Abstract
Non-hazardous waste generated in Metropolitan Lima and Callao is transported to the Modelo Callao landfill for safe disposal. The accumulation of waste constitutes a significant source of fungal particles released into the atmosphere, posing a potential health risk to nearby populations. The aim of this research was to evaluate the concentration of outdoor fungal particles, considering environmental conditions (temperature, relative humidity, wind speed, and direction) during summer and winter seasons in the 18 de octubre settlement and Chillón Avenue, areas located in the vicinity of the Modelo Callao Landfill in Ventanilla during 2022. The gravitational method was used for sampling. The highest concentrations were detected at 150 and 200 m from the landfill, where a kindergarten and a local park are located. Fifteen fungal genera were identified in both seasons. The predominant fungi were Aspergillus spp. (46.09 %), Penicillium spp. (23.29 %) and Alternaria spp. (11.33 %). The average concentrations during summer and winter were 297.21 CFU/m3 and 471.69 CFU/m3, respectively. Based on these findings, we recommend that residential areas be located beyond 200 m from the landfill to minimize exposure to fungal aerosols. Additionally, we propose the implementation of an action plan to improve air quality in the areas surrounding the final disposal infrastructure.
Keywords: Air pollution, Bioaerosols, Waste management, Landfill disposal, Waste disposal, Fungal particles
1. Introduction
Landfills are a widely employed waste management method, necessary for effectively handling waste and reducing the associated environmental impacts of safe disposal [1]. Their operation involves the controlled containment and storage of solid waste using covering materials [2]. However, the increasing construction of landfills in recent years has raised concerns due to their potential impact. Despite advances in management practices, surrounding areas often face issues such as soil degradation, groundwater contamination, land instability, the onset of erosive processes, landscape alteration, unpleasant odours, and air pollution [3].
Waste disposal in landfills and tips remains a persistent challenge, leading to environmental issues such as particle emissions, foul odours, leachate production, gaseous emissions (for example, CH4, CO, CO2, H2S, NO2, SO2, HCl, and HF), toxic organic microcontaminants (dioxins and polycyclic aromatic hydrocarbons), dissemination of bioaerosols, and an increase in scavenging birds, insects, and rodents [[4], [5], [6], [7]].
Globally, waste disposal is distributed as follows: 33 % of waste is sent to open dumps, 25 % to unspecified landfills, 13.5 % is recycled, 11 % is incinerated, 7.7 % is disposed of in sanitary landfills with gas collection, 5.5 % is composted, 4 % is deposited in controlled landfills, and 1 % is treated using other methods [8]. In Europe, several countries send more than 20 % of their waste to landfills, including Malta, Cyprus, Romania, Portugal, Spain, Hungary, Croatia, Slovakia, Czechia, Latvia, France, and Poland. The case of Malta is concerning as more than 70 % of municipal waste ends up in landfills owing to a lack of measures for material and/or energy recovery from waste [9]. Contrarily, in sub-Saharan Africa, South Asia, and the Middle East, half the waste is transported to open dumps [8].
In Peru, during the year 2023, approximately 23.3 million tonnes of waste were deposited in landfills, of which over 4 million tons were originated from the Metropolitan Lima and Callao provinces [10]. The country has 650 waste facilities, including five landfills located in the capital city of Lima and the provinces of Callao: Huaycoloro, Portillo Grande, Modelo Callao, and Chilca [11]. Despite these efforts, selective waste collection has only been implemented in two districts in the country, causing waste from other municipalities to be transported to landfills without segregation or potential recovery. Municipal waste, composed of a mixture of organic and inorganic materials, is a source of pollution in the final disposal infrastructure. This waste releases bioaerosols into the environment through decomposition, fragmentation, and disintegration.
Bioaerosols are assemblages of suspended particles of biological origin including fungi, viruses, parasites, bacteria, mites, and pollen [12,13]. These particles range in size from 10 nm to 100 μm and could have detrimental effects on both human health and crops [14,15]. Fungal particles, a component of bioaerosols, originate from organic matter, soil, plants, and animals and can have health impacts owing to their resistance to desiccation in the environment. These particles are found in the air as spores, hyphal fragments, or parts of sporophores, remaining suspended until they reach areas with less turbulence, where the sedimentation velocity exceeds the wind speed [16]. Landfills function as microbial reservoirs due to the decomposition of organic waste, releasing fungal microorganisms into the soil. In this environment, these organisms actively participate in biogeochemical cycles and act as decomposers. During waste turning and compaction operations, fungal particles become airborne and can remain suspended in the atmosphere until encountering a suitable growth substrate. It is noteworthy that, whilst air always contains fungal spores, waste management facilities may increase their concentration when appropriate conditions exist, particularly with the continuous influx of organic materials.
Fungi are found in both indoor and outdoor environments, including landfills and nearby communities, where waste accumulation, decomposition, compaction, and confinement occur [17]. For instance, the Northern Landfill in Poland recorded an outdoor air concentration of fungal particles at 10,000 CFU/m³ [18]. A landfill in Taiwan reported an average concentration of 11,000 CFU/m³ [19]. In Dehradun, India, an average of 4600 CFU/m³ was detected [20], while Londrina, Brazil recorded 2738.3 CFU/m³ [16]. The Municipal Landfill of Colombia showed a range of 730 to 1830 CFU/m³ [21]. The results obtained exceed the following standards: World Health Organization (500 CFU/m3), Polish Standard (3000–5000 CFU/m3 for uncontaminated air; 5000–10,000 CFU/m3 for moderately contaminated air; and values greater than 10,000 CFU/m3 for heavily contaminated air), Omeliansky (500 CFU/m3), and China Scientific Ecology Center (CSEC) (500 CFU/m3) [[22], [23], [24], [25]].
The Modelo Callao Landfill, situated in Ventanilla, Peru. Despite its proximity to residential areas, including the 18 de octubre settlement, no previous studies have determined the level of fungal contamination in the surrounding atmosphere. The lack of knowledge regarding their health effects has generated inconsistencies within the Peruvian regulatory framework, especially concerning the permissible distance between landfills and nearby populations, which should exceed 500 m. There is no established regulation for measuring microbiological parameters, which hinders environmental monitoring of outdoor fungal concentrations. Moreover, the exposure of populations residing near landfills could be exacerbated by informal occupation of land within or around solid waste disposal facilities. Further research is required to determine the environmental impact of disposal sites on residential areas.
The current study aimed to determine the air fungal concentrations and their association with environmental conditions during the summer (January–March) and winter (June–August) seasons in the 18 de octubre settlement and Chillón Avenue, situated around the Modelo Callao Landfill in Ventanilla. These seasons were chosen because Peru is geographically situated in the Intertropical Zone of the Earth, causing both seasons to be more distinctly pronounced than the others.
2. Materials and methods
2.1. General characteristics of the landfill
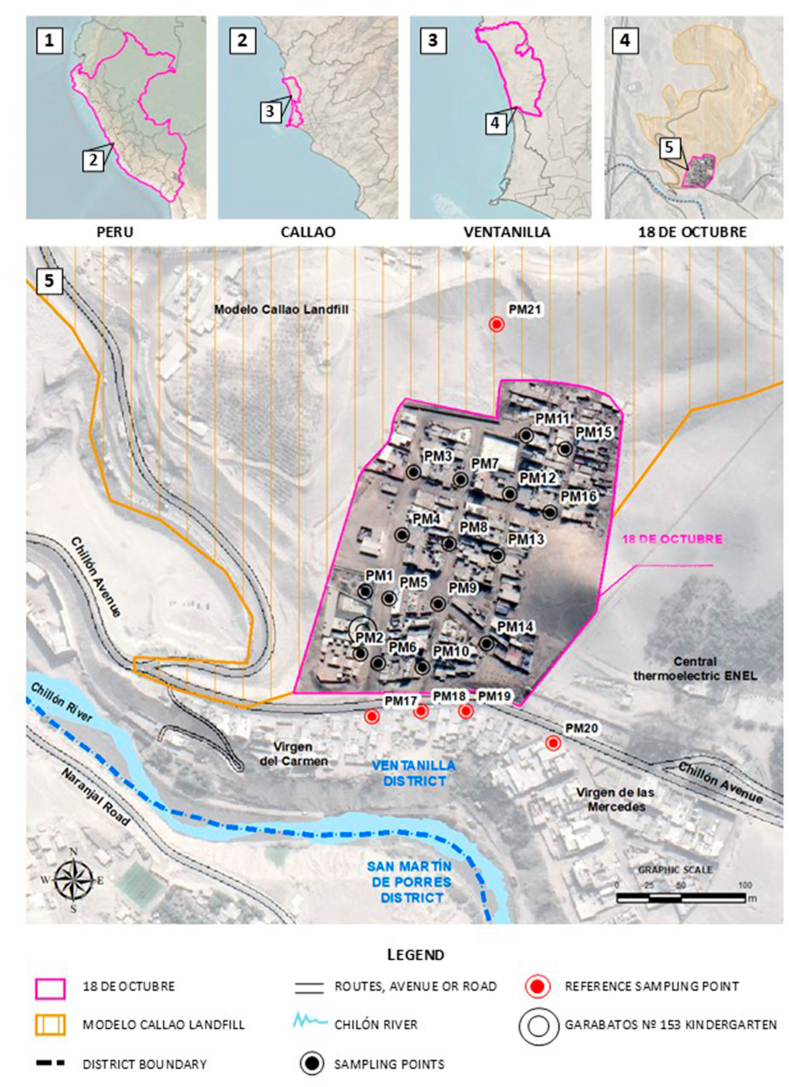
The Modelo Callao Landfill is one of the most important final disposal sites in the Callao and Metropolitan Lima areas (Fig. 1). Until 2003, this area was a waste dump known as the “La Cucaracha”. It is located in the southern area of the Ventanilla district, in the Constitutional Province of Callao, at kilometre 19 of the Callao – Ventanilla route, in the area known as “Parque Porcino”. The landfill is situated on the right bank of the Chillón River [26] and is bordered by a Thermal Power Plant to the east, Chillón Avenue to the south, and landfills to the west and north. Three settlements are located in the surroundings: Virgen de las Mercedes, 18 de octubre, and Virgen del Carmen.
