Abstract
Relevance
Spontaneous abortion (SAB) and recurrent pregnancy loss (RPL) occur alone or concurrently with increasing incidences recently. Scutellaria baicalensis Georgi (SBG) has been used to prevent pregnancy loss for thousands of years, which is recognized as a “pregnancy-stabilizing herb” in ancient China. Baicalin (BA) and its metabolite baicalein (BE) are the main bioactive flavonoids in the root of SBG.
Methods
In this study, we focused particularly on the metabolism, toxicology, and pharmacological effects of BA at the maternal-fetal interface based on the biological process prediction by network pharmacology. Focused on the systematic review of BA's regulatory mechanisms of immune homeostasis, cell proliferation and invasion, programmed cell death, inflammatory microenvironment, angiogenesis, oxidative stress and vascular remodeling at the maternal-fetal interface, it was found that BA exerts its biological effects to treat SAB and RPL through multiple perspectives and targets. We also critically elucidated the limitations of using BA from a clinical perspective.
Results
We explored the bioavailability, targeting and efficacy of BA from a new perspective (optimization of the BA delivery system, organoid studies based on BA, potential effects of BA on uterine flora and bioactive components). Finally, we propose a multimodal stereo sequencing study of biologically active components based on pathological dynamics incorporating single-cell RNA sequencing, spatially resolved transcriptomics, and single-cell multimodal omics to delve deeper into the fetal-preserving mechanism of BA and to promote the application of BA in clinical practice.
Keywords: Baicalin, Traditional Chinese medicine, Recurrent pregnancy loss, Spontaneous abortion, Critical review
The list of chemical compounds in the article
Chemical compounds studied in this article Baicalin (PubChem CID: 64982).
Baicalein (PubChem CID: 5281605).
1. Introduction
Spontaneous abortion (SAB) is the loss of a pregnancy before 20 weeks of gestation and affects up to 20 %–30 % of all pregnancies [1]. Recurrent pregnancy loss (RPL) is defined as the failure of two or more clinically recognized pregnancies before 20–24 weeks of gestation and includes embryonic and fetal losses [2]. SAB and RPL both are distress pregnancy disorder experienced by ∼2.5 % of women trying to conceive [3]. Several pathologies have been identified. The causes could be female age, anatomical and chromosomal abnormalities, genetic, endocrinological, placental anomalies, infection, smoking, alcohol consumption and psychological factors. Even with advanced assistant reproductive technology, the etiology of RPL caused by non-embryonic factors is complex, and different etiologies often coexist in PRL women, which makes it difficult to diagnosis and treatment [4,5].
Scutellaria baicalensis Georgi (SBG), a famous traditional herbal medicine applied in treating pregnancy-related disease for over 1000 years, is regarded as a “pregnancy-stabilizing herb”. Studies have shown that Baicalin (BA) is one of the main chemical components of SBG [[5], [6], [7]] and the quality marker (Q-marker, a standardization of a quality control system for traditional Chinese medicine (TCM) [8]) of SBG. BA and its metabolite baicalein (BE) are the main bioactive flavonoids that can be extracted from the dried roots of SBG. Studies have demonstrated that Chinese herb prescriptions consisting of BA can improve the levels of progesterone and β-human chorionic gonadotropin (β-hCG), plasma prothrombin time (PPT), activated partial thromboplastin time (APTT). However, there is still a notable lack of understanding of the pharmacology and toxicology of BA in preventing SBA/RPL.
In this review, we critically evaluated the pharmacological properties, toxicology and safety of BA. We also summarized information of previous experiments and mechanisms of BA in the prevention of SAB based on the prediction biochemical process by network pharmacology. We also analyzed the differences in experimental designs, outcomes and limitations of previous studies, and provide future directions for the clinical applications of BA (Flow figure was detailed in Fig. 1).
Fig. 1.
Multi-level framework of BA in the treatment of SAB/RPL. Notes: BA: Baicalin. SAB: Spontaneous abortion. BE: metabolite baicalein. RPL: Recurrent pregnancy loss. SBG: Scutellaria baicalensis Georgi.
2. Characteristics of baicalin
SBG is widely distributed in Northern, Northeastern, and Northwestern China, and is commonly found in extensive areas north of the Yangtze River [9,10]. The best quality SBG with the richest components is produced at Linyi city in Shandong province, and followed by Yuncheng city in Shanxi province [[11], [12], [13], [14]]. Studies have shown that flavonoids are the main bioactive chemical components of SBG, with the most representative components being BA and its main metabolite BE [15,16]. And according to the identification criteria of Q-marker, BA is considered to be an important chemical major component in SBG [17]. The proposed chemical structures of the metabolites and metabolic pathways of BA are shown in Fig. 2 A-E. As a glycosidic flavone, BA is too polar to traverse the lipid bilayer by passive diffusion and is thus poorly absorbed by the intestinal tract. It has also been found that BA has low bioavailability (2.2 %). Moreover, according to recent studies, BA exhibits different pharmacokinetic properties under different pathological conditions due to the fact that pathological conditions may alter the functionality of many enzymes and transporters in vivo. Thus, the pharmacokinetics of BA needs further research, especially under sensitive and appropriate detection methods [18].
Fig. 2.
Map showing the metabolic pathways of BA. (A) Lipinski's Rule-of-Five for BA and its metabolite BE. (B). Chromatograms for BA in a double blank plasma sample, blank plasma sample with internal standard and a LLOQ plasma sample. (C–D) Proposed metabolic pathways of baicalin in vivo. (E) Interconversion pathways of BA and BE in vivo. Abbreviations: BA, Baicalin; BE, Baicalein; MW, Molecular weight; PSA, polar surface area; RB, rotatable bonds; HBA, hydrogen bond acceptors; HBD, hydrogen bond donors; GUS, β-glucuronidase; UGT, Uridine Diphosphate Glycosyltransferase; LLQQ, Lower limit of quantification.
3. Pharmacological prediction of SA/RPL treated with BA
To identify the target genes of SAB/RPL when treated by BA, we performed a prediction study by network pharmacology (NP), a common method in Complementary and Alternative Medicine (CAM) [19]. A NPgto predict the target regulatory network was conducted based on the BA and BE considering of the drug-likeness for oral delivery [20].
3.1. Prediction of the mechanisms of baicalin-treated abortion by network pharmacology
3.1.1. Regulatory target network prediction of BA
The targets of BA and BE were introduced into the Swiss Target Prediction database (http://www.swisstargetprediction.ch/), the HERB database (http://herb.ac.cn/) and the PUBCHEM database (https://pubchem.ncbi.nlm.nih.gov/) (Fig. 3A–B). Differentially expressed genes (DEGs) were then identified, including Cyclin-dependent kinase 1 (CDK1), Xanthine dehydrogenase (XDH), Lysine-specific demethylase 4D-like (KDM4E), Arachidonate 15-lipoxygenase (ALOX15), and Arachid-onate 12-lipoxygenase (ALOX12) (Fig. 3C). Moreover, screening of the protein-protein interaction (PPI) network (minimum required interaction score: highest confidence = 0.900) [21] by STRING (https://string-db.org), Cytoscape network analysis and the Cytohubba algorithm [22], revealed that steroid receptor coactivator (SRC), mitogen-activated protein kinase (MAPK), estrogen receptor alpha (ESR1), epidermal growth factor receptor erbB1(EGFR) and FYN may represent the core target genes of BA (Fig. 3D).
Fig. 3.
Predictive NP of the prevention from miscarriage by BA. (A) The target network of BA (from SWISS). (B) The target network of BE (from SWISS). (C) GO enrichment analysis. (D) KEGG enrichment analysis of BA regulated targets (from metascape). (E) Related biological processes at maternal-fetal interface. Notes: NP, Network pharmacology. BA, baicalin. BE, baicalein. GO, Gene Ontology. BP, biological process. CC: Cellular components. MF: Molecular function.
3.1.2. GO/KEGG enrichment analysis of SAB/RPL regulated targets by BA
Metascape (https://metascape. org/gp/) was utilized to analyze the Gene Ontology (GO) database (http://geneontology.org/) and predicted the SBG acting on SAB/RPL physiological and pathological processes at the maternal-fetal interface. By summarizing the enrichment of BA/BE, 119 molecular function (MF), 112 cellular components (CC), 354 biological processes (BP) (Fig. 3C). The BPs identified in recent studies include Relation of hormone levels (0010817), Negative regulation of immune system process (0002683), Negative regulation of cell population proliferation (0008285), Positive regulation of cell death (0010942), Regulation of inflammatory response (0050727) and Angiogenesis (0001525) (details are shown in Table 1).
Table 1.
