictions, a feature associated with the active stage of the disease, were observed in 1.57% of our cases, a finding corroborated by Waśkiel et al. [15], who noted them in 4% of cases [4].
Yellow dots were identified in 2.38% of our cases, displaying a lower prevalence compared to studies by Lacarrubba et al. [21], Kibar et al. [22], Mane et al. [19], and Khunkhet et al. [23], where yellow dots ranged from 6% to 100%. This disparity may be attributed to the active stage and short duration of the disease in our study, as Miteva and Tosti [24] suggest that yellow dots are absent in children due to underdeveloped sebaceous glands before puberty.
Short vellus hairs were present in 39.6% of our cases, aligning with findings in studies by Ekiz et al. (50%) [25] and Waśkiel et al. (61%) [15]. Considered a potential sign of spontaneous remission or successful treatment, short vellus hairs indicate the non-destructive nature of the disease. Pigtail hairs were noted in 14.28% of our cases, akin to the observations in studies by Waśkiel et al. (21%) [15] and Rakowska et al. (23%) [16].
Upright regrowing hairs were observed in 23% of our cases, consistent with the findings of Rakowska et al. [16], who reported them in 44% of cases.
The presence of short vellus hairs, pigtail hairs, and upright regrowing hairs collectively signifies ongoing hair regrowth [16, 21].
In our study, tinea capitis emerged as the second most prevalent diagnosis (40.5%), with gray patch being the predominant type, followed by black dot and kerion. Similar patterns were reported by Abu El-Enin et al. [26], though Grover et al. [27] identified the black dot as the most common clinical type. Variations in clinical type prevalence may be attributed to differences in race, culture, or climate conditions.
In our study, 5% of the cases exhibited more than one clinical type of tinea capitis, categorized as the mixed type. Grover et al. [27] and Sharma et al. [8] also reported mixed patterns in 10% and 6% of cases, respectively. Tinea capitis was more prevalent in males, consistent with Ayaya et al.’s [28] study in Kenya. This higher prevalence among boys may be attributed to factors such as shorter hair, facilitating easier spore spread, and the use of infrequently disinfected barbing instruments and cap exchange [29].
In TC patients, short broken hairs were universally detected in all cases (100%) in our study, mirroring Ekiz et al.’s [25] findings. Rakowska et al. [16] reported broken hairs in 87% of cases, suggesting that short broken hairs may be a non-specific trichoscopic finding in tinea capitis but could indicate disease severity [30].
Additionally, black dots were observed in 19.70% of our tinea capitis cases, akin to Ekiz et al.’s [25] findings of 13.3%. However, it is crucial to note that black dots and short broken or dystrophic hairs are non-specific to tinea capitis and can also be present in conditions like alopecia areata and trichotillomania [4]. Comma-shaped hairs were identified in 46.91% of our cases, a finding consistent with studies by Rakowska et al. (50% of cases) [16] and Ekiz et al. (66.7% of patients) [25]. Spiral or corkscrew hair manifested in 25.9% of our cases, aligning with observations by Ekiz et al. (80.0% of patients) [25]. Zigzag-shaped hairs were evident in 34.5% of our cases, mirroring the findings of Rakowska et al. [16], who reported a similar occurrence in 50% of cases. I-hairs were observed in a low percentage (4.93%) of cases in our study, consistent with the results reported by Amer et al. [31], who found i-hairs in 5% of tinea capitis cases. However, these hair features are not exclusive to tinea capitis and can also be present in patients with alopecia areata and trichotillomania [32]. Diffuse scaling was prevalent in 75.3% of our cases, while perifollicular scaling was observed in 72.8% of cases, aligning with a study by Park et al. [18], where perifollicular scaling was found in 88.9% of cases and diffuse scaling in 100% of cases. Perifollicular erythema and pustules were present in 54.32% and 34.56% of cases in our study, respectively.
In our study, the KOH test yielded positive results in 92.6% of tinea capitis cases, nearly mirroring the findings of Grover et al. [27]. This concordance may be attributed to the potential for false-negative KOH tests, even among experienced observers, due to inadequate sample collection or artifacts [33].
Trichotillomania accounted for 8% of cases in our study, with a slight male predominance, contradicting previous reports that predominantly affect females [34]. The atypical sex distribution in our cases may be attributed to the small sample size analyzed. Trichotillomania trichoscopic findings included broken hair at different levels and black dots in 93.75% and 75%) of our patients, respectively. This aligns with Elmas & Metin [35] study, which found similar trichoscopic findings in trichotillomania patients, including broken hairs (100%) and black dots (85%). Other findings, such as tulip hairs, mace hairs, flame hair, V-sign, and burnt matchstick sign, were also noted in trichotillomania cases.