Fig. 1.
Modelo Callao Landfill.
The landfill has a storage capacity of 1200–2000 m3 per day and a useful lifespan of 30 years. Currently, this landfill has an approximate surface area of 962,321.88 m2 [27]. The solid waste infrastructure includes a reception booth, weighing area, final disposal cell for solid waste, leachate storage and recirculation system, gas evacuation and/or control drains, maintenance areas (heavy machinery, electric welding, tire shops, and air compressors), sanitary barriers, and septic tanks.
Environmental impacts include particulate matter emissions, leachate generation, alteration of the natural landscape, release of polluting gases, presence of vectors, and soil and groundwater contamination. The concessionaire company has introduced green areas in the gatehouse zone, considering species such as Hibiscus tiliaceus, Ficus microcarpa, and Ficus benjamina. Additionally, a prefabricated concrete wall has been placed around the 18 de octubre settlement.
Currently, landfills face challenges such as storage capacity in the final disposal cells, excessive dust generation in the vicinity of Chillón Avenue, land invasion by the 18 de octubre settlement, informality of solid waste operating and recycling companies, release of polluting gases and unpleasant odours at different times of the day, and public concern regarding potential contamination of the Chillón River [26].
2.2. Description of waste management
The concessionaire company of the Modelo Callao Landfill is primarily responsible for the final disposal of waste, including the sweeping, manual collection, and transport of municipal and non-municipal waste. Its operational process includes a daily inspection of incoming vehicles to prevent the entry of hazardous waste, detailed recording of waste data, unloading of non-hazardous waste in designated areas, and compacting the waste into uniform layers of earth approximately 4 m high using heavy machinery such as crawler tractors. The last stage is crucial because it reduces the volume of waste and extends the operational lifespan of landfills.
This facility receives waste from 16 districts: Comas, Ancón, San Miguel, Puente Piedra, Pueblo Libre, Magdalena del Mar, La Molina, Carabayllo, Santa Rosa, Callao, Mi Perú, La Perla, La Punta, Bellavista, Ventanilla, and Carmen de La Legua Reynoso [10]. Furthermore, non-hazardous waste from non-municipal sources is disposed of on the premises. Waste of municipal origin is received without prior segregation, whereas waste from other sources is transported by specialised companies and is subject to more rigorous controls.
2.3. Air sampling
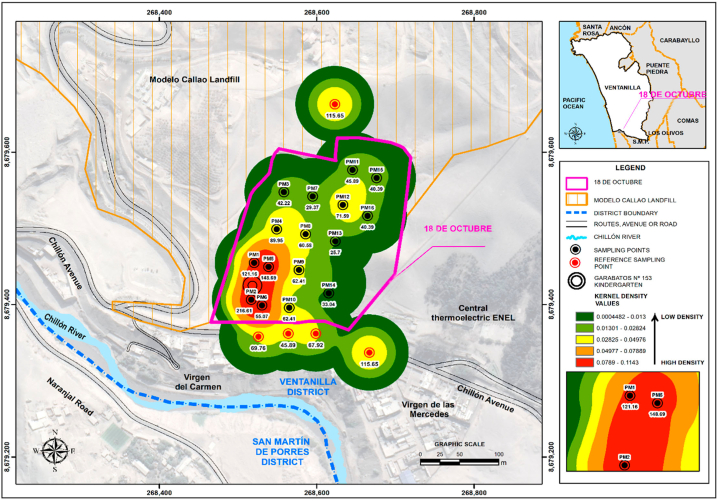
Air sampling was performed at the 18 de octubre settlement in Ventanilla, around the Modelo Callao Landfill. The sampling considered 21 points with three replicates, located 50, 100, 150, and 200 m from the landfill site. Sixteen points were established in the 18 de octubre settlement, four on Chillón Avenue (whose street is around the final disposal infrastructure), and one in the waste discharge area of the landfill (Fig. 2).
Fig. 2.
Spatial location of the Modelo Callao Landfill and sampling points.
Sampling was conducted during the summer (January–March) and winter (June–August) seasons using a gravitational and non-volumetric method with consecutive sampling. Air monitoring was carried out from January to August 2022 between 7:00 a.m. and 5:00 p.m., using 81 Petri plates with chloramphenicol Sabouraud Agar [28] placed for 30 min at a height of 1.50 m above ground level to simulate breathing during sampling [29] in both the seasons. Thereafter, the Petri plates were collected in a cooler with a gel pack and incubated for seven days at 28 °C. There were eight samples in total: four collected during the summer season (January 28, February 12, February 26, and March 16) and four during the winter (June 26, July 9, July 23, and August 21), amounting to 668 Petri plates. Sabouraud Agar supplemented with chloramphenicol was used for fungal isolation and cultivation. Chloramphenicol inhibits bacterial growth and facilitates the selective growth of fungi [30].
2.4. Measurement of meteorological variables
The meteorological parameters measured were temperature (°C) and relative humidity (%) using an HTC-2 thermohygrometer, and the wind speed (m/s) was determined using a Lutron anemometer at each point. A meteorological station was installed 100 m from the landfill site to measure wind speed and direction during the summer and winter months to generate wind roses using the WRPLOT software.
2.5. Microbiological analysis
Petri plates with chloramphenicol Sabouraud Agar were examined at 3, 5, and 7 d to avoid overlap of the fungal colonies [31]. In some cases, the colonies were too small, necessitating an additional 7 days of incubation to visualise their structure under a microscope. A drop of lactophenol blue was placed on the slide [30,31], and the fungal material was removed from the colonies using adhesive tape. Fungal genera were identified considering macroscopic (growth rate, appearance, texture, edges, colours) and microscopic (type of hyphae, spores, conidiophores) characteristics, using the BASEBiO database [32] descriptions of species [33] and the book “Micología General” [34].
2.6. Fungal concentration
The number of colony-forming units (CFU) of fungi, excluding yeasts, present on each Petri plates was counted after 3, 5, and 7 days of incubation. Subsequently, the concentration was determined using the Omeliansky conversion equation [22,25,35].
Equation 1:
Where:
2.7. Geospatial distribution of concentrations
The kernel density tool allows the analysis, visualisation, and identification of spatial data, enabling the prediction of pollution event patterns in high-risk zones and locations [36,37]. It is commonly used to analyse crime patterns, urban planning, disease incidence, traffic congestion, and accidents. Google Earth Pro was used to delineate the study area and sampling points. Subsequently, the results of the seasonal concentrations (summer and winter) were consolidated in a Microsoft Excel 2019 spreadsheet, and the shapefile “puntos_concentracion” (concentration points) was created. Thereafter, the “kernel density” tool in ArcGIS software was employed, considering the following parameters: the input file “puntos_concentracion”, the “concentration summer” field with a search radius of 50 m, the area units in square meters, and the planar method. Finally, the summer and winter density maps were generated [38].
2.8. Statistical analysis
The relative abundance of fungal genera was determined per season using Infostat software. Statistical analysis of meteorological variables (temperature, relative humidity, and wind speed) and fungal concentrations was performed using Paleontological Statistics (PAST) version 4.2 [39] to obtain the total concentrations, means, and standard deviations of the sampling and seasonal data. Subsequently, a correlation analysis was conducted between the concentrations of fungi and the meteorological variables measured during sampling. Finally, a permutational multivariate analysis of variance (PERMANOVA) was performed using the number of CFU of the fungal genera identified from the monitoring data [40].
3. Results
3.1. Seasonal meteorological conditions
Meteorological variables were measured to characterize the environmental conditions during sample collection (Table 1): during the summer season, average temperatures ranged from 22.51 °C to 28.00 °C, with relative humidity reaching up to 77 % and wind speeds between 0.82 m/s and 1.06 m/s. In the winter season, average temperatures ranged from 17.01 °C to 19.68 °C, with relative humidity up to 82.33 % and wind speeds ranging from 0.79 m/s to 1.26 m/s.
Table 1.
Environmental conditions of the monitoring period.
| Seasons | Monitoring | Average ∗Concentration | ∗Temperaturea (°C) | ∗Relative humiditya (%) | ∗Wind speed (m/s) a |
|---|---|---|---|---|---|
| Summer | 1 | 45.46 | 24.45 ± 2.15 | 67.4 ± 8.43 | 1.06 ± 0.30 |
| 2 | 61.19 | 23.69 ± 2.23 | 70.24 ± 7.72 | 0.82 ± 0.75 | |
| 3 | 86.02 | 22.51 ± 1.64 | 76.86 ± 5.78 | 0.84 ± 0.60 | |
| 4 | 104.55 | 28.00 ± 1.21 | 46.24 ± 1.22 | 0.83 ± 0.52 | |
| Winter | 5 | 111.54 | 17.54 ± 0.45 | 80.24 ± 2.74 | 1.13 ± 0.57 |
| 6 | 226.93 | 17.01 ± 0.44 | 82.33 ± 1.83 | 1.26 ± 0.49 | |
| 7 | 66.43 | 19.68 ± 1.68 | 69.14 ± 4.91 | 0.79 ± 0.30 | |
| 8 | 66.78 | 17.21 ± 0.44 | 73.62 ± 0.80 | 0.93 ± 0.45 |
Mean ± standard deviation.