Prediction of active biochemical process of BA in preventing SBA/PRL.
| GO | Major BP | FDR | Gene Ratio |
Expression patterns in SA or RPL | Regulatory evidence in this review |
|---|---|---|---|---|---|
| 0010817 | Regulation of hormone levels | 9.84 | 18/532 | Regardless of etiology, sex hormone expression level is markedly decreased in a large proportion of patients with SAB/RPL | 4.1 The effects of different abortion induction models |
| 0002683 | Negative regulation of immune system process | 9.42 | 17/487 | Many types of decidual immune cell occupancies and functions are significantly altered in patients with SAB or RPL | 4.2 Regulation of decidual immune cells at the maternal fetal interface by BA on SAB/RPL |
| 0008285 | Negative regulation of cell population proliferation | 5.81 | 16/787 | The proliferation and invasion of trophoblast cells and decidual cells were significantly reduced in patients with SAB or RPL | 4.3 Cell proliferation and cell invasion of BA |
| 0010942 | Positive regulation of cell death | 9.76 | 19/621 | Programmed death of trophoblast cells or deciduate cells is abnormally activated in patients with SAB or RPL | 4.4 BA regulates programmed cell death at the maternal fetal interface |
| 0050727 | Regulation of inflammatory response | 10.43 | 17/414 | Overactivation of maternal and fetal interfacial inflammatory systems in patients with SAB or RPL (↑) | 4.5 Inflammation and immune homeostasis |
| 0001525 | Angiogenesis | 2.44 | 7/326 | The angiogenic capacity of decidual tissue was significantly reduced in more patients with SAB or RPL (↓) | 4.6 Angiogenesis and vascular remodeling |
The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment results suggested that the mechanism of BA/BE in treating SAB/RPL was significantly related to the NF-kappa B signaling pathway (hsa04064), Apoptosis (hsa04210), MAPK signaling pathway(hsa04010), VEGF signaling pathway(hsa04370), PI3K-Akt signaling pathway(hsa04151) and mTOR signaling pathway(hsa04150) (details was shown in Table 1 and Fig. 3E).
3.2. Limitations of network pharmacology in the studies of SAB/RPL
NP has been widely applied and recommended for investigating CAM-disease target genes. As mentioned previously, we have screened the core target genes and related pathways of BA for the treatment of SAB/RPL by network pharmacology. Nevertheless, there are several limitations to this approach. First, we should be cautious when using results derived from NP due to the fact that the databases are updated slowly, which can limit the comprehensiveness and credibility of research results [23]. Second, the dosage of herbs is always ignored when evaluating pharmacology and toxicology, making it difficult to identify regulatory relationships between the component and the targe genes [24]. Third, NP analysis is based on database screening. Whether the predicted genes work on the target organs or target cells need further investigation [25]. Finally, NP analysis can rarely reflect the actual mechanisms at the maternal-fetal interface during BA treatment. This is due to the clear heterogeneity between patients and the dynamic process that occurs during embryo implantation.
4. Mechanisms of BA in the treatment of SAB/PRL
As shown in Table 1, the predicted active BPs are crucial to maintain successful pregnancy. According to the prediction by the NP, we reviewed the four main active BPs of BA in preventing SBA/PRL. To focus on the mechanisms of BA/BE, we only summarized the modeled-animal trials (summarized in Table 2).
Table 2.
Experimental study on BA for anti-abortion.
| Study | Object of study | Dosage of BA | Intervention time | Modeling method | pregnancy diseases | Biological Processes∗ |
Related Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|---|
| ① | ② | ③ | ④ | |||||||
| Tian, 2022 [25] | HTR-8/Svneo | 100 μmol/L | 48 h after drug intervention | 25 mmol/L glucose | gestational diabetes | + | LDH, SOD, cleared caspase3, Bax, miR NC, and miR 451 | |||
| Qiao, 2022 [26] |
BALB/c, CBA/J and DBA/2 mice | 25~100 mg/kg, OA | 1.0~14.0 dpc (14 days) | CBA/J × DBA/2 pregnant mice | RPL | + | RR, TNFα, IL14, IL10, CD4+/CD8+, IFN-γ, p-PI3K, p-AKT | |||
| Lai, 2022 [27] |
RSA patients BALB/c, CBA/J and DBA/2 mice |
25~100 mg/kg, OA | 0.5~12.5 dpc (12 days) | CBA/J × DBA/2 pregnant mice | RPL | + | RR, DC cells, HLA-DR/MHC-II, CD80, CD86, CD274, 33D1, SAT5-ID2, IL6, IL10, IL12, TGF-β | |||
| Li, 2021a [28] |
2-cell stage embryos of K-M mice | 4 mg/Ml, OA | 116 h post-hCG injection | 42 °C for 1 h at 112 h post-hCG injection | heat stress∗ | + | Blastocyst hatchability, c-fos, ERK1/2, AP-1, mtDNA, TFAM, Bax, Bcl-2 and caspase-3), c-Jun | |||
| Li, 2021b [29] |
GDM patients HTR-8/Svneo |
0.5, 10, 20, 50 and 100 μM | 2 h | high-glucose (25 mmol/L) stimulation | GDM | + | + | + | Cell viability, proliferation, Apoptosis, TNF-α, IL-1β, IL-6, miRNA-17-5p, Mfn1/2, NF-κB | |
| Lu, 2021 [30] |
SD gestation Rat | 5 mg/kg Tail vein injection |
1.0~10.0 dpc (10 days) | LPS (1.0 μg/kg) | SAB | + | RR, TNFα, IL1β, CD86, CD206, p-PI3K, p-AKT | |||
| Xu, 2021 [31] |
Wistar gestation Rat | 50 mg/kg intraperitoneally injection | 16.0~21.0 dpc (5 days) | Nitro L Arginine Methyl Ester (100 mg/kg) 13.0~21.0dpc |
PE | + | Tail artery blood pressure, 24 h urine protein level, Beclin 1, LC3-II and P62 | |||
| Wang, 2021 [32] |
SD gestation Rat Primary culture of PE rat trophoblasts | 25~100 μg/mL | 48 h after intervention with medicated serum | As above | PE | + | Cell morphology, Cell proliferation, cell invasiveness, GCM-1, miRNA-30d | |||
| Zhao, 2021 [33] |
HTR-8/Svneo HUVECs |
1, 5, 25 μM | 48 h after drug intervention | Ang II(20 μM) | HDCP | + | + | Cell proliferation, cell invasiveness, cell viability (HTR-8/SVneo and HUVECs); cell apoptosis ROS, MMP2, MMP9, VEGF, LncRNA NEAT1, miR-205-5p, HIF-1α | ||
| Li, 2020 [34] |
In Vitro Fertilized Eggs of Pigs | 8 mg/L OA | Intervention for 48, 120 and 168 h respectively | No modeling | IVF-ET | + | Changes of cleavage rate, mulberry sac rate, blastocyst rate and NO expression in different time periods | |||
| Jiao, 2020 [35] |
BALB/c, CBA/J and DBA/2 mice | 0.4 mL/mg | 2.0~9.0 dpc (7 days) | CBA/J × DBA/2 pregnant mice | RSA | + | Macrophage (CD14), FSH, E2, PRL, TNF-α, IL-4, TLR4, NFκB, MyD88 | |||
| Yang, 2019 [36] |
BALB/c, CBA/J and DBA/2 mice | 0.4 mL/mg OA |
1.0~14.0 dpc (14 days) | CBA/J × DBA/2 pregnant mice | RSA | + | RR, CD4+, CD8+, IFN-γ, IL4, Th1, Th2 | |||