Tractional alopecia trichoscopic findings included vellus hair and interfollicular erythema in 100% of cases, perifollicular casts in 28.57% of cases, black dots in 7.14% of cases, hair diameter diversity in 57.14% of cases, empty hair follicles in 21.4% of cases, and coiled hair and perifollicular pustules in 14.2% of cases. Mualla [36] reported similar findings, with vellus hair observed in 100% of tractional alopecia cases, broken hairs in 68%, yellow dots in 68%, and black dots in 48%. However, Tosti et al. [37] reported a higher occurrence of perifollicular casts (81%) compared to our results.
Congenital triangular alopecia accounted for 1.5% of cases in our study, with all cases being unilateral, consistent with Yin Li and Yesudian’s [38] reported predominance of unilateral involvement. The most common findings in congenital triangular alopecia were vellus hair length diversity in 100% of cases and white hairs in 33.3% of cases, visible only through trichoscopy. Karadağ et al. [20] found short vellus hair, vellus hair length diversity, and white hair in 100% of their cases.
Androgenetic alopecia trichoscopic findings included hair diameter diversity in affected areas, with more than 20% vellus hair and the presence of single hair bilosebaceous units. These findings aligned with other studies diagnosing androgenetic alopecia in children, such as Rossi et al. [39], where hair shaft miniaturization and hair shaft diameter diversity greater than 20% were diagnostic criteria for androgenetic alopecia.
Conclusion
In conclusion, the routine use of trichoscopy in the clinical evaluation of scalp and hair disorders will enhance diagnostic capabilities beyond simple clinical inspection by revealing specific features of disease, leading to better management.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Micali G, Verzì AE, Quattrocchi E, Ng CY, Lacarrubba F. Dermatoscopy of Common Lesions in Pediatric Dermatology. Dermatol Clin. 2018 Oct;36(4):463–472. doi: 10.1016/j.det.2018.05.012. Epub 2018 Aug 16. [DOI] [PubMed] [Google Scholar]
- 2.Rudnicka L, Olszewska M, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008 Jul;7(7):651–4. [PubMed] [Google Scholar]
- 3.Bhandary DJ, Girisha BS, Mahadevappa BN. Clinico-Dermoscopic Pattern of Beard Alopecia Areata: A Cross-Sectional Study. Indian Dermatol Online J. 2019 Nov 1;10(6):644–649. doi: 10.4103/idoj.IDOJ_508_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudnicka L, Olszewska M, Rakowska A. Atlas of trichoscopy: dermoscopy in hair and scalp disease. Springer Science & Business Media; 2012. [Google Scholar]
- 5.Rakowska A, Rudnicka L. Dermoscopy in General Dermatology. CRC Press; 2018. Hair disorders (trichoscopy) pp. 229–240. [Google Scholar]
- 6.Olszewska M, Rudnicka L, Rakowska A. Atlas of trichoscopy: Dermoscopy in hair and scalp disease. Springer Science & Business Media; 2012. [Google Scholar]
- 7.Rudnicka L, Rakowska A, Kerzeja M, Olszewska M.Hair shafts in trichoscopy: clues for diagnosis of hair and scalp diseases Dermatol Clin 2013Oct314695–708., x 10.1016/j.det.2013.06.007 [DOI] [PubMed] [Google Scholar]
- 8.Sharma MK, Gupta S, Kumar R, Singhal AK, Jain SK, Sharma M. A Clinico-Epidemiological Study of Scalp Hair Loss in Children (0–18 Years) in Kota Region, South-East Rajasthan. Indian J Dermatol. 2019 Jul–Aug;64(4):285–291. doi: 10.4103/ijd.IJD_393_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu L, Liu KX, Senna MM. A Practical Approach to the Diagnosis and Management of Hair Loss in Children and Adolescents. Front Med (Lausanne) 2017 Jul 24;4:112. doi: 10.3389/fmed.2017.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortés GA, Mardones VF, Zemelman DV. Caracterización de las causas de alopecia infantil [Aetiology of childhood alopecia] Rev Chil Pediatr. 2015 Jul–Aug;86(4):264–9. doi: 10.1016/j.rchipe.2015.06.015. Spanish. Epub 2015 Aug 19. [DOI] [PubMed] [Google Scholar]