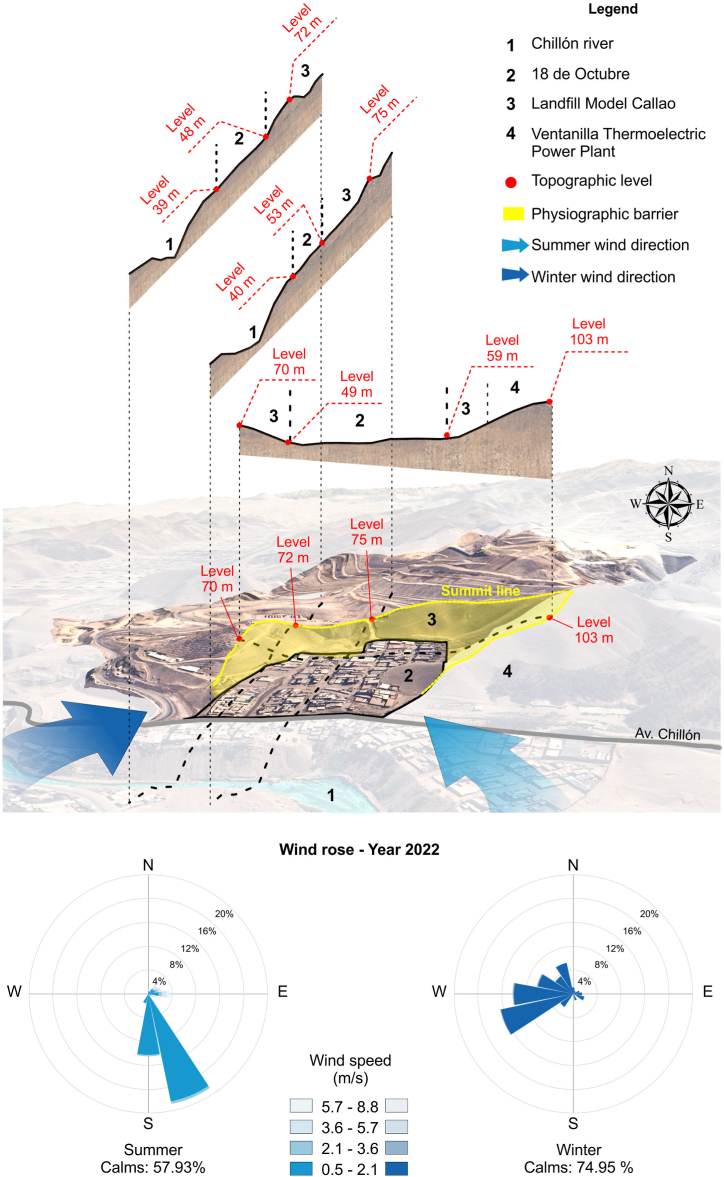
As illustrated in Fig. 3, during the summer, air masses primarily originated in the Chillón River Basin to the south and southeast, moving northward and northeastward at speeds up to 2.1 m/s. Additionally, in summer, air masses from the eastward and northeastwards, where the solid waste landfill is located, reached maximum speeds of 8.8 m/s, moving towards the west and southwest, specifically towards points 1, 2, 5, and 6 of the 18 de octubre settlement. During the winter season, two predominant patterns were observed: first, air masses from the southwest and west moved towards the northeast and eastward at speeds between 0 m/s and 3.6 m/s; second, winds originating in the northeast advanced towards the southwest.
Fig. 3.
Environmental conditions and wind direction in the 18 de octubre settlement from January to August 2022.
3.2. Characterization of airborne fungi
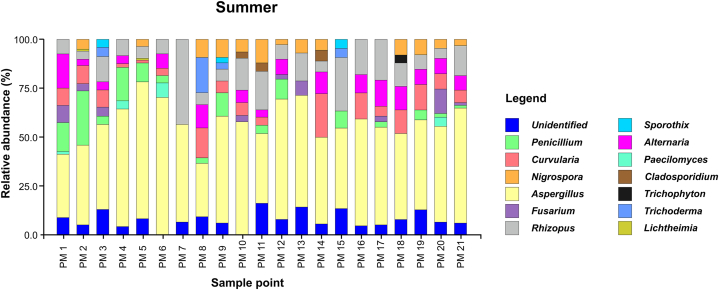
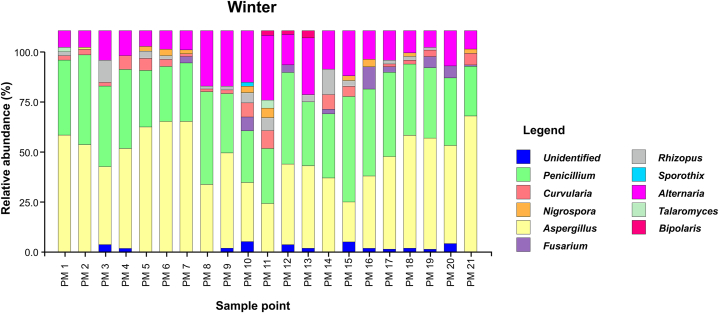
Fungal genera that were present in the sampling area during both seasons (summer “s" and winter “w") were Aspergillus spp. (s: 50.12 %, w: 43.51 %), Penicillium spp. (s: 9.18 %, w: 32.17 %), Alternaria spp. (s: 6.71 %, w: 14.23 %), Curvularia spp. (s: 6.82 %, w: 2.89 %), Rhizopus spp. (s: 10.35 %, w: 2.22 %), Fusarium spp. (s: 2.82 %, w: 1.85 %), Nigrospora spp. (s: 3.76 %, w: 1.11 %), and Sporothrix spp. (s: 0.35 %, w: 0.07 %) (Fig. 4, Fig. 5).
Fig. 4.
Fungal genera identified in the summer season (January–March) during 2022.
Fig. 5.
Fungal genera identified in the winter season (June–August) during 2022.
3.3. Fungal concentrations
The average fungal concentrations were 297.21 CFU/m3 and 471.69 CFU/m3 during summer and winter, respectively, not exceeding the threshold levels established by Omeliansky, the Polish Standards, the World Health Organization, and China Scientific Ecology Center (Table 2).
Table 2.
Seasonal concentrations.
| Sample point | Average summer concentrationa (CFU/m3) | Average winter concentrationa (CFU/m3) |
|---|---|---|
| PM1 | 121.16 ± 94.89 | 97.29 ± 49.94 |
| PM2 | 216.61 ± 180.41 | 167.05 ± 67.40 |
| PM3 | 42.22 ± 43.39 | 110.14 ± 70.43 |
| PM4 | 89.95 ± 51.00 | 113.81 ± 75.24 |
| PM5 | 148.69 ± 177.59 | 100.96 ± 33.04 |
| PM6 | 55.07 ± 47.02 | 111.98 ± 67.40 |
| PM7 | 29.37 ± 18.96 | 124.83 ± 129.84 |
| PM8 | 60.58 ± 11.01 | 132.17 ± 61.43 |
| PM9 | 62.41 ± 59.50 | 102.80 ± 92.30 |
| PM10 | 62.41 ± 45.07 | 117.48 ± 127.32 |
| PM11 | 45.89 ± 26.39 | 93.62 ± 138.98 |
| PM12 | 71.59 ± 41.27 | 106.47 ± 91.61 |
| PM13 | 25.70 ± 28.44 | 108.31 ± 97.48 |
| PM14 | 33.04 ± 41.75 | 82.61 ± 97.67 |
| PM15 | 40.39 ± 39.08 | 80.77 ± 60.25 |
| PM16 | 40.39 ± 61.87 | 111.98 ± 78.71 |
| PM17 | 69.76 ± 49.62 | 135.84 ± 69.01 |
| PM18 | 45.89 ± 58.86 | 108.31 ± 112.22 |
| PM19 | 67.92 ± 103.39 | 143.18 ± 140.67 |
| PM20 | 115.65 ± 183.26 | 102.80 ± 118.55 |
| PM21 | 115.65 ± 104.60 | 223.96 ± 98.61 |
Mean ± standard deviation.