| Guo, 2019 [37] |
In Vitro Fertilized Eggs of Pigs (2–4 cell stage) | 0, 0.05, 0.1, 0.5 and 1.0 μg/mL | Intervention for 44 h respectively | No modeling | IVF-ET | + | + | Apoptosis, oocyte quality, embryo competence in vitro, BAX,BMP 15, GDF9, CyclinB, CDK1, PTGS1/2, PTX3, TNFAIP6 and ΔΨm | ||
| Liang, 2018 [38] |
embryonated eggs HUVECs |
6 μM (chicken embryo); 40 mg/kg (mice) | Once every 3 days, 3 times in total | high-glucose (50 mM) stimulation | GDM | + | + | Apoptosis, Autophagy, SOD, GSH-Px, MQAE, GABAA, p62, | ||
| Liu, 2018 [39] |
HTR-8/SVneo cells | 0.0, 0.05, 0.1∗∗, and 0.5 μM | 24 h after drug intervention | No modeling | SAB | + | + | Cell Viability, Invasiveness MMP-2/MMP9, NF-Κb p65 | ||
| Hou, 2018 [40] |
JEG-3 trophoblast cells | 1.5625, 3.125, and 6.25μg/ml | 16 h after drug intervention | 8 h hypoxia and 16 h reoxygenation | PE | + | + | Cell apoptosis, cell vitality, cell invasiveness, CASP3, CASP9 | ||
| Ma, 2018a [41] |
K-M mice | 0.4 mL/mg OA |
4.0~9.0 dpc (5 days) | LPS (0.1 μg) | SAB | + | RR, TNF-α, IL-10 | |||
| Ma, 2018b [42] |
K-M mice | 0.4 mL/mg OA |
4.0~9.0 dpc (5 days) | LPS (0.1 μg) | SAB | + | IR, IFN-γ, IL-4, IL-10, Th1/Th2 | |||
| Lai, 2016 [43] |
RSA patients BALB/c, CBA/J and DBA/2 mice |
25~100 mg/kg OA |
0.5~12.5 dpc (12 days) | CBA/J × DBA/2 pregnant mice | RSA | + | CD11c+,CD274/CD275,33D1、CD205,IL-10,pDC/cDC,MHC-II+ | |||
| Guan, 2016 [44] |
ICR mice | 0.25, 0.50, and 1.0 mg/mL | 0.5~6.5 dpc (6 days) | Listeria spp (5 × 106/mL) | SAB | + | RR, IL-1β | |||
| Wang, 2016 [45] |
Wistar gestation Rat | 50, 100 mg/kg (I.h) | 16.0~21.0 dpc (5 days) | Nitro L Arginine Methyl Ester (100 mg/kg) 13.0~21.0dpc |
PE | + | + | Tail artery blood pressure, 24 h urine protein level, Mitochondrial morphology, angiogenesis, XIAP, CASP9 | ||
| Qi, 2016 [46] |
Mouse prokaryotic embryo | 2,4, and 8 μg/mL | 24 h after drug intervention | No modeling | SA | + | + | Dnmt3a, Dnmt1,GJA-1、CDH1、BCL-2, HSP70, BAX, CASP3 | ||
| Chen, 2014 [47] |
K-M mice | 40 mg/kg OA |
2.0~7.0 dpc (5 days) | Ru486 (3.33 mg/kg) | SA | + | RR, number of implanted blastocysts, β-catenin, LRP6, DKK1 and Wnt4, Progesterone,estradiol | |||
| Zhang, 2015 [48] |
K-M mice | 100 mg/kg OA |
1.0~8.0 dpc (7 days) | Female mice were injected with PMSG and hCG | COH | + | + | Adhesion, p-GSK3β, GSK3β, β-catenin, FUT4, SP5, Wnt, | ||
| Qi, 2016 [46] |
2-cell stage embryos of KM mice | 4 μg/mL | Intervention for 24, 48, 72, 96, 120 and 168 h respectively | No modeling | IVF-ET | + | + | Blastocyst development rate, BCL-2, HSP70 and GJA1 | ||
| Wang, 2014 [49] |
Decidual cells in mice | 4 μg/mL OA |
48 h after drug intervention | LPS (0~1000 ng/Ml) | SAB | + | RR, Decidual damage scenario, TNFα, Progesterone | |||
| K-M mice | 0.25, 1.25 and 2.5 mg/mL ((0.1 mL/10 g) | 7.0~8.0 dpc (2 days) | LPS (0.1 mL/10 g) | + | ||||||
| Liu, 2013 [50] |
Human chorionic trophoblast cells | 0.05 g/mL (Dilute the stock solution to 1 million times) | 24 h after drug intervention | No modeling | SAB | + | Cell Viability,TNF | |||
| Zhang, 2010 [51] |
K-M mice, EEC |
40, 400, and 800 μg/mL OA |
0.5~3.5 dpc (2 days) | LPS (0.1–0.6 μg) | SAB | + | IR, Th1, Th2, IFN-γ, IL-10 and NOS activity. | |||
| Ma, 2009 [52] |
K-M mice | 10, 20, 50 mg/kg OA |
1.0~7.0 dpc (7 days) | Bromocriptine (0.075 mg) | SAB | + | RR, IFN-γ, IL-10, Th1/Th2 and Progesterone | |||
| Qin, 2007 [53] |
Human decidual cells | Not applicable | 24 h after drug intervention | TNF- α (0.5, 2.0, 10.0, 50.0 μg/L) | SAB | + | Decidual apoptosis | |||
| Jiang, 2007 [54] |
BALB/c mice | 2.5 mg/mL,0.4 mL/d | 4.0~7.0 dpc (4 days) | Ru486 (90μg/0.6 mL) I.h | SAB | + | RR, IR, CD80+ 、CD86+ | |||
| Che, 2006 [55] |
BALB/c mice | 2.5 mg/mL,0.4 mL/d | 4.0~7.0 dpc (4 days) | ①Ru486 (90μg/0.6 mL) I.h; ②LPS (0.1 μg) I.h |
SAB | + | RR, IR, IL-2, IL-4, IL-10,IFNγ | |||
Notes:①Regulation of cell proliferation and invasion. ②Regulation of Programmed Cell Death (PCD). ③Regulation of inflammation and immune homeostasis. ④Regulation of angiogenesis and vascular remodeling.∗ Heat stress causes oxidative damage and induces excessive cell apoptosis and thus affects the development and/or even causes the death of preimplantation embryos. Abbreviation: COH, Controlled ovarian hyperstimulation. AKT, protein kinase B. AP1, activator protein 1. BA, Baicalin. BE, Baicalein. BCL2, B-cell lymphoma 2. BMP15, Bone Morphogenetic Protein 15. CASP3, cysteinyl aspartate specific proteinase(caspase) 3. CDK1, cyclin-dependent kinases 1. CDH1, E-Cadherin. DPC, days of pregnancy. DKK1, Dickkopf-1. EEC, Endometrial epithelial cells. FUT4, Fucosyltransferase 4. GDM, gestational diabetes mellitus. GDF9, Growth Differentiation Factor 9. GJA1, Gap junction alpha-1. GSK-3β, Glycogen synthase kinase-3 beta. HDCP, hypertensive disorder complicating pregnancy. HUVECs, human umbilical vein endothelial cells. HSP70, heatshockprotein-70. IFN-γ, Interferon-γ. IL2, Interleukins-2. IR, implantation rate. I.P, intraperitoneal injection. IVF-ET, In vitro fertilization embryo transfer. K-M mice, Kun Ming mice. LPS, Lipopolysaccharide. LRP6, Low Density Lipoprotein Receptor Related Protein 6. MMP-2, Matrix metalloproteinase-2. NF-κB, Nuclear factor kappa-B. NOS, Nitric Oxide Synthase. OA, oral administration. RR, Resorption rate. RSA, Recurrent spontaneous abortion. ROS, Reactive Oxygen Species. PI3K, Phosphatidylinositol3-kinase. PTX3, Pentraxin 3. PE, Preeclampsia. SAB, spontaneous abortion. TNFα, Tumor necrosis factor alpha. TNFAIP6, Tumor necrosis factor α Inducible Protein 6. TGF-β, Transforming growth factor-β. TFAM, Transcription Factor A. Mitochondrial. XIAP, X-linked inhibitor of apoptosis protein.
4.1. Regulation of decidual immune cells at the maternal-fetal interface by BA
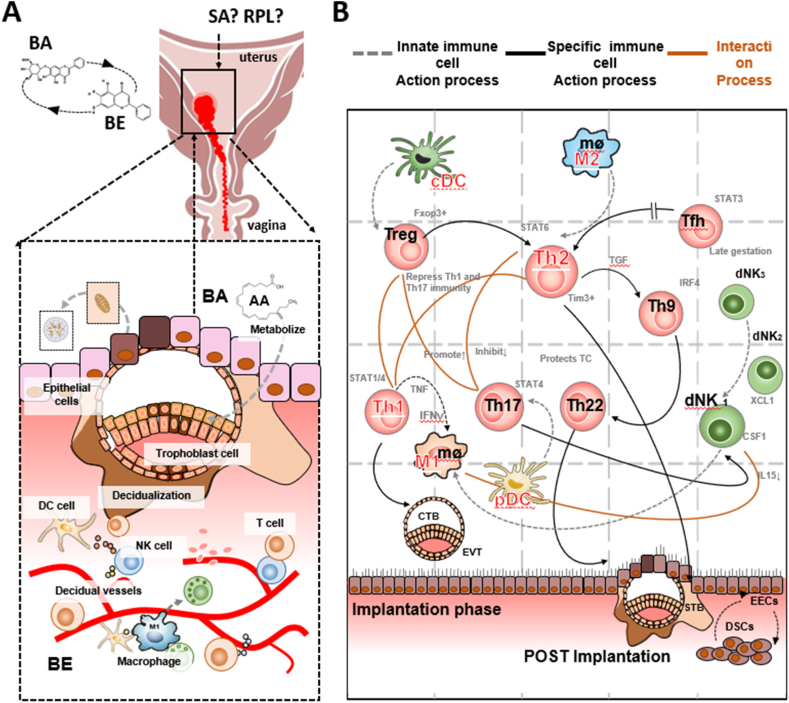
Since the fetus is semi-allogeneic, the maternal immune system should exhibit tolerance of the fetus while maintaining the defense against infection. Thus, the microenvironment of the maternal-fetal interface, which consists of decidual stromal cells, trophoblast cells and decidual immune cells, should play a key role in maintaining pregnancy. Various subsets of material immune cells constitute the decidual immune system, such as natural killer (NK) cells, macrophages, T cells, B cells, and dendritic cells (DC). An imbalance of the microenvironment or functional changes may contribute to SAB or RPL [56](Fig. 4, Fig. 5).
Fig. 4.