- 11.Conti R, Colucci R, Arunachalam M, Berti S, Fabroni C, De Martino M, Dragoni F, Lazzeri L, Pisaneschi L, Moretti S. Hair and Scalp Disorders in a Tuscan Pediatric Dermatological Outpatient Clinic: A Clinical and Epidemiological Evaluation. Med Princ Pract. 2016;25(1):67–71. doi: 10.1159/000439466. Epub 2015 Oct 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shetty VM, Shanmukhappa AG, Nataraj HV, Aradhya SS. Hair Loss in Children: A Clinicoetiological Study from South India. Int J Trichology. 2021 Nov–Dec;13(6):17–25. doi: 10.4103/ijt.ijt_56_19. Epub 2021 Nov 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Atış G, Eroğlu SS, Güldiken G. Is There a Pathogenetic Relationship Between Alopecia Areata and Familial Mediterranean Fever? Indian J Dermatol. 2022 Nov–Dec;67(6):835. doi: 10.4103/ijd.ijd_312_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nnoruka EN, Obiagboso I, Maduechesi C. Hair loss in children in South-East Nigeria: common and uncommon cases. Int J Dermatol. 2007 Oct;46(Suppl 1):18–22. doi: 10.1111/j.1365-4632.2007.03457.x. [DOI] [PubMed] [Google Scholar]
- 15.Waśkiel A, Rakowska A, Sikora M, Olszewska M, Rudnicka L. Trichoscopy of alopecia areata: An update. J Dermatol. 2018 Jun;45(6):692–700. doi: 10.1111/1346-8138.14283. Epub 2018 Mar 22. [DOI] [PubMed] [Google Scholar]
- 16.Rakowska A, Maj M, Zadurska M, et al. Trichoscopy of Focal Alopecia in Children - New Trichoscopic Findings: Hair Bulbs Arranged Radially along Hair-Bearing Margins in Aplasia Cutis Congenita. Skin Appendage Disord. 2016 Sep;2(1–2):1–6. doi: 10.1159/000445721. Epub 2016 Apr 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inui S, Nakajima T, Itami S. Scalp dermoscopy of androgenetic alopecia in Asian people. J Dermatol. 2009 Feb;36(2):82–5. doi: 10.1111/j.1346-8138.2009.00593.x. [DOI] [PubMed] [Google Scholar]
- 18.Park J, Kim JI, Kim HU, Yun SK, Kim SJ. Trichoscopic Findings of Hair Loss in Koreans. Ann Dermatol. 2015 Oct;27(5):539–50. doi: 10.5021/ad.2015.27.5.539. Epub 2015 Oct 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mane M, Nath AK, Thappa DM. Utility of dermoscopy in alopecia areata. Indian J Dermatol. 2011 Jul;56(4):407–11. doi: 10.4103/0019-5154.84768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karadağ Köse Ö, Güleç AT. Clinical evaluation of alopecias using a handheld dermatoscope. J Am Acad Dermatol. 2012 Aug;67(2):206–14. doi: 10.1016/j.jaad.2011.08.019. Epub 2011 Oct 22. [DOI] [PubMed] [Google Scholar]
- 21.Lacarrubba F, Verzì AE, Errichetti E, Stinco G, Micali G. Darier disease: Dermoscopy, confocal microscopy, and histologic correlations. J Am Acad Dermatol. 2015 Sep;73(3):e97–9. doi: 10.1016/j.jaad.2015.04.066. [DOI] [PubMed] [Google Scholar]
- 22.Kibar M, Aktan Ş, Lebe B, Bilgin M. Trichoscopic findings in alopecia areata and their relation to disease activity, severity and clinical subtype in Turkish patients. Australas J Dermatol. 2015 Feb;56(1):e1–6. doi: 10.1111/ajd.12102. Epub 2013 Aug 29. [DOI] [PubMed] [Google Scholar]
- 23.Khunkhet S, Vachiramon V, Suchonwanit P. Trichoscopic clues for diagnosis of alopecia areata and trichotillomania in Asians. Int J Dermatol. 2017 Feb;56(2):161–165. doi: 10.1111/ijd.13453. [DOI] [PubMed] [Google Scholar]
- 24.Miteva M, Tosti A. Hair and scalp dermatoscopy. J Am Acad Dermatol. 2012 Nov;67(5):1040–8. doi: 10.1016/j.jaad.2012.02.013. Epub 2012 Mar 8. [DOI] [PubMed] [Google Scholar]