3.4. Geospatial distribution of concentrations
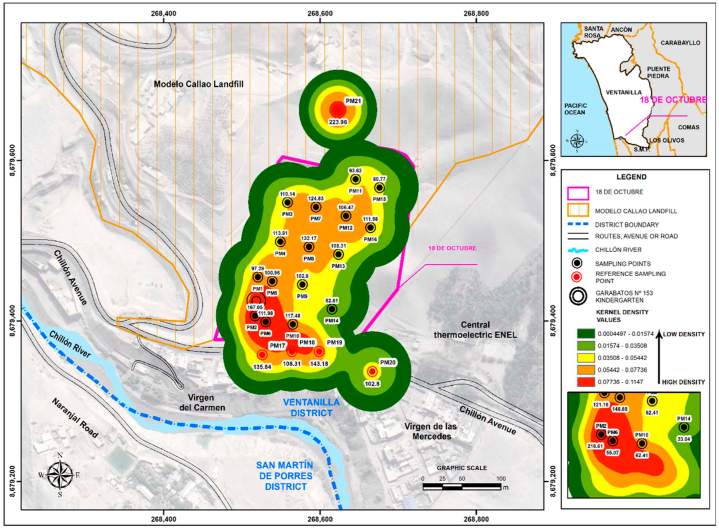
The density graphs represent the average concentrations found during summer and winter, identifying the outliers with the highest concentrations. In summer (Fig. 6), the outliers were: point 1 (121.16 CFU/m3), point 2 (216.61 CFU/m3), point 4 (89.95 CFU/m3), point 5 (148.69 CFU/m3), point 20 (115.65 CFU/m3), and point 21 (115.65 CFU/m3). During winter (Fig. 7), the outliers were: point 2 (167.05 CFU/m3), point 7 (124.83 CFU/m3), point 8 (132.17 CFU/m3), point 10 (117.48 CFU/m3), point 17 (135.84 CFU/m3), point 19 (143.18 CFU/m3) and point 21 (223.96 CFU/m3). The heat maps indicated high-risk areas of contamination within 150–200 m of the landfill boundary (represented by red zones), including Garabatos No. 153 kindergarten which exhibited elevated concentrations in both seasons.
Fig. 6.
Fungal concentrations during the summer season of 2022 (January–March).
Fig. 7.
Fungal concentrations during the winter season of 2022 (June–August).
3.5. PERMANOVA analysis
The Bray-Curtis analysis revealed significant seasonal variations (Permanova, p-value <0.05) with F = 11.69. Simper revealed that the differences were due to the presence of a higher and a lower number of colonies of Aspergillus spp. and Rhizopus spp., respectively, in summer, whereas in winter, the most representatives were Aspergillus spp. and Penicillium spp. These seasonal patterns were consistent across all monitoring sessions, with the exception of sessions 5 and 7, which showed no significant distinctions (Table 3).
Table 3.
Comparison tests with Bonferroni p-values.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | |
| 2 | 1 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | |
| 3 | 0.0028 | 0.0028 | 1 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | |
| 4 | 0.0028 | 0.0028 | 1 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | |
| 5 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | 0.1176 | 0.0168 | |
| 6 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | |
| 7 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | 0.1176 | 0.0028 | 1 | |
| 8 | 0.0028 | 0.0028 | 0.0028 | 0.0028 | 0.0168 | 0.0028 | 1 |
4. Discussions
4.1. Influence of organic waste on the proliferation of airborne fungi
Inadequate management of organic waste can contribute to the proliferation of airborne fungi, as observed in various localities, including the present research in Peru. The World Bank reports the global waste composition as follows: 44 % green and food waste, 17 % paper and cardboard, 12 % plastic, 5 % glass, 4 % metals, 2 % rubber and leather, 2 % wood, and 14 % others [8]. The composition of municipal waste in Peru exhibits the following distribution: 57.2% organic, 21.2% inorganic, 14.1% non-recoverable and 7.5% hazardous. Organic waste constitutes the largest fraction, encompassing food remnants (dairy products, dried food, meat and fish bones, bread and bakery leftovers, and fruit and vegetable peeling) and garden refuse such as weeds, leaves, grass clippings, hedge trimmings and dead plant material generated through site clearing and maintenance in inhabited areas [[41], [42]]. Sanitary waste arises from activities involving bodily fluids or bathroom waste such as urine, sanitary towels, disposable paper, and other materials used in such facilities. Biocontaminated waste, on the other hand, contains pathogenic biological agents from contact with sick individuals receiving home care, including food remnants, personal protective equipment, disposable tissues, and any item directly exposed to those suffering from an illness [43,44].
This nutrient-rich composition provides an ideal substrate for fungal proliferation. Fungi are ubiquitous microorganisms that proliferate rapidly in the presence of organic compounds in the atmosphere that serve as nutrients for their growth and development. The presence of fungi in the air can be attributed to both local sources and the decomposition of the organic waste deposited in sanitary landfills [45]. Environmental conditions in landfills, including oxygen levels, moisture content and temperature variations, promote microbial growth and accelerate decomposition processes, resulting in atmospheric spore release despite the application of a daily cover material to the waste. For instance, oxygen availability in upper landfill strata facilitates aerobic decomposition, generating thermal gradients and moisture variations that enhance fungal proliferation. These containment systems are engineered to prevent wind-blown waste and dust dispersal. However, their effectiveness depends on both composition and application methodology, as porous materials may allow the release of microscopic spores during waste management operations. The sampling plates collected during monitoring exhibited abundant dust particles and an unpleasant odour, likely due to organic remnants. Accelerated fungal growth was observed on nearly all Petri plates after 3 and 5 days of incubation. Genera such as Aspergillus (Aspergillus niger, Aspergillus flavus, Aspergillus nidulans), Rhizopus, Curvularia, Alternaria, Fusarium, Nigrospora, and Penicillium were identified. These microorganisms can degrade organic waste in landfills. Several studies have identified the following genera in different landfills worldwide: Aspergillus, Micrococcus, Penicillium, Fusarium, Acremonium, Alternaria, Trichoderma, Curvularia, Rhizopus, Geotrichum [46], Dreschslera, Nigrospora, Paecilomyces, Gliocladium, Eurotium, Cunninghamella [47], Sporobolomyces, Trichophyton, and Wallemia [48]. These findings are consistent with the genera identified and suggest a common pattern in the microbial biodiversity associated with solid waste disposal sites
The geographical location of the study area, situated near the Chillón River basin and Pacific Ocean, facilitates the dispersion of particulate matter from the soil into the atmosphere due to wind variability. This phenomenon contributes to the aerial propagation of fungal spores, thereby increasing the exposure risk for neighbouring communities [45].
4.2. Concentrations of fungal particles found
The research revealed that the average concentration of airborne fungi was higher during the winter season (471.69 CFU/m³) compared to the summer season (297.21 CFU/m³). Fungal aerosol concentrations did not exceed in the 18 de Octubre settlement and the surrounding area of the Modelo Callao Landfill, including those set by the World Health Organization, Polish regulations, Omeliansky, and the CSEC [49].
Various studies have compared their results with those of Polish standards. For instance, a municipal waste landfill in Poland determined that the concentration of airborne fungi within the infrastructure ranged from 121 to 18,290 CFU/m³, and in the surrounding areas, it was 4550 CFU/m³; these values do not exceed Polish air pollution standards [49]. On the other hand, at the Barycz landfill, fungal concentrations ranging between 112 and 16,445 CFU/m³ were detected, surpassing the threshold of 5000 CFU/m³, thus classifying the environment as “microbiologically contaminated” [50]. These differences in the results could be attributed to the methods used for measuring concentrations and the fungal identification techniques.
Qualitative analysis of fungal aerosols presents limitations such as subjectivity, lack of universal standards, and difficulty in detecting rare fungi. In this study, 80 colonies could not be identified: 61 in summer and 19 in winter. The main challenges include the secretion of liquids by certain fungi, slow growth of some colonies, difficulty distinguishing the complete structure of minute specimens, and the presence of samples in which only spores were observed. Although concentrations were generally higher in winter, the rapid proliferation of Chrysonilia sitophila during certain monitoring periods made it difficult to identify other species [29]. Growth showed marked seasonal variations: slow summer development necessitated additional cultures in the test tubes, whereas rapid winter growth facilitated colony identification. Molecular methods can improve identification and reduce the time required, although they present challenges, such as the concentration and recovery of biological material and the selection of genomic regions for amplification or sequencing [51].
These results are important because the indoor air quality of homes near landfills can be affected by their proximity. Airborne fungi can enter homes through doors, windows, open gardens, skylights, ventilation systems, or air conditioning [52]. The highest concentrations of fungi in outdoor air were found near a nursery and a local park at 150 and 200 m, respectively, which is particularly concerning as even low concentrations can pose health risks to immunocompromised children, such as those with acute leukaemia or organ and/or transplant recipients, who are susceptible to respiratory diseases. Infections in this group of patients manifested in the lungs, skin, and paranasal sinuses [53,54,55].
In the 18 de octubre settlement, the only existing vegetation cover was limited to the park and a section of the kindergarten, consisting of grass, two palm trees, one tara tree, and one molle tree. The streets are unpaved and composed of uncovered soil, with considerable vehicle traffic in front of these public infrastructures. The high concentrations could also be attributed to the presence of dust generated by landfill operations such as the transport of units with waste to the discharge area and compaction. Microbial particles can be quickly transferred over long distances, depending on the particle size [56]. In one study, fungal concentrations were higher when the hospital was located near areas without vegetation cover (291.5 CFU/m³) and lower in urban areas (11.8 CFU/m³) and green spaces (103 CFU/m³). These results confirm that areas without vegetation cover exhibit higher airborne fungal concentrations and to mitigate this, green spaces should be created within a radius of 0.5–3 km [57]. The reason for the low concentrations at Points 13, 14, 15, and 16 could be that the houses had more than two storeys and their streets were narrow. Additionally, this could be attributed to the existence of a chain of hills 75 and 103 m high, which are conditioning factors that prevent particles from settling on the Petri plates at the mentioned points.