Regulatory networks of BA on immune cells at the maternal fetal interface in SAB/RPL patients. Notes: M1/M2: Decidual macrophages can be classified into M1 and M2 macrophages according to pro-inflammatory and anti-inflammatory states. CTB: cytotrophoblast, EECs: endometrial epithelial cells, EECs. DSCs: decidual stromal cell. AA: arachidonic acid. DC: dendritic cell. NK: natural killer cell. TfH: Follicular helper T cells. Th: helper T cell. EVT: extravillous trophoblast.
Fig. 5.
Main regulatory mechanisms of BA against SAB/RPL.
4.1.1. Decidual CD4+ T cells
During early pregnancy, decidual T lymphocytes account for about 10–20 % of immune cells, while CD 4+Th cells account for 30–45 %. T helper (Th) dichotomy Th1/Th2 immune response is incorporated with a concept of regulatory T cell (Treg) immunity and expends to the Th1/Th2/Th17 and Treg cell paradigm. When the balance of Th1/Th2 and Th17/Treg is damaged, the poor decidualization is accompanied with adequate endometrial vascularity, leading to impaired implantation. Research has shown that the propensity for Th2 over Th1 immune response is recognized by administrated BA and that the ratio of CD4+/CD8+ was ameliorated to prevent from RPL [36].
4.1.2. Macrophages
Macrophages (Mø), a type of antigen presenting cells, consists of 20∼25 % of maternal-fetal immune cells. Decidual macrophages can be classified into M1 and M2 macrophages according to pro-inflammatory and anti-inflammatory states. Recent studies show that BA can improve the estrogen level and anti-inflammatory states in maintaining the balance of maternal-fetal interface through inhibition of endometrial Mø infiltration and regulation of TLR4/NF-κB signaling pathway activity [35].
4.1.3. Decidual dendritic cells
Dendritic cells (DCs) are important maternal fetal interface immune cells discovered recently. They provide fetal antigens to decidual T cells through their powerful antigen presentation function. DC-SIGN + immature decidual cells and CD83+ mature decidual cells were found. Researchers have demonstrated a mutual transformation and regulation between DCs and macrophages. It has also been reported that the content of ILT4+ DCs in peripheral blood decreases in patients with recurrent abortion [57]. Recent studies indicate that BA could alleviate embryo resorption of RSA mice by reversing conventional DCs to plasmacytoid DCs and functional molecule expression via inhibiting the STAT5-ID2 pathway. These studies indicate that DCs play an important role in the pathogenesis of RSA and BA might be used for treating RPL [27].
4.1.4. Decidual natural killer(dNK) cells
As the most abundant leukocytes during pregnancy, natural killer (NK) cells are recruited and activated by ovarian hormones and have pivotal roles throughout pregnancy. During the first trimester, NK cells represent up to 50–70 % of decidua lymphocytes. Differently from peripheral-blood NK cells, dNK cells are poorly cytolytic. They release cytokines/chemokines that induce trophoblast invasion, tissue remodeling, embryonic development, and placentation. NK cells can also shift to a cytotoxic identity and carry out immune defense if infected in utero by pathogens [58]. Although there are no direct reports on BA's regulation of NK cells in decidual or trophoblast tissues, a large number of Chinese herbal compounds containing BA suggest that they can improve the immune state by adjusting the balance between peripheral blood NK cells and endometrial NK cells and regulating related factors to treat patients with RPL or RIF [59]. However, more evidence is needed.
4.1.5. Myeloid derived suppressor cells
Myeloid derived suppressor cells (MDSC), in particular polymorphonuclear myeloid derived suppressor cells (PMNMDSC), play a significant role in maintaining the maternal-fetal tolerance and preventing the invasion of the normal human cytotrophoblast cells [60]. PMN-MDSCs can mediate immune tolerance by inhibiting NK cytotoxicity, inducing Treg cell proliferation and polarizing CD4+ T cells into Th2 cells. Though there is still a lack of evidence linking BA and MDSC of decidua or trophoblastic tissue, in recent years it has been suggested that treatment with BA could ameliorate Lesional tissue immunosuppressive environment by downregulation of PD-L1 expression and proportion of MDSCs and upregulation of percent of CD4+ and CD8+ T cells in lesional tissue [61]. Therefore, this category of cells also deserves attention.
4.2. Regulation of cell proliferation and cell invasion
Early embryonic development is very similar to tumor development. Trophoblast cell proliferation is often accompanied by invasion; this process refers to the ability of cells to migrate from one region to another through the extracellular matrix [62]. Cell invasiveness often predicts the ability of embryo implantation and the function of endometrium decidualization.
BA is significantly more effective than water extracts of BA at the same dose with regards to promoting trophoblast cell proliferation [50]. BA can significantly inhibit the expression of Mir-30d and up-regulate GM-1 protein to promote the proliferation and invasion of placental trophoblast cells in rats with preeclampsia [32]. BE can promote the migration and invasion of HTR-8/SVneo cells through the NF-κB pathway, which is very important for successful pregnancy [39].
4.3. Regulation of programmed cell death (PCD)
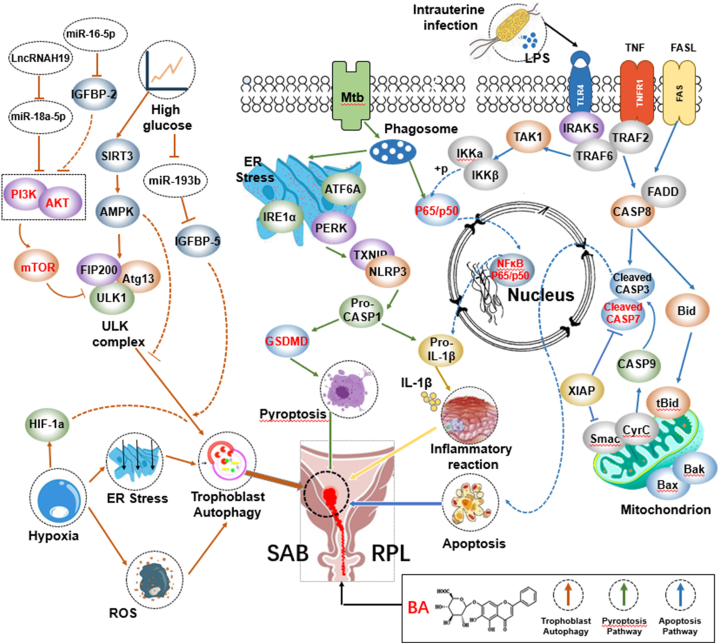
PCD is a physiological and active “suicide” phenomenon in which cells die at particular points during the development process. PCD has since been recognized and accepted as a distinctive and important mode of cell death, which involves the genetically determined elimination of specific cells [63]. During embryo implantation, PCD is conductive to the invasion of trophoblast cells into the endometrial cells and decidualization, However, overactivated PCD during pregnancy is not conducive to the survival of the embryo. Therefore, homeostasis is critical during PCD [64].There are many types of PCD which can manifest as apoptosis, autophagy-dependent death, pyroptosis, ferroptosis, and programmed cell necrosis at the maternal-fetal interface [65]. BA has obvious advantages in the regulation of PCD. As detailed below, it is believed that the mechanism of BA is mainly regulated from three types, including apoptosis, autophagy and pyroptosis.
4.3.1. Apoptosis
Apoptosis refers to the spontaneous and orderly death of cells controlled by genes in order to maintain a stable internal environment. The process can be divided into four steps: reception of apoptosis signal, interaction between apoptosis-regulating molecules, activation of proteolytic enzyme (Caspase) and continuous reaction of death. BA can reverse the mitochondrial pathway of excessive cell apoptosis [45] and cell autophagy [31]. In vitro studies have shown that BA can regulate Gap junction alpha-1 (Gja1), E-cadherin (Cdh1), b-cell lymphoma-2(bcl-2) and heatshockprotein70 (HSP70) to inhibit cell apoptosis to improve the development ability of mouse embryos cultured in vitro [28,37,46]. BA can also play an anti-apoptotic effect on gestational diabetes mellitus (GDM) by regulating the mirNA-17-5p-Mfn1/2-Nf-κb pathway [29].
4.3.2. Autophagy
Autophagy refers to the process by which lysosomes or vacuole intima directly encase and degrade substrates. Three main forms of autophagy: micro-autophagy, macro-autophagy, and Chaperone-mediated autophagy (CMA) have been observed. Autophagy regulates the crosstalk between these cells at the maternal-fetal interface as well. Aberrant autophagy is found in villi, decidual stromal cells, peripheral blood mononuclear cells in SAB/RPL patients, although the findings are inconsistent among different studies [66]. In vivo studies show that BA could inhibit X-1inked inhibitor of apoptosis protein (XIAP), microtubule associated protein 1 light chain (LC3-II) and cysteine containing aspartation-9 (Caspase-9) in the placental trophoblast cells of preeclampsia animal models [31].