- 25.Ekiz O, Sen BB, Rifaioğlu EN, Balta I. Trichoscopy in paediatric patients with tinea capitis: a useful method to differentiate from alopecia areata. J Eur Acad Dermatol Venereol. 2014 Sep;28(9):1255–8. doi: 10.1111/jdv.12246. Epub 2013 Aug 24. [DOI] [PubMed] [Google Scholar]
- 26.Abu El – Enin A, Khedr M, Abu El-Ata A. Tinea Capitis In Assuit Governorate: (A Clinical and Mycological Study) The Egyptian Journal of Hospital Medicine. 2007;29(1):738–744. doi: 10.21608/ejhm.2007.17715. [DOI] [Google Scholar]
- 27.Grover C, Arora P, Manchanda V. Tinea capitis in the pediatric population: a study from North India. Indian J Dermatol Venereol Leprol. 2010 Sep–Oct;76(5):527–32. doi: 10.4103/0378-6323.69078. [DOI] [PubMed] [Google Scholar]
- 28.Ayaya SO, Kamar KK, Kakai R. Aetiology of tinea capitis in school children. East Afr Med J. 2001 Oct;78(10):531–5. doi: 10.4314/eamj.v78i10.8963. [DOI] [PubMed] [Google Scholar]
- 29.Uneke C, Ngwu B, Egemba O. Tinea Capitis And Pityriasis Versicolor Infections Among School Children In The South-Eastern Nigeria: The Public Health Implications. The Internet Journal of Dermatology. 2005;4(2) [Google Scholar]
- 30.El-Taweel AE, El-Esawy F, Abdel-Salam O. Different trichoscopic features of tinea capitis and alopecia areata in pediatric patients. Dermatol Res Pract. 2014;2014:848763. doi: 10.1155/2014/848763. Epub 2014 Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amer M, Helmy A, Amer A. Trichoscopy as a useful method to differentiate tinea capitis from alopecia areata in children at Zagazig University Hospitals. Int J Dermatol. 2017 Jan;56(1):116–120. doi: 10.1111/ijd.13217. Epub 2016 Sep 22. [DOI] [PubMed] [Google Scholar]
- 32.Malakar S, Mehta PR. “i hair”: A prognostic marker in alopecia areata & trichotillomania. Indian J Dermatol. 2017 Nov–Dec;62(6):658–660. doi: 10.4103/ijd.IJD_337_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhurat R, Shukla D, Agrawal S, Chitalia J, Ghate S, Jage M. Tinea Capitis Presenting as Diffuse Hair Loss and Significance of Trichoscopy: Four Case Reports. Skin Appendage Disord. 2021 Jun;7(4):286–291. doi: 10.1159/000513315. Epub 2021 May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melo DF, Lima CDS, Piraccini BM, Tosti A. Trichotillomania: What Do We Know So Far? Skin Appendage Disord. 2022 Jan;8(1):1–7. doi: 10.1159/000518191. Epub 2021 Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elmas ÖF, Metin MS. Trichoscopic findings of trichotillomania: new observations. Postepy Dermatol Alergol. 2020 Jun;37(3):340–345. doi: 10.5114/ada.2020.96295. Epub 2020 Jul 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polat M. Evaluation of clinical signs and early and late trichoscopy findings in traction alopecia patients with Fitzpatrick skin type II and III: a single-center, clinical study. Int J Dermatol. 2017 Aug;56(8):850–855. doi: 10.1111/ijd.13599. Epub 2017 Mar 30. [DOI] [PubMed] [Google Scholar]
- 37.Tosti A, Iorizzo M, Piraccini BM. Androgenetic alopecia in children: report of 20 cases. Br J Dermatol. 2005 Mar;152(3):556–9. doi: 10.1111/j.1365-2133.2004.06279.x. [DOI] [PubMed] [Google Scholar]
- 38.Yin Li VC, Yesudian PD. Congenital Triangular Alopecia. Int J Trichology. 2015 Apr–Jun;7(2):48–53. doi: 10.4103/0974-7753.160089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rossi A, D’Arino A, Pigliacelli F, Caro G, Muscianese M, Fortuna MC, Carlesimo M. The diagnosis of androgenetic alopecia in children: Considerations of pathophysiological plausibility. Australas J Dermatol. 2019 Nov;60(4):e279–e283. doi: 10.1111/ajd.13079. Epub 2019 Jun 6. [DOI] [PubMed] [Google Scholar]