4.3. Fungal community and health implications
During the summer (January to March 2022), 14 genera were identified, with Aspergillus spp. (50.12 %) and Rhizopus spp. (10.35 %) being the most predominant. The winter months, 11 genera were detected, with Aspergillus spp. (43.51 %) and Penicillium spp. (32.17 %) standing out as the most predominant. A significant proportion of the fungi, representing 7.18 %, could not be identified at the genus level in summer, suggesting the presence of a fungal diversity that is yet to be characterised, whereas, in winter, the unidentified fungi accounted for 1.41 %. This highlights the need for more advanced techniques and additional studies for a more precise identification.
Aspergillus spp. are the most predominant, representing nearly half of the fungal community. This cosmopolitan microorganism has a size ranging from 2 to 5 μm in the air and exhibits a high adaptability to a range of environmental conditions [58]. Rhizopus spp., the second most frequent genus during the summer season, is common in soil and decaying plant material, including humid environments (23 °C to 27 °C), with spores up to 13 μm in length [59]. Penicillium spp. had greater predominance in winter, with an optimal growth temperature of 17 °C to 18 °C [60] and conidia measuring 3.0–3.5 μm [61], notable for its ability to degrade various organic materials [62]. Aspergillus spp. and Penicillium spp. release several spores (less than 5 μm) into the air due to their asexual and sexual reproduction.
Alternaria spp., found on the aerial parts of trees, food, textiles, and other materials, is notable for its versatility as a saprophyte, endophyte, and phytopathogen [63]. Its spores range in size from 10 to 20 μm [64]. Its adaptability allows it to thrive in ecosystems characterised by high temperatures and both dry and wet seasons. Trichoderma spp., detected exclusively during summer, has a limited presence due to its reduced biological activity in low temperatures. It grows rapidly growth in warm and dry environments (25 °C to 30 °C) [[65], [66], [67]], and thrives in soil with organic matter and adequate moisture, where it acts as a natural decomposition agent [65,68].
Bipolaris spp. produces large conidia measuring 6–39 μm and can survive in a dormant state in soil, cereal stubble, and grains for up to four years. Its optimal growth temperature is between 25 °C to 35 °C [[64], [65], [66]]. Curvularia spp., with conidia sizes of 8.8–30 μm, grows at temperatures between 20 °C to 35 °C and commonly inhabits dead plant material [69,70] Fusarium spp. is found in warmer regions and can survive in temperate climates in soil and decomposing organic matter [71]. It produces both macroconidia (23–54 × 3–4.5 μm) and microconidia (5–12 × 2.3–3.5 μm) [72], with an optimal temperature range of 23 °C to 25 °C [71]. Talaromyces spp. is a dimorphic fungus that releases pigments (yellow, orange, and red) and has been identified in soil, dust, and leaf litter [72], growing in a range of 25 °C–37 °C, with conidia sizes ranging from 1.5 to 8 μm [73,74]. The detection of phytopathogenic fungi such as Curvularia spp., Alternaria spp., Fusarium spp., Nigrospora spp., and Bipolaris spp. [75]. in the study zone, an area without agricultural activity, indicates that their origin could be linked to the adjacent landfill.
The fungal genera that can cause health effects in immunocompromised patients include Aspergillus spp. which were present at all monitoring points and are associated with allergic diseases, sinusitis, and asthma [76]. Alternaria spp. is linked to skin diseases, fungal keratitis, and paranasal sinusitis, while Curvularia spp. is associated with fungal keratitis, sinusitis, and onychomycosis [66]. Although Talaromyces spp. is uncommon in South America, its discovery is relevant for health professionals, as it can cause systemic infection affecting the lungs, bone marrow, and skin [77]. Bipolaris spp. can lead to rhinorrhoea and nasal congestion, especially in immunocompromised individuals [77], Sporothrix spp. is a dimorphic fungus that causes sporotrichosis [78], Lichtheimia spp., belonging to the Mucorales group, is involved in the decomposition of organic matter and can cause mucormycosis, along with Rhizopus spp [44]. Although infections caused by Fusarium spp., Curvularia spp., Alternaria spp., and Bipolaris spp. are uncommon, they can occur in patients with delayed grafting [49]. For example, approximately 10,000 annual hospitalisations in the United States for aspergillosis occur due to the inhalation of Aspergillus flavus spores in people with weakened immune systems [77]. The presence of potentially pathogenic fungi highlights the need for mycological surveillance of urban environments, particularly near landfill sites.
4.4. Influence of meteorological factors
Meteorological variables affect the persistence, transport, distribution, and fluctuation of fungal concentrations in the environment. In this study, during summer, temperature and wind speed were the determining factors in the concentrations and abundance of microorganisms, whereas in winter, relative humidity became more relevant.
All fungi found are mesophilic, thriving in temperature ranges between 20 °C and 30 °C. In summer, a greater diversity of fungal genera was observed, which could be related to the adaptability of fungi to moderate temperatures, creating an ideal environment for their development and favouring the diversity of the fungal community. For example, Aspergillus includes both mesophilic and thermophilic species that tend to proliferate in warm and humid climates. In winter, the presence of the genus Penicillium spp. was notable, which is capable of adapting to lower temperatures, allowing it to survive and grow at an average temperature of 17.86 °C during winter, as its optimal growth range is between 17 °C to 18 °C [60]. Paradoxically, the high concentrations of Alternaria spp. spores found in winter could be attributed to the physiological characteristics of the spores that allow for their survival in the coldest months.
Fungi are more resistant to desiccation than bacteria and remain airborne for longer periods, despite high temperatures and low relative humidity [71,72]. For example, when maximum concentrations were found, relative humidity ranged between 60 % and 70 %, wind speed was below 1 m/s, and temperature varied between 25 °C and 30 °C [73,74]. Aspergillus spp. and Penicillium spp. are well adapted to high temperatures and humidity levels above 80 % [73,74]. The presence of fungi depends on the season. For instance, fungal concentrations were higher during summer and autumn in the Kuopio landfill in Finland [74], whereas in Tijuana, concentrations were higher during summer and lower in winter [76]. The average relative humidity in summer ranged from 46.24 % to 76.86 %, however, this variable does not influence fungal growth [86]. Meanwhile, in winter, relative humidity and concentrations were higher than in summer, but other studies suggest that humidity levels above 80 % promote greater fungal growth. During winter, relative humidity reached up to 82.33 %, allowing for a greater abundance of fungi such as Penicillium spp. In contrast, during summer, when relative humidity dropped below 70 %, a higher prevalence of Aspergillus spp. was observed. This predominance is attributed to their remarkable ability to adapt to warm and relatively dry environments.
Wind speed and atmospheric instability also influence the concentration of airborne fungi. During summer, rising warm air masses generate clear skies, whereas in winter, cold air masses settle in the atmospheric boundary layer, forming clouds with vertical development and causing the accumulation of fungal particles in the study area. Moreover, the wind direction and speed can affect the dispersion of pollutants. In summer, the dispersion was more active owing to higher wind speeds, whereas, in winter, pollutants remained more concentrated near their sources owing to calmer conditions. All particles suspended in the air settled even under calm conditions. The seasonal dynamics of fungi are determined by a complex interaction between meteorological and environmental factors; therefore, it is recommended to investigate fungal ecology in urban environments.
Linear regression analyses between meteorological variables (temperature, wind speed, and relative humidity in winter) and fungal concentrations showed statistically significant results. However, the relationship obtained does not establish a clear causality, and the results are not necessarily applicable to other contexts because of the variability in meteorological factors between different environments. Furthermore, the quality and quantity of available meteorological data are insufficient for robust comparison, especially because factors such as land use, pollution sources, and atmospheric stability have not been considered. Therefore, longitudinal statistical analyses should be conducted to establish reliable relationships.
4.5. Recommendations
The action plan to address fungal contamination in the air is categorised into five key areas (Table 4): emission source management, capacity building, monitoring of meteorological variables and fungal microorganisms, management of economic instruments, and development of public infrastructure. For the identified sensitive areas, specifically the Garabatos N°153 Kindergarten and the local park, the following mitigation measures are proposed: paving of urban roads adjacent to the Modelo Callao Landfill and the 18 de octubre settlement; establishing vegetative barriers along the landfill perimeter; implementing HEPA filtration systems and air purification in the educational facility; and enhancing the park with deciduous tree species capable of dust retention in their foliage.
Table 4.