4.3.3. Pyroptosis
Pyroptosis, also known as inflammatory necrosis of cells, is a programmed cell death, which is manifested by the continuous expansion of cells until the membrane bursts, resulting in the release of cell contents and the activation of a strong inflammatory response. It is involved in the regulation of host defense against pathogens and immune inflammatory processes, and is crucial in maintaining the balance of immune inflammation in the body. Activation of inflammasome can mediate the formation of Gasdermin shear body and participate in pyroptosis. Recently, increasing evidence suggests that pyroptosis and inflammasome are key to the physiological processes of pregnancy and the occurrence and development of many pregnancy diseases. Although there are no reports of the effects of BA on pyroptosis of basic cells in pregnancy, most studies suggest that BA reduces pyroptosis by inhibiting the PERK/TXNIP/NLRP3 axis, and therefore may be a new host-directed therapy (HDT) drug [67].
4.4. Regulation of inflammation and immune homeostasis
A balance between pro-inflammatory and anti-inflammatory cytokines is key to embryo implantation and immune tolerance [68]. However, the innate immunity system and specific immune systems can create an imbalance in the inflammatory environment after stimulation by pathological factors. This imbalance can change the quantity of decidual immune cytokines and induce temporal-spatial development disorders and disturb maternal-embryo interface communication, thus leading to SAB [69,70]. BA has significant pharmacological effects in regulating inflammation. Firstly, BA can inhibit the levels of interferon (IFN)-γ and interleukin (IL)-2 in the uterine tissue through the PI3K/AKT signaling pathway, and can also activate Th2 cells to secrete IL-4, IL-10 and reduce the activity of NOS. BA can also promote the Th1/Th2 balance to influence the development of the Th2 type and effectively reduce the CD4+/CD8+ ratio [26,36,41]. Secondly, BA can also inhibit macrophage polarization through the TLR4/Nf-κb signaling pathway [35] and improve the inflammatory microenvironment at the maternal-fetal interface. Last but not least, it has been found that conventional dendritic cells can be reversed into plasmacytoid dendritic cells and that functional molecule expression can be inhibited by inhibition of the STAT5-Id2 pathway, thereby reducing embryonic resorption in RSA mice [27].
In addition, we focused on the effects of the arachidonic acid metabolic system on the reproductive system and the maternal fetal interface through NP. Researchers have suggested that the three metabolic pathways importantly on female pathological pregnancy. As early as 20 years ago, some scholars proposed that BA is an inhibitor of arachidonic acid (AA) metabolic pathway lipoxyase (LO), which has a significant protective effect on mouse inflammatory models such as liver injury caused by bacterial lipopolysaccharide. At present, a large number of reports suggest that baicalin has obvious curative effect on LPS-induced abortion mice, and its mechanism is also related to the regulation of arachidonic acid.
4.5. Regulation of oxidative stress
In physiological states, there is an oxidation/antioxidation balance in the body that can provide a low-stress environment for the placenta. In pathological states, when the body is stimulated by various internal and external factors, excessive mitochondrial reactive oxygen species (ROS) are produced and the oxidative/antioxidant balance in the body is impaired. As a result, oxidative stress is initiated, which in turn causes cellular damage. There is a large body of evidence that an abnormal increase or rapid fluctuation of ROS has a deleterious effect on the trophoblast function, leading to a variety of adverse pregnancies [71,72]. Oxidative stress can lead to spontaneous abortion by reducing the oocytes and compromising sperm DNA [73]. In the fetus, oxidative stress can affect fetal growth and development by impairing placental function, leading to inflammatory responses and vascular dysfunction in the placenta. In addition, oxidative stress can directly damage fetal DNA, proteins, and lipids and increase the risk of pregnancy disorders in the fetus. Dysregulation of signaling pathways of cellular antioxidant defense mechanisms has been reported to be associated with adverse birth outcomes such as miscarriage, pre-eclampsia and intrauterine fetal growth retardation [74].
BA contains two phenolic hydroxyl groups that act as hydrogen or electron donors, and has potent antioxidant and anti-inflammatory effects. Studies have shown that BA can play a protective role by reducing damage to cells and tissues caused by oxidative stress. BA scavenges free radicals in the body, regulates the activity of antioxidant enzyme systems and reduces the production of oxidative substances, thereby alleviating the adverse effects of oxidative stress on pregnancy. It was found that the inflammatory response and oxidative stress were alleviated, protease expression was reduced and MMP expression was downregulated in infected premature rats after baicalein intervention, indicating that BA can effectively improve the degree of infection, reduce the infiltration of inflammatory cells into the placenta, and improve the adverse pregnancy outcomes [75,76].
4.6. Regulation of angiogenesis and vascular remodeling
Angiogenesis refers to the development and formation of new blood vessels from existing capillaries or postcapillary veins, mainly including the degradation of vascular basement membrane and the activation, proliferation and migration of vascular endothelial cells in the activation phase [77]. Angiogenesis is regulated by the balance of pro-angiogenic factors, hypoxia-inducible factors, transforming growth factors and their inhibin networks at the maternal-fetal interface. There are important pathological changes such as vascular reduction and vascular endothelial cell structure destruction in the decidual tissue during SAB/RPL [78,79].
Vascular endothelial growth factor (VEGF) contributes to the process of embryo implantation by enhancing embryo development, improving endometrial receptivity, and facilitating the interactions between the developing embryo and the endometrium. There is a correlation between the alteration of VEGF expression and reproductive failure, including recurrent implantation failure (RIF) and RPL [78]. Studies have found that, VEGF mRNA was decreased in the decidua and villi of RSA. BA has long been confirmed to increase VEGF expression and angiogenesis by activating the ERRα/PGC-1α pathway [80]. BA can inhibit the interaction between Mir-205-5p and MMP9 or VEGF by up-regulating lncRNA NEAT1, which can in turn reduce vascular endothelial injury [33].
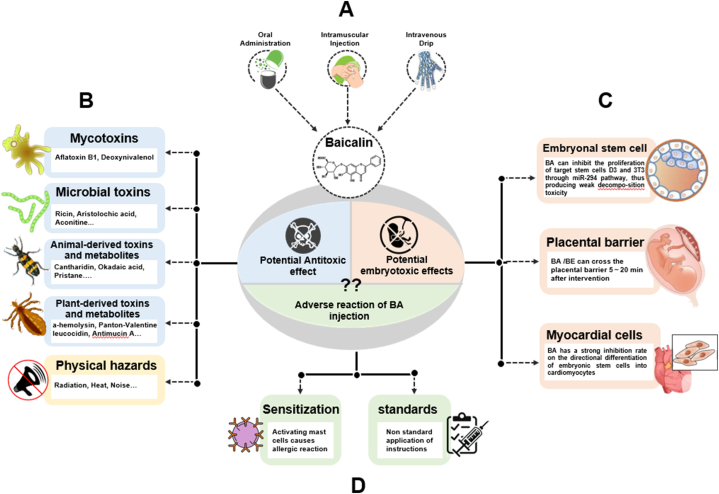
5. Toxicological effects and safety evaluation
BA is considered a safe, natural and effective culinary herbal medication, but it is essential to pay attention to the dosage and duration of treatment. The chemical drug instructions published by the National Medical Products Administration in 2002 pointed out that BA capsules should be 250mg/tablet, 2 tablets/time and, 3 times/day when taken for oral administration; 60–120 mg/time, once a day for intramuscular injection; and 300–600 mg added to 250 mL of 10 % glucose solution for intravenous injection [81,82](Fig. 6A). A study investigated the pharmacokinetics, safety and tolerability of BA after a multiple-ascending-dose protocol in healthy Chinese volunteers, and found that in dose range of 200–800 mg, multiple-dose oral BA administration was safe and well tolerated [83] (Fig. 6B). However, dose proportionality was inconclusive.
Fig. 6.
Antitoxic and embryotoxic potential effects of BA. (A) Administration routes of BA commonly used in the clinic. (B) Protective and therapeutic effects of BA on natural toxicity and physical hazards. (C) Potential embryotoxicity of BA. (D) Problems related to BA injection.
The injection of BA has become a significant focus of research due to the high efficacy of this route of administration. However, adverse reactions caused by injections of Chinese herbs containing BA often involve multiple systems, organs and tissues. For example, Shuanghuanglian, a Chinses herb formular contains BA, is known to cause an BA-induced allergenic effect [83]. BA can also activate mast cells and increase the levels of IgE and IgG antibodies [84]. In addition, particles in the injection, non-standard instructions, overdose and other factors can also lead to the aggravation of adverse reactions. Considering safety and efficacy, the application and promotion of BA-containing injections have been questioned and continue to be investigated. Moreover, clinical reports on BA focus more on the treatment of hepatitis and pneumoniac [[85], [86], [87], [88]].
For the embryotoxicity, the flavonoids of SBG were detected in pregnant rats at different stages, except for those in middle pregnancy, thus indicating potential in utero exposure in case of oral SBG administration [87]. Studies have shown that BA, wogonoside and wogonin could penetrate the placental barrier in 5 min, while BE and oroxylin A could penetrate in 20 min [89,90]. Excessive levels of BA are toxic and can downregulate the expression levels of miR-290 clusters thereafter to inhibit the proliferation of mouse D3 embryonic stem cells (ESCs) [91,92].Researchers have assessed the embryotoxicity of baicalin using embryonic stem (ES) cell tests in vitro and find that the ability of ES cells to differentiate into myocardial cells gradually decreases with 40 mg/L of BA and that the rate of inhibition was 69 %. According to estimations of embryo toxicity, BA exhibited only weak levels of embryotoxicity [93]. However, the cut-off dosage for BA needs to be further explored (Fig. 6C).
Moreover, a report investigated the use of BA capsules when combined with labetalol hydrochloride injection and show that thus combination had a significant effect in the treatment of severe preeclampsia by improving renal function and blood fluidity [94] (Fig. 6D).
6. Outlook on the research of BA in preventing SAB/RPL
Although clinical evidence supports the efficacy of BA in preventing SAB/RPL, the current understanding of the mechanism and transformation of BA in SAB/RPL is still far from complete. We believe that there are still a lot of application potential and related directions in BA delivery system, organoid research, uterine flora research and spatiotemporal omics research.
6.1. Optimization of the BA delivery system
The administration of BA is inefficient due to its low bioavailability. Multiple delivery strategies for BA have been invented, including nanoscale techniques, phospholipid complexes, solid dispersions, inclusion complexes and micelles [[95], [96], [97], [98], [99]]. Compared with BA alone, the modification of BA can improve its solubility and solubility, which in turn can further improve its targeting ability and therapeutic effect [100].
6.2. BA-based organoid studies
Organoids are three-dimensional (3D) cell cultures that contain some of the key properties of their representative organs,share a similar spatial organization with the corresponding organ, and reproduce some functions of the corresponding organ. Organoids could provide a highly physiologically relevant system [101]. Advances in human stem cell and organoid technology have been applied to extra-embryonic tissues to develop trophoblast cell lines that can grow in two dimensions (2D) and 3D and differentiate into EVTs, meaning that the currently used “trophoblast” cell lines should soon become new research trends [102]. Therefore, the development of an organoid research system will be of great significance for investigating the pathogenesis of BA on preeclampsia, fetal growth restriction, stillbirth and recurrent miscarriage.
6.3. Potential effect of BA on uterine flora
The relationship between endometrial microbiota and implantation attracted professional focus. Amplification and Sequencing of the bacteria-specific 16S ribosome gene (16S rRNA) using technologies such as Next Generation Sequencing (NGS) has confirmed the presence of the uterine microbiome. The potential mechanism of microbiota affecting implantation is unknown. The takeaway from the gut microbiome is that the uterine microbiome may regulate the subpopulations of immune cells needed for implantation. The differentially abundant microbiome may be attributed to the immune tolerance through binding to the NOD-like receptor [103]. Studies suggest that BA and its metabolites also inhibite the expression of pro-inflammatory cytokines, TNF-α and IL-1β, and the activation of NF-κB in LPS-stimulated peritoneal macrophages [104]. However, the regulation of uterine flora by BA is unclear and still worthy of further investigation.
6.4. Temporal and spatial genomics of bioactive components
The “disease-gene-target-drug” network pharmacological patterns shown in many biological research studies and CAM network biology research is usually static, thus meaning that these outcomes do not conform to the occurrence and development of disease. During the peri-implantation period, there are multiple biological process balance transitions at the maternal-fetal interface. During the localization, adhesion and invasion of blastocyst cells, with associated changes in pro-inflammatory and anti-inflammatory states of the decidua, the involved subsets of cells and related genes show dynamic network changes at each time point [105].
Routine sequencing obtains the “average value” of the overall tissue expression, and the effect of CAM components or metabolic factors at the spatiotemporal level may lead to negative results by NP. The incorporation of single-cell RNA sequencing, spatially resolved transcriptomics and single-cell multimodal omics (including the physiological characteristics of the skin-mucosal barrier, blood-brain barrier and placental barrier) can helps to study the pharmacological mechanism of BA from macro to organism, from microscopic to cellular, integrating multi-scale observation and identification, to carry out spatio-temporal genomics research, and to establish a three-dimensional sequencing system for biologically active ingredients. It has great potential for application in the discovery of pharmacodynamic substances, construction of action networks and elucidation of integrated regulatory mechanisms [106,107] (Fig. 7A–C).
Fig. 7.
Overview of spatiotemporal biological network analysis patterns. (A) Future cross-integration patterns of clinical medicine and multidisciplinary analysis. (B) Schematic representation of the pharmacological study of spatiotemporal networks for BA. (C) Technology schematic diagram of three major single-cell omics technologies: single-cell RNA sequencing, spatially resolved transcriptomics and single-cell multimodal omics.
7. Conclusion
In summary, fertility preservation is a common global issue that determines human reproduction and prosperity. Based on the biological activity, specific pharmacokinetics, network analysis and related experimental studies of BA, we conclude that BA is effective in the prevention and treatment of SAB and RPL with the following potential mechanisms: (i) the promotion of trophoblast cell proliferation and invasion, (ii) reduced cell apoptosis at the maternal-fetal interface, (iii) the maintenance of inflammatory immune homeostasis, and (iv) the promotion of decidual angiogenesis, ultimately facilitating the continuous process of decidualization. This suggests that BA exerts its fetal-sparing effects through multiple pathways and targets, and the key molecular mechanisms of its action can be further verified by animal or cellular experiments. In fact, although clinicians have recognized the efficacy of BA in preserving fertility, there are still few high-quality clinical reports on BA, and clinicians and reproductive medicine practitioners in the relevant fields can conduct large-sample, multicentre clinical trials to confirm its safety and efficacy and provide high-quality guidance for the clinic.
In addition, due to the low bioavailability of BA, there are few clinical toxicology studies and the current study design is not rigorous enough. We can optimise our research protocols to improve the bioavailability of the active ingredients of SBG through the aforementioned stereo-sequencing system of bioactive components based on the dynamic pattern of pathology, starting from spatio-temporal genomic analysis, to provide a more rigorous and objective view of the efficacy of BA for fertility preservation, and further promote the application of BA in clinical practice. Therefore, our work constructs a research blueprint for the in-depth study of the efficacy of BA for fertility preservation and its mechanism, and provides some scientific basis for the development of new effective fertility-preserving drugs in the future.
Funding
This research is supported by the National Natural Science Foundation of China (No. 82205176, No. 81973901), Sichuan Provincial Department of Science (No.2021YJ0257) and Technology China Postdoctoral Science Foundation (No. 2021MD703798).
Ethics declaration
Review and/or approval by an ethics committee as well as informed consent was not required for this study because this literature review only used existing data from published studies and did not involve any direct experimentation/studies on living beings.
Data availability statement
No data was used for the research described in the article. No data associated in this article has been deposited into a publicly available repository.
CRediT authorship contribution statement
Linwen Deng: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Yue Jin: Writing – original draft, Methodology, Formal analysis, Data curation. Xiaoyan Zheng: Methodology, Data curation. Yi Yang: Data curation, Conceptualization. Yong Feng: Data curation. Hang Zhou: Writing – original draft, Conceptualization. Qian Zeng: Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Summary acronym list
| Abbreviations | |
|---|---|
| SAB | Spontaneous abortion |
| RPL | Recurrent pregnancy loss |
| SBG | Scutellaria baicalensis Georgi |
| BA | Baicalin |
| BE | baicalein |
| β-hCG | β-human chorionic gonadotropin |
| PPT | plasma prothrombin time |
| APTT | activated partial thromboplastin time |
| NP | network pharmacology |
| CAM | Complementary and Alternative Medicine |
| DEGs | Differentially expressed genes |
| CDK1 | Cyclin-dependent kinase 1 |
| ALOX15 | Arachidonate 15-lipoxygenase |
| ALOX12 | Arachid-onate 12-lipoxygenase |
| PPI | protein-protein interaction |
| SRC | steroid receptor coactivator |
| MAPK | mitogen-activated protein kinase |
| ESR1 | estrogen receptor alpha |
| EGFR | epidermal growth factor receptor erbB1 |
| GO | Gene Ontology |
| CC | cellular components |
| MF | molecular function |
| BP | biological processes |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| NK | natural killer |
| DC | dendritic cells |
| Th | T helper |
| Mø | Macrophages |
| dNK | Decidual natural killer |
| MDSC | Myeloid derived suppressor cells |
| PMNMDSC | polymorphonuclear myeloid derived suppressor cells |
| PCD | programmed cell death |
|
XIAP |
X-1inked inhibitor of apoptosis protein |
| HDT | host-directed therapy |
| AA | arachidonic acid |
| LO | Lipoxyase |
| VEGF | Vascular endothelial growth factor |
| RIF | recurrent implantation failure |
| ESCs | embryonic stem cells |
| 3D | three-dimensional |
| NGS | Next Generation Sequencing |
| 16S rRNA | 16S ribosome gene |
Acknowledgement statement
We wish to extend our appreciation to EditSprings (https://www.editsprings.cn/) for providing the expert linguistic services. We appreciate Zeng Peng Ph.D. (Department of Pathology and Pathophysiology, School of Basic Medicine, Tongji Medical College of HUST) for the contribution to methodological suggestions of net-work analysis and guidance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e38633.