Action plan to address fungal contamination.
| N° | Description of the measure | Institutions responsible for executing the proposals in Peru |
|---|---|---|
| A. Emission source management: Minimize the concentration of aerofungal organisms originating from final disposal infrastructures, informal recyclers, and solid waste operating companies. | ||
| 1 | Establish air quality standards for biological contaminants (biological parameters for solid waste infrastructures such as landfills, secure landfills, composting plants, recycling facilities, and incineration plants). | MINSA - MINAM |
| 2 | Incorporate an environmental management plan into the corrective environmental management instruments for solid waste infrastructures that includes specific measures to address the presence of biological contaminants in the air. | MINSA – MINAM |
| 3 | Enact an ordinance prohibiting the accumulation of non-hazardous waste in residential properties for commercial use. | Municipality of Ventanilla |
| 4 | Enact an ordinance establishing mandatory municipal waste segregation in the district of Ventanilla. | Municipality of Ventanilla |
| 5 | Enact an ordinance prohibiting informal occupation in areas influenced by final disposal infrastructures. | Municipality of Ventanilla |
| 6 | Introduce solid waste containers at critical points. | Municipality of Ventanilla |
| 7 | Implement the source segregation and selective collection programme for solid waste. | Municipality of Ventanilla |
| B. Capacity building: Increase the number of professionals in the public and private sectors with knowledge of aerofungal air quality management. | ||
| 1 | Enact an ordinance prohibiting solid waste operating companies from transporting waste in open-topped vehicles. | Municipality of Ventanilla MINAM |
| 2 | Inspect and ensure that operating companies have vehicles that prevent the emission of fungal particles into the air. | Regional Government of Callao |
| 3 | Include the emission of biological contaminants due to the lack of a closed cargo area in vehicle infractions. | SUTRAN |
| 4 | Train staff in the monitoring of fungal aerosols in educational, hospital, and residential areas. | MINAM – MINSA |
| C. Capacity building: Generate representative and comparable information on aerofungal air quality. | ||
| 1 | Raise awareness and provide training for residents, public and private organisations. | MINSA – Regional Government of Callao |
| 2 | Disseminate information about the effects of exposure to airborne fungal particles. | MINSA – Regional Government of Callao |
| 3 | Conduct health campaigns to detect diseases caused by airborne fungal microbes. | MINSA |
| 4 | Communicate health issues through an environmental health alert system. | Municipality of Ventanilla |
| 5 | Promote scientific research on airborne fungi. | National and private universities |
| D. Monitoring of meteorological variables and aerofungal microorganisms. | ||
| 1 | Implement a surveillance system with low-cost sensors for meteorological variables. | SENAMHI |
| 2 | Establish a technical standard on sampling methods for monitoring airborne fungal particles. | INACAL – MINSA – MINAM |
| 3 | Continuously monitor airborne fungi in special protection zones. | SENAMHI– MINSA – OEFA |
| E. Management of economic instruments: Establish economic mechanisms for emissions reduction. | ||
| 1 | Establish financial penalties for transport route infractions. | MEF – MINSA – MTC – Gobierno Regional del Callao |
| 2 | Establish financial penalties for infractions of solid waste environmental management instruments. | MEF – MINSA – MINAM – SENACE – Callao Regional Government |
| F. Management of public infrastructure: Improve the living conditions of the inhabitants. | ||
| 1 | Implement road paving infrastructure in high-traffic areas of the 18 de octubre settlement and Chillón Avenue, with emphasis on urban zones adjacent to the landfill site. | MVCS – MEF – Callao Regional Government - Municipality of Ventanilla |
| 2 | Implement green spaces in the 18 de octubre settlement and Chillón Avenue. | Callao Regional Government - Municipality of Ventanilla |
| 3 | Implement HEPA filtration systems and air purification in the educational facility. | MINEDU - Callao Regional Government - Municipality of Ventanilla |
| 4 | Establish vegetative barriers comprising locally-adapted species that demonstrate effective particulate matter retention and pollutant tolerance. | Landfill concessionaire company |
∗ MINSA: Ministerio de Salud, MINAM: Ministerio de Ambiente, SUTRAN: Superintendencia de Transporte Terrestre de Personas, Carga y Mercancías, SENAMHI: Servicio Nacional de Meteorología e Hidrología del Perú, INACAL: Instituto Nacional de Calidad, OEFA: Organismo de Evaluación y Fiscalización Ambiental, MINEDU: Ministerio de Educación, MEF: Ministerio de Economía y Finanzas, MTC: Ministerio de Transporte y Comunicaciones, SENACE: Servicio Nacional de Certificación Ambiental para las Inversiones Sostenibles, MVCS: Ministerio de Vivienda, Construcción y Saneamiento
4.6. Limitations and future research
Peruvian regulations have established that solid-waste disposal infrastructure must be located at least 500 m from populated areas or surface water sources [87]. However, the Modelo Callao Landfill does not comply with this regulation. Located on the right bank of the Chillón River, this landfill lacks paved roads within the nearby 18 de octubre settlement and along Chillón Avenue, which could exacerbate air pollution in the area. Similarly, Mexican laws stipulate that the final disposal site must be situated no less than 500 m from any continuously flowing surface water, lake, or lagoon (NOM-083-SEMARNAT-2003, 2004) [78]. This study recommends that the minimum distance for a disposal site should be greater than 200 m to minimize exposure to bioaerosols, considering the prevailing winds and physiography of the area. For example, a study in the United Kingdom demonstrated that compostable organic waste generates bioaerosols and communities located 250 m from waste disposal facilities may be exposed to high levels of bioaerosols [89]. The distance established for housing and educational establishments depends on factors, such as the absence of vegetation cover, lack of vegetation, proximity to the final disposal infrastructure, and meteorological variables, all of which warrant further investigation.
The limitations of this research are as follows: the methodology used, particularly the sedimentation technique, involves exposing Petri plates to the environment without applying specific particle diameter filters. Consequently, particles larger than 2.5 μm could be deposited due to wind action and vehicle movement. Additionally, there could be a selection bias in the study area because monitoring points on Gambetta Avenue, Ventanilla, and Peru were not considered, and no previous studies have evaluated the biological quality of the air. However, no molecular studies have evaluated the species or mycotoxins of the identified genera. Neither the district nor the provincial municipality have established air quality monitoring stations, and there are no historical data on meteorological variables. Therefore, a meteorological station was installed to conduct the study. A key strength of this study is that it is the first of its kind in the area to obtain a variety of samples that allowed the identification of several fungal genera that could affect the health of local inhabitants. A high-risk contamination zone was determined, and this information can be used by district authorities to improve road paving and increase green areas in the zone.
5. Conclusions
This research concludes that the fungal composition of the air in the vicinity of the Modelo Callao landfill presents various genera such as Aspergillus spp. (46.09 %), Penicillium spp. (23.29 %), Alternaria spp. (11.33 %), Rhizopus spp. (5.37 %), Curvularia spp. (4.41 %), Fusarium spp. (2.23 %), Nigrospora spp. (2.14 %), Paecilomyces spp. (0.36 %), Bipolaris spp. (0.18 %), Cladosporium spp. (0.18 %), Sporothrix spp. (0.14 %), Lichtheimia spp. (0.05 %), Talaromyces spp. (0.14 %), and Trichophyton spp. (0.05 %). Factors influencing airborne fungal concentrations included the accumulation of organic waste in the landfill, meteorological variables (relative humidity, wind speed, temperature, and wind direction), the presence of hills surrounding the study area, vehicle movement (motorcycles and cars), sampling at different collection points, summer and winter seasons, and lack of road paving implementation. Fungal concentrations were higher during the winter season, with Aspergillus spp. and Penicillium spp. being predominant. Point 2 (east: 268517, north: 8679407), located near the Garabatos Nursery School and 200 m from the landfill, showed average concentrations of 216.61 CFU/m3 in summer and 167.05 CFU/m3 in winter. Although these concentrations are relatively low and the fungi identified are commonly found in ambient air worldwide, the proximity of landfills to residential areas and educational facilities warrants consideration. The concentration of fungal particles affects both outdoor and indoor air quality. Therefore, establishments such as schools, hospitals, nurseries, markets, and centres for the elderly should not be located near landfills. Future urban planning should consider maintaining a buffer zone of at least 200 m between landfills and these sensitive areas to minimize the potential exposure to bioaerosols, particularly for vulnerable populations. The landfill represents a potential source of bioaerosol emissions in the study area and surroundings. However, further research is needed to establish definitive links between the observed fungal concentrations and specific health risks.
Funding
The Vicerrectorado of the San Ignacio de Loyola University financed the project POLARIS 2019–2. I am grateful to the University for providing me with the financial incentive to carry out this research work.
Data availability
Data are included in the supplementary material.
Informed consent
Informed consent was obtained from all individuals included in this study.
Ethical approval
All participants provided informed consent to participate in the study.
CRediT authorship contribution statement
Diana Isabel Rios Valle: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Erika Yovana Gonzales Medina: Writing – review & editing, Visualization, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization. Orlando Advíncula Zeballos: Writing – review & editing, Visualization, Validation, Software, Formal analysis, Data curation.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Diana Isabel Rios Valle reports financial support and article publishing charges were provided by San Ignacio de Loyola University. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e38186.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cerminara G., Cossu R. Solid Waste Landfilling. Elsevier; 2018. Waste input to landfills; pp. 15–39. [DOI] [Google Scholar]
- 2.Cossu R. Solid Waste Landfilling. Elsevier; 2018. Multibarrier principles in landfilling; pp. 53–72. [DOI] [Google Scholar]
- 3.Cossu R., Pivato A., Barausse A. Solid Waste Landfilling: Concepts, Processes, Technologies. Elsevier; 2018. Environmental impacts assessment; pp. 939–954. [DOI] [Google Scholar]
- 4.Yolande Y., Macklin A., Kibble Andrew, Pollitt Frances. 2011. Impact on Health of Emissions from Landfill Sites : Advice from the Health Protection Agency. [Google Scholar]
- 5.Vaverková M.D. Landfill impacts on the environment— review. Geosciences. Oct. 2019;9(10) doi: 10.3390/geosciences9100431. [DOI] [Google Scholar]
- 6.Reinhart D., Stegmann R. Solid Waste Landfilling: Concepts, Processes, Technologies. Elsevier; 2018. Physical/chemical reactions in landfills; pp. 117–138. [DOI] [Google Scholar]
- 7.Njoku P.O., Edokpayi J.N., Odiyo J.O. Health and environmental risks of residents living close to a landfill: a case study of thohoyandou landfill, Limpopo province, South Africa. Int. J. Environ. Res. Publ. Health. Jun. 2019;16(12) doi: 10.3390/ijerph16122125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaza S.Y.L.C., Bhada-Tata P., Van Woerden F. What a waste 2.0: a global snapshot of solid waste management to 2050. 2018. http://hdl.handle.net/10986/30317 Washington.