Contributor Information
Hang Zhou, Email: zh6463@cdutcm.edu.cn.
Qian Zeng, Email: 15715428@qq.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
figs4.
figs5.
References
- 1.Zhao Y., Zhao Y., Fan K., Jin L. Association of history of spontaneous or induced abortion with subsequent risk of gestational diabetes. JAMA Netw. Open. 2022;5(3) doi: 10.1001/jamanetworkopen.2022.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender Atik R., Christiansen O.B., Elson J., Kolte A.M., Lewis S., Middeldorp S., et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. 2018;(2) doi: 10.1093/hropen/hoy004. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimitriadis E., Menkhorst E., Saito S., Kutteh W.H., Brosens J.J. Recurrent pregnancy loss. Nat. Rev. Dis. Prim. 2020;6(1):98. doi: 10.1038/s41572-020-00228-z. [DOI] [PubMed] [Google Scholar]
- 4.Kaur M., Kaur R., Chhabra K., Khetarpal P. Maternal candidate gene variants, epigenetic factors, and susceptibility to idiopathic recurrent pregnancy loss: a systematic review. Int. J. Gynecol. Obstet. 2023;162(3):829–841. doi: 10.1002/ijgo.14701. [DOI] [PubMed] [Google Scholar]
- 5.Huisman P., Krogh J., Nielsen C.H., Nielsen H.S., Feldt-Rasmussen U., Bliddal S. Thyroglobulin antibodies in women with recurrent pregnancy loss: a systematic review and meta-analysis. Thyroid. 2023;33(11):1287–1301. doi: 10.1089/thy.2023.0292. [DOI] [PubMed] [Google Scholar]
- 6.Singh S., Meena A., Luqman S. Baicalin mediated regulation of key signaling pathways in cancer. Pharmacol. Res. 2021;164 doi: 10.1016/j.phrs.2020.105387. [DOI] [PubMed] [Google Scholar]
- 7.Zhou S.N., Deng R.X., Zhao S., Liu P. Determination of eight flavonoids in Scutellaria baicalensis Georgi by HPLC. Chem. Res. Appl. 2022;34(8):1920–1926. [Google Scholar]
- 8.Ren J.L., Zhang A.H., Kong L., Han Y., Yan G.L., Sun H., et al. Analytical strategies for the discovery and validation of quality-markers of traditional Chinese medicine. Phytomedicine. 2020;67 doi: 10.1016/j.phymed.2019.153165. [DOI] [PubMed] [Google Scholar]
- 9.Huang Q., Wang M., Wang M., Lu Y., Wang X., Chen X., et al. Scutellaria baicalensis: a promising natural source of antiviral compounds for the treatment of viral diseases. Chin. J. Nat. Med. 2023;21(8):563–575. doi: 10.1016/s1875-5364(23)60401-7. [DOI] [PubMed] [Google Scholar]
- 10.Qian J.X., Meng W.W., Zhao J.C., Wang Y.H., Jin Y., Zhan Z.L. Herbal textual research on Scutellariae radix in famous classical formulas. Chin. J. Exp. Tradit. Med. Formulae. 2023;29(5):84–93. doi: 10.13422/j.cnki.syfjx.20220458. [DOI] [Google Scholar]
- 11.Chen Z.Y., Zheng K., Han G.M., Zhuang L. Effect of vacuum steam moistening medicine method on quality of Scutellaria baicalensis slices from different regions. Acta Chin. Med. Pharmacol. 2022;50(2):44–49. doi: 10.19664/j.cnki.1002-2392.220034. [DOI] [Google Scholar]
- 12.Wang W.T., X. Cao Y., Hu S.Y., Cui L.J., Ren H.B., Yang L., et al. Comparative analysis of 5 flavonoids in Scutellaria baicalensis from different origin. J. Chin. Med. Mater. 2021;44(7):1691–1696. doi: 10.13863/j.issn1001-4454.2021.07.026. [DOI] [Google Scholar]
- 13.Wu X.M., Shao J., Sun H.J., Gan X., Yu M.J., Q. Ji Y. Analysis of the constituents of baicalensisin different producing areas based on UPLCLTQ-qrbitrap metabolomics. Asia Pac. Tradit. Med. 2021;17(8):45–49. [Google Scholar]
- 14.Yang Y., Wang Z.Q., Reng L.L., Xu Q.S., Cao L.J., Li T.X. Study on the change law of major pharmacological components in Scutellariae Radix membranaceus from different habitats, J. Tianjin Coll. Tradit. Chin. Med. 2020;39(3):324–329. [Google Scholar]
- 15.Huang Y.P., Wu D.Z., Wang S. Research progress on pharmacological action of Scutellariae radix and its drug pair. China Pharm. 2022;31(15):129–133. [Google Scholar]
- 16.Baradaran Rahimi V., Askari V.R., Hosseinzadeh H. Promising influences of Scutellaria baicalensis and its two active constituents, baicalin, and baicalein, against metabolic syndrome: a review. Phytother Res. 2021;35(7):3558–3574. doi: 10.1002/ptr.7046. [DOI] [PubMed] [Google Scholar]
- 17.Li W., Xu W. Research progress on pharmacological effects of baicalin, Res. Integr. Tradit. Chin. Med. West. Med. 2022;14(3):193–196. [Google Scholar]
- 18.Huang T., Liu Y., Zhang C. Pharmacokinetics and bioavailability enhancement of baicalin: a review. Eur. J. Drug Metab. Pharmacokinet. 2019;44(2):159–168. doi: 10.1007/s13318-018-0509-3. [DOI] [PubMed] [Google Scholar]
- 19.Zeng P., Yi Y., Su H.F., Ye C.Y., Sun Y.W., Zhou X.W., et al. Key phytochemicals and biological functions of Chuanxiong rhizoma against ischemic stroke: a network pharmacology and experimental assessment. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.758049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shu H., Ding F., Zhou J., Xue Y., Zhao D., Zeng J., et al. Boosting single-cell gene regulatory network reconstruction via bulk-cell transcriptomic data. Briefings Bioinf. 2022;23(5) doi: 10.1093/bib/bbac389. [DOI] [PubMed] [Google Scholar]
- 21.Haag S.M., Gulen M.F., Reymond L., Gibelin A., Abrami L., Decout A., et al. Targeting STING with covalent small-molecule inhibitors. Nature. 2018;559(7713):269–273. doi: 10.1038/s41586-018-0287-8. [DOI] [PubMed] [Google Scholar]
- 22.Liu X., Hong Z., Liu J., Lin Y., Rodríguez-Patón A., Zou Q., et al. Computational methods for identifying the critical nodes in biological networks. Briefings Bioinf. 2020;21(2):486–497. doi: 10.1093/bib/bbz011. [DOI] [PubMed] [Google Scholar]
- 23.Zeng P., Su H.F., Ye C.Y., Qiu S.W., Tian Q. Therapeutic mechanism and key alkaloids of Uncaria rhynchophylla in Alzheimer's disease from the perspective of pathophysiological processes. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.806984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng P., Shi Y., Wang X.M., Lin L., Du Y.J., Tang N., et al. Emodin rescued hyperhomocysteinemia-induced dementia and Alzheimer's disease-like features in rats. Int. J. Neuropsychopharmacol. 2019;22(1):57–70. doi: 10.1093/ijnp/pyy090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian Y.Q., Ren H.L., Liang A.J. Protective effects of baicalin on high glucose-induced trophoblast cell injury through modulation of miR-451. Chin. Tradit. Pat. Med. 2022;44(4):1314–1317. doi: 10.3321/j.issn:1673-8225.2007.19.039. [DOI] [Google Scholar]
- 26.Qian Q.R., Wang J.P., Zhang Y.R., Zhang Y.Y. Effects of baicalin on regulating immune status of uterine maternal and fetal interface in RSA mice based on PI3K/Akt signaling pathway. Chin. J. Inf. Tradit. Chin. Med. 2022;39(8):8–13. doi: 10.19656/j.cnki.1002-2406.20220802. [DOI] [Google Scholar]
- 27.Lai N., Fu X., Hei G., Song W., Wei R., Zhu X., et al. The role of dendritic cell subsets in recurrent spontaneous abortion and the regulatory effect of baicalin on it. J Immunol. Res. 2022;2022 doi: 10.1155/2022/9693064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J., Wang Y., Wu T., Li S., Sun Y.N., Liu Z.H. Baicalein suppresses high glucose-induced inflammation and apoptosis in trophoblasts by targeting the miRNA-17-5p-Mfn1/2-NF-κB pathway. Placenta. 2022;121:126–136. doi: 10.1016/j.placenta.2022.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Li H., Cong X., Sui J., Jiang Z., Fu K., Huan Y., et al. Baicalin enhances the thermotolerance of mouse blastocysts by activating the ERK1/2 signaling pathway and preventing mitochondrial dysfunction. Theriogenology. 2022;178:85–94. doi: 10.1016/j.theriogenology.2021.11.007. [DOI] [PubMed] [Google Scholar]