- 9.Eurostat Landfill rate of waste excluding major mineral wastes. https://ec.europa.eu/eurostat/databrowser/view/ten00138/default/bar?lang=en [Online]. Available:
- 10.MINAM Sigersol municipal: Disposición final de Lima Metropolitana y la Provincia Constitucional del Callao. https://sistemas.minam.gob.pe/SigersolMunicipal/#/accesoLibre/disposicion [Online]. Available.
- 11.MINAM, Inventario Nacional de Áreas Degradadas por Residuos Sólidos Peru: Plataforma digital única del Estado. 2024. https://www.gob.pe/institucion/oefa/normas-legales/5677147-00052-2024-oefa-dsis 1–12.
- 12.Pillai S., Ricke S. Bioaerosols from municipal and animal wastes: background and contemporary issues. Can. J. Microbiol. 2002;48(8):681–696. doi: 10.1139/w02-070. [DOI] [PubMed] [Google Scholar]
- 13.Mainelis G. Bioaerosol sampling: classical approaches, advances, and perspectives. Aerosol. Sci. Technol. May 2020;54(5):496–519. doi: 10.1080/02786826.2019.1671950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gollakota A.R.K., et al. Elsevier Inc.; Nov. 01, 2021. Bioaerosols: Characterization, Pathways, Sampling Strategies, and Challenges to Geo-Environment and Health. [DOI] [Google Scholar]
- 15.Archana J., Surendra S., Qin W., Yuanfu L., Jingshan S. A review of plant leaf fungal diseases and its environment speciation. Bioengineered. Jan. 2019;10(1):409–424. doi: 10.1080/21655979.2019.1649520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hei C., et al. Assessment of airborne particles and bioaerosols concentrations in a waste recycling environment in Brazil. Sci. Rep. Dec. 2020;10(1) doi: 10.1038/s41598-020-71787-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gladding T.L., Gwyther C.L. A study of the potential release of bioaerosols from containers as a result of reduced frequency residual waste collections. Sci. Total Environ. Jan. 2017;576:481–489. doi: 10.1016/j.scitotenv.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 18.Pagalilauan H.A.M., Paraoan C.E.M., Vital P.G. Detection of pathogenic bioaerosols and occupational risk in a Philippine landfill site. Arch. Environ. Occup. Health. Mar. 2018;73(2):107–114. doi: 10.1080/19338244.2017.1299087. [DOI] [PubMed] [Google Scholar]
- 19.Huang C.-Y., Lee C.-C., Li F.-C., Ma Y.-P., Su H.-J.J. The seasonal distribution of bioaerosols in municipal landfill sites: a 3-yr study. Atmos. Environ. 2002;36:4385–4395. doi: 10.1016/s1352-2310(02)00322-9. [DOI] [Google Scholar]
- 20.Agarwal S., Mandal P., Srivastava A. Quantification and characterization of size-segregated bioaerosols at municipal solid waste dumping site in Delhi. Procedia Environ Sci. 2016;35:400–407. doi: 10.1016/j.proenv.2016.07.021. [DOI] [Google Scholar]
- 21.Morgado W., Castillo M., Parody A., Viloria A., Arrieta M., Kamatkar S. In: Data Mining and Big Data. Y, Tan Ying T.Q., Shi, editors. Springer International Publishing; 2018. Concentrations and size distributions of fungal bioaerosols in a municipal landfill; pp. 244–253. [DOI] [Google Scholar]
- 22.Borrego S., Molina A. vol. 1. AUGM DOMUS; 2014. http://revistas.unlp.edu.ar/domus/issue/view/99 (Comportamiento de la aeromicrobiota en dos depósitos del Archivo Nacional de la República de Cuba durante 7 años de estudio). [Online]. Available: [Google Scholar]
- 23.Zawiślak K., et al. Microbiological analysis and concentration of organic dust in an herb processing plant. Pol. J. Environ. Stud. 2019;28(5):3505–3511. doi: 10.15244/pjoes/93705. [DOI] [Google Scholar]
- 24.Li Y., Fu H., Wang W., Liu J., Meng Q., Wang W. Characteristics of bacterial and fungal aerosols during the autumn haze days in Xi’an, China. Atmos. Environ. Dec. 2015;122:439–447. doi: 10.1016/j.atmosenv.2015.09.070. [DOI] [Google Scholar]
- 25.Rodríguez J. Evaluación aeromicrobiológica del depósito del Centro de Documentación del Museo Nacional de la Música de Cuba Aeromicrobiological Evaluation in repository of the Documentation Center of the National Museum of Music of Cuba. Geo-conservación. 2016;9:116–126. [Google Scholar]
- 26.Minsa R.D. 2005. Peru: Portal Interactivo de Fiscalización Ambiental. No 1326-2005/DIGESA/SA. [Google Scholar]
- 27.MINAM . 2023. Tribunal de Solución de Controversias Ambientales: Resolución No 014 - 2023- MINAM/TSCA; pp. 1–20. [Google Scholar]
- 28.Black W. A comparison of several media types and basic techniques used to assess outdoor airborne fungi in Melbourne, Australia. PLoS One. Dec. 2020;15(12 December) doi: 10.1371/journal.pone.0238901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Camargo Y., Borja H., Muñoz M., Vergara-Vásquez E., Vélez-Pereira A. Assessment of fungal aerosols in a public library with natural ventilation. Aerobiologia. Mar. 2023;39(1):37–50. doi: 10.1007/s10453-022-09772-5. [DOI] [Google Scholar]
- 30.Mentese S., Otkun M.T., Palaz E. Comparison of dichloran rose bengal chloramphenicol and Sabouraud dextrose agar with cycloheximide and chloramphenicol for airborne mold sampling. Aerobiologia. Jun. 2017;33(2):211–219. doi: 10.1007/s10453-016-9462-2. [DOI] [Google Scholar]
- 31.Abbasi F., Jalili M., Samaei M., Mokhtari A., Azizi E. The monitoring of fungal contamination in indoor air of two hospitals in shiraz. Journal of Environmental Health and Sustainable Development. 2019;4(4):879–884. doi: 10.18502/jehsd.v4i4.2020. [DOI] [Google Scholar]
- 32.Instituto Nacional de Seguridad y Salud en el Trabajo, “Fichas de agentes biológicos - BASEBiO.” Accessed: August. 19, 2023. [Online]. Available: https://www.insst.es/agentes-biologicos-basebio/hongos?p_p_id=com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_MZAulrtDx9R8&p_p_lifecycle=0&p_p_state=normal&p_p_mode=view&_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_MZAulrtDx9R8_redirect=%2Fagentes-biologicos-basebio%2Fhongos&_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_MZAulrtDx9R8_delta=20&p_r_p_resetCur=false&_com_liferay_asset_publisher_web_portlet_AssetPublisherPortlet_INSTANCE_MZAulrtDx9R8_cur=2.
- 33.University of Adelaide Descriptions of species from the following genera are provided. https://www.adelaide.edu.au/mycology/fungal-descriptions-and-antifungal-susceptibility/alphabetical-index [Online]. Available:
- 34.Estrada G., Ramírez M. vol. 1. Universidad Católica de Manizales; 2019. https://www.ucm.edu.co/micologia-general/#dearflip-df_186519/1/ (Micología General). Manizales, Colombia. [Google Scholar]
- 35.Nageen Y., et al. Analysis of culturable airborne fungi in outdoor environments in Tianjin, China. BMC Microbiol. Dec. 2021;21(1) doi: 10.1186/s12866-021-02205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davarashvili S., et al. Application of the double kernel density approach to the analysis of cancer incidence in a major metropolitan area. Environ. Res. Oct. 2016;150:269–281. doi: 10.1016/j.envres.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Thakali L., Kwon T.J., Fu L. Identification of crash hotspots using kernel density estimation and kriging methods: a comparison. Journal of Modern Transportation. Jun. 2015;23(2):93–106. doi: 10.1007/s40534-015-0068-0. [DOI] [Google Scholar]
- 38.Lin Y., Chu H., Wu C., Chang T., Chen C. Hotspot analysis of spatial environmental pollutants using kernel density estimation and geostatistical techniques. Int. J. Environ. Res. Publ. Health. 2011;8(1):75–88. doi: 10.3390/ijerph8010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammer Ø., Harper D., Ryan P. Past: paleontological Statistics software package for education and data analysis. Palaeontol. Electron. 2001;4(1):1–9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm [Online]. Available: [Google Scholar]
- 40.Anderson M. Wiley StatsRef: Statistics Reference Online; Wiley: 2017. Permutational Multivariate Analysis of Variance (PERMANOVA) pp. 1–15. [DOI] [Google Scholar]
- 41.Liu X., Xie Y., Sheng H. Elsevier B.V.; Mar. 01, 2023. Green Waste Characteristics and Sustainable Recycling Options. [DOI] [Google Scholar]
- 42.Everitt H., van der Werf P., Seabrook J.A., Wray A., Gilliland J.A. The quantity and composition of household food waste during the COVID-19 pandemic: a direct measurement study in Canada. Socioecon Plann Sci. 2022;82(Aug) doi: 10.1016/j.seps.2021.101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ministerio de Salud . 2018. Resolución Ministerial No 1295-2018-MINSA; pp. 1–88.https://cdn.www.gob.pe/uploads/document/file/234853/Resoluci%C3%B3n_Ministerial_N__1295-2018-MINSA.PDF?v=1544722781 País. [Online]. Available: [Google Scholar]
- 44.Ferguson R.M.W., et al. Size fractionation of bioaerosol emissions from green-waste composting. Environ. Int. 2021;147(Feb) doi: 10.1016/j.envint.2020.106327. [DOI] [PubMed] [Google Scholar]
- 45.Nair A.T. Springer Science and Business Media B.V; Jun. 01, 2021. Bioaerosols in the Landfill Environment: an Overview of Microbial Diversity and Potential Health Hazards. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao M., Yan X., Qiu T., Han M., Wang X. Variation of correlations between factors and culturable airborne bacteria and fungi. Atmos. Environ. Mar. 2016;128:10–19. doi: 10.1016/j.atmosenv.2015.12.008. [DOI] [Google Scholar]
- 47.Kuzyk A.C., Burbidge T., Mydlarski P.R. Cutaneous Emmonsia infection in a renal transplant recipient. JAAD Case Rep. May 2021;11:44–46. doi: 10.1016/j.jdcr.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibrahim A., Spellberg B., Walsh T., Kontoyiannis D. Pathogenesis of mucormycosis. Clin. Infect. Dis. Feb. 2012;54(SUPPL. 1):S16–S22. doi: 10.1093/cid/cir865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Breza-Boruta B. The assessment of airborne bacterial and fungal contamination emitted by a municipal landfill site in Northern Poland. Atmos. Pollut. Res. Nov. 2016;7(6):1043–1052. doi: 10.1016/j.apr.2016.06.011. [DOI] [Google Scholar]
- 50.Frączek K., Kozdrój J., Górny R.L., Cyprowski M., Gołofit-Szymczak M. Fungal air contamination in distinct sites within a municipal landfill area. Int. J. Environ. Sci. Technol. Dec. 2017;14(12):2637–2648. doi: 10.1007/s13762-017-1344-9. [DOI] [Google Scholar]
- 51.Mbareche H., Morawska L., Duchaine C. Taylor and Francis Inc; Jul. 03, 2019. On the Interpretation of Bioaerosol Exposure Measurements and Impacts on Health. [DOI] [PubMed] [Google Scholar]
- 52.Parija S., Shivaprakash M., Jayakeerthi S. Evaluation of lacto-phenol cotton blue (LPCB) for detection of Cryptosporidium, Cyclospora and Isospora in the wet mount preparation of stool. Acta Trop. Mar. 2003;85(3):349–354. doi: 10.1016/S0001-706X(02)00265-6. [DOI] [PubMed] [Google Scholar]
- 53.Xia J., Wang Z., Li T., Lu F., Sheng D., Huang W. Immunosuppressed patients with clinically diagnosed invasive fungal infections: the fungal species distribution, antifungal sensitivity and associated risk factors in a tertiary hospital of anhui province. Infect. Drug Resist. 2022;15:321–333. doi: 10.2147/IDR.S351260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garnacho-Montero J., Barrero-García I., León-Moya C. Fungal infections in immunocompromised critically ill patients. Journal of Intensive Medicine. Jul. 2024;4(3):299–306. doi: 10.1016/j.jointm.2024.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bhatt V.R., Viola G.M., Ferrajoli A. Invasive fungal infections in acute leukemia. Ther Adv Hematol. 2011;2(4):231–247. doi: 10.1177/2040620711410098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frączek K., Rózycki H., Ropek D. Statistical analyses of bioaerosol concentration at municipal landfill site. Ecological Chemistry and Engineering S. Jun. 2014;21(2):229–243. doi: 10.2478/eces-2014-0018. [DOI] [Google Scholar]
- 57.Abbasi F., Jalili M., Samaei M.R., Mokhtari A.M., Azizi E. Effect of land use on cultivable bioaerosols in the indoor air of hospital in southeast Iran and its determination of the affected radius around of hospital. Environ. Sci. Pollut. Control Ser. 2021;28(10):12707–12713. doi: 10.1007/s11356-020-10357-3. [DOI] [PubMed] [Google Scholar]
- 58.Paulussen C., et al. John Wiley and Sons Ltd; Mar. 01, 2017. Ecology of Aspergillosis: Insights into the Pathogenic Potency of Aspergillus fumigatus and Some Other Aspergillus Species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bautista-Baños S., Bosquez-Molina E., Barrera-Necha L.L. Postharvest Decay: Control Strategies. Elsevier Inc.; 2014. Rhizopus stolonifer (soft rot) pp. 1–44. [DOI] [Google Scholar]
- 60.Yadav A.N., et al. New and Future Developments in Microbial Biotechnology and Bioengineering: Penicillium System Properties and Applications. Elsevier; 2017. Biodiversity of the genus penicillium in different habitats; pp. 3–18. [DOI] [Google Scholar]
- 61.Pitt J.I. Food Spoilage Microorganisms. Elsevier Ltd; 2006. Penicillium and related genera; pp. 437–450. [DOI] [Google Scholar]
- 62.Wolny-Koład K. Microbiological quality of air in free-range and box-stall stable horse keeping systems. Environ. Monit. Assess. May 2018;190(5) doi: 10.1007/s10661-018-6644-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kasprzyk I., et al. Air pollution by allergenic spores of the genus Alternaria in the air of central and eastern Europe. Environ. Sci. Pollut. Control Ser. Jun. 2015;22(12):9260–9274. doi: 10.1007/s11356-014-4070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kilic M., et al. The effects of meteorological factors and Alternaria spore concentrations on children sensitised to Alternaria. Allergol. Immunopathol. May 2010;38(3):122–128. doi: 10.1016/j.aller.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 65.Yao X., Guo H., Zhang K., Zhao M., Ruan J., Chen J. Trichoderma and its role in biological control of plant fungal and nematode disease. Front. Microbiol. 2023;14 doi: 10.3389/fmicb.2023.1160551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zin N.A., Badaluddin N.A. Faculty of Agriculture, Ain-Shams University; Dec. 01, 2020. Biological Functions of Trichoderma Spp. For Agriculture Applications. [DOI] [Google Scholar]
- 67.Argumedo-Delira R., Alarcón A., Ferrera-Cerrato R., Almaraz J.J., Peña-Cabriales J.J. Tolerance and growth of 11 Trichoderma strains to crude oil, naphthalene, phenanthrene and benzo[a]pyrene. J Environ Manage. 2012;95(SUPPL) doi: 10.1016/j.jenvman.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 68.Yao X., Guo H., Zhang K., Zhao M., Ruan J., Chen J. Frontiers Media S.A; 2023. Trichoderma and its Role in Biological Control of Plant Fungal and Nematode Disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sutton D.A., Rinaldi M.G., Sanche S.E. Chapter 14 - dematiaceous fungi. Clinical Mycology. 2009:329–354. doi: 10.1016/B978-1-4160-5680-5.10014-3. [DOI] [Google Scholar]
- 70.Katushova M., Beloshapkina O., Tarakanov R., Shipulin R., Dzhalilov F. IOP Conference Series: Earth and Environmental Science. IOP Publishing Ltd; Feb. 2021. Fungi of the genus Curvularia sp. - new pathogens of turfgrass in Russia. [DOI] [Google Scholar]
- 71.Goncharov A.A., Glebova A.A., Tiunov A.V. Elsevier B.V.; Jan. 01, 2020. Trophic Interactions between Fusarium Species and Soil Fauna: A Meta-Analysis of Experimental Studies. [DOI] [Google Scholar]
- 72.Ye R., Xu S., Wang Q., Fu X., Dai H., Lu W. Fungal diversity and its mechanism of community shaping in the milieu of sanitary landfill. Front. Environ. Sci. Eng. Aug. 2021;15(4) doi: 10.1007/s11783-020-1370-6. [DOI] [Google Scholar]
- 73.Rajeshkumar K.C., Yilmaz N., Marathe S.D., Seifert K.A. Morphology and multigene phylogeny of Talaromyces amyrossmaniae, a new synnematous species belonging to the section Trachyspermi from India. MycoKeys. Jan. 2019;45:41–56. doi: 10.3897/mycokeys.45.32549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wei W., et al. Talaromyces marneffei suppresses macrophage inflammation by regulating host alternative splicing. Commun. Biol. Dec. 2023;6(1) doi: 10.1038/s42003-023-05409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Charya L., Garg S. Advances in Biological Science Research: A Practical Approach. Elsevier; 2019. Advances in methods and practices of ectomycorrhizal research; pp. 303–325. [DOI] [Google Scholar]
- 76.L. Martínez-Hernández, C. Haydee Caro-Sánchez, and A. Bonifaz, “Infecciones por Fusarium Fusarium Infections.”.
- 77.Claffey C. El hongo entre nosotros: Aspergillus. Nursing. Sep. 2014;31(5):63–64. doi: 10.1016/j.nursi.2014.10.019. española. [DOI] [Google Scholar]
- 78.Diario Oficial de la Federación . México; 2004. NORMA Oficial Mexicana NOM-083-SEMARNAT-2003.https://www.dof.gob.mx/nota_detalle.php?codigo=658648&fecha=20/10/2004&print=true [Online]. Available: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are included in the supplementary material.