- 30.Lu X.H. Effects of baicalin on uterine macrophages in LPS-induced abortion animal model by regulating PI3K/AKT pathway and its mechanism. J. Southeast Univ. 2021;40(5):683–689. doi: 10.3969/j.issn.1671-6264.2021.05.020. [DOI] [Google Scholar]
- 31.Xu Q.L., Wang J.F., Xu H., Niu Y.X., Dai X.W. Autophagy of placental cells in the pathogenesis of preeclampsia rats and the interventional effect of baicalin. J. Wenzhou Med. Univ. 2021;51(9):755–758. doi: 10.3969/j.issn.2095-9400.2021.09.012. [DOI] [Google Scholar]
- 32.Wang F., Xu J., Gao Y.N., Cui L.N., Fu X.H. Mechanism of baicalin on proliferation and invasion of placental trophoblast in preeclampsia rats, J. Zhejiang Univ. Tradit. Chin. Med. 2021;45(12):1358–1364+1373. doi: 10.16466/j.issn1005-5509.2021.12.017. [DOI] [Google Scholar]
- 33.Zhao L., Xiong M., Liu Y. Baicalin enhances the proliferation and invasion of trophoblasts and suppresses vascular endothelial damage by modulating long non-coding RNA NEAT1/miRNA-205-5p in hypertensive disorder complicating pregnancy. J. Obstet. Gynaecol. Res. 2021;47(9):3060–3070. doi: 10.1111/jog.14789. [DOI] [PubMed] [Google Scholar]
- 34.Li W.M., Mao H. Effects of icariin and baicalin on NO levels in vitro fertilization embryo culture medium of pigs. Chin. J. Anim. Husb. Vet. Med. 01) 2020:45–46. CNKI:SUN:XMKX.0.2020-01-031. [Google Scholar]
- 35.Cui W., Cui H.M., Huo Y.L., Wang Y.J. Effects of baicalin on the activation of toll like receptor 4/NF-KB signaling pathway inMice with recurrent abortion. World Journal of Traditional Chinese Medicine. 2020;15(6):846–849. doi: 10.3969/j.issn.1673-7202.2020.06.007. [DOI] [Google Scholar]
- 36.Yang X.X., Ma Y.N., Ma X.J., Xu B. Effect of baicalin on fetal protection and regulation of uterine immune microenvironment in recurrent spontaneous abortion mice. J. Changchun Univ. Chin. Med. 2019;35(1):124–127. doi: 10.13463/j.cnki.cczyy.2019.01.037. [DOI] [Google Scholar]
- 37.Guo Q. YanBian University; 2019. Effects of Baicalin on in Vitro Maturation of Pig Oocytes and in Vitro Development of Pig Embryos. [Google Scholar]
- 38.Liang J.X. Jinan University; 2018. Baicalin Administration Attenuates Hyperglycemia-Induced Malformation of Cardiovascular System. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu J., Lv S.S., Fu Z.Y., Hou L.L. Baicalein enhances migration and invasion of extravillous trophoblasts via activation of the NF-κB pathway. Med. Sci. Monit. 2018;24:2983–2991. doi: 10.12659/msm.909722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou P.Q. Shanxi Medical University; 2018. Protective Effect of Baicalin on H/R Damage in JEG-3 Trophoblast Cells. [Google Scholar]
- 41.Ma Y.N., Yang X.X. Mechanism research of Baicalin regulating the local immunomodulatory mechanism of uterus by LPS induced abortion mice. J. Tianjin Univ. Tradit. Chin. Med. 2018;37(4):314–317. doi: 10.11656/j.issn.1673-9043.2018.04.13. [DOI] [Google Scholar]
- 42.Ma Y.N., Yang X.X. Effect of maternal fetal interface of Baicalin on immune abortion mice induced by LPS micro environment. J. Changchun Univ. Chin. Med. 2018;34(2):231–234. doi: 10.13463/j.cnki.cczyy.2018.02.009. [DOI] [Google Scholar]
- 43.Lai N.N. University; of Jinan: 2016. The Theraputic Effect and Mechanisms of Baicalin on URSA via Regulating DC Differentiation and Function in. [Google Scholar]
- 44.Guan C.K., Zhang J.L. Effect of baicalin on mouse embryo loss rate after Listeria monocytogenes infection. Sci. Technol. Innovation. 2016;30:35. CNKI:SUN:HLKX.0.2016-30-034. [Google Scholar]
- 45.Wang Y.H., Song J., Dong J.P., Yang T.T., Hao M. Effect of baicalin on mitochondrial apoptosis pathway of placental trophoblast in preeclampsia rat model. Chin. J. Prev. Med. 2016;19(12):933–939. doi: 10.3760/cma.j.issn.1007-9408.2016.12.010. [DOI] [Google Scholar]
- 46.Qi X., Li H., Cong X., Wang X., Jiang Z., Cao R., et al. Baicalin increases developmental competence of mouse embryos in vitro by inhibiting cellular apoptosis and modulating HSP70 and DNMT expression. J. Reprod. Dev. 2016;62(6):561–569. doi: 10.1262/jrd.2016-047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen J.G., Chen T., Ding Y., Han L., Zhou F.Y., Chen W.Z., et al. Baicalin can attenuate the inhibitory effects of mifepristone on Wnt pathway during peri-implantation period in mice. J. Steroid Biochem. Mol. Biol. 2015;149:11–16. doi: 10.1016/j.jsbmb.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y.M., Zhang Y.Y., Bulbul A., Shan X., Wang X.Q., Yan Q. Baicalin promotes embryo adhesion and implantation by upregulating fucosyltransferase IV (FUT4) via Wnt/beta-catenin signaling pathway. FEBS Lett. 2015;589(11):1225–1233. doi: 10.1016/j.febslet.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 49.Wang X., Zhao Y., Zhong X. Protective effects of baicalin on decidua cells of LPS-induced mice abortion. J Immunol. Res. 2014 doi: 10.1155/2014/859812. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu H.P., Wang R.G. The influence of baicalin and the water extract of Scutellaria on growth of CTB in vitro. Guidi. J. Tradit. Chin. Med. Pharm. 2013;19(6):14–16. doi: 10.13862/j.cnki.cn43-1446/r.2013.06.045. [DOI] [Google Scholar]
- 51.Zhang X.S. Hebei Agricultural University; 2010. Protective Effects of Baicalin on Mouse Uterus Inimplantation. [Google Scholar]
- 52.Ma A.T., Zhong X.H., Liu Z.M., Shi W.Y., Du J., Zhai X.H., et al. Protective effects of baicalin against bromocriptine induced abortion in mice. Am. J. Chin. Med. 2009;37(1):85–95. doi: 10.1142/s0192415x09006709. [DOI] [PubMed] [Google Scholar]
- 53.Qin M.C., Wang R.G., Li C.M., Liu X.L., Qin L.H., Liu H.P. Establishment of decidual cell apoptotic models induced by tumor necrosis factor alpha and effects of baicalin on decidual cell apoptosis. Chin. J. Tissue Eng. Res. 2007;19:3793–3796. doi: 10.3321/j.issn:1673-8225.2007.19.039. [DOI] [Google Scholar]
- 54.Jiang G.J., Che S.F., Zhou B.H., Liu Z.P., Zhong X.H. The anti-abortion effects of Scutellaria baicalensis Georgi and its components onRU486 inducing abortion and CD80+/CD86+ cells of spleen in mice. Acta Vet. Zootech. Sin. 2007;10:1131–1135. doi: 10.3321/j.issn:0366-6964.2007.10.022. [DOI] [Google Scholar]
- 55.Chen S.F. Hebei Agricultural University; 2006. Effects of Scutellaria baicalensis Georgi and Components on Uterus Tissue Th1/Th2 Immunity under Different Abortion Models. [Google Scholar]
- 56.Yang F., Zheng Q., Jin L. Dynamic function and composition changes of immune cells during normal and pathological pregnancy at the maternal-fetal interface. Front. Immunol. 2019;10:2317. doi: 10.3389/fimmu.2019.02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu S., Wei H., Li Y., Huang C., Lian R., Xu J., et al. Downregulation of ILT4(+) dendritic cells in recurrent miscarriage and recurrent implantation failure. Am. J. Reprod. Immunol. 2018;80(4) doi: 10.1111/aji.12998. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X., Wei H. Role of decidual natural killer cells in human pregnancy and related pregnancy complications. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.728291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bao W.J. Shandong University of Traditional Chinese Medicine; 2021. Study on the Expression of NK Cells and Related Factors in Patients with Kidney Deficiency and Blood Stasis Type of Recurrent Implantation Failure and Intervention of Bushen Huoxue Decoction. [Google Scholar]
- 60.Shah N.K., Xu P., Shan Y., Chen C., Xie M., Li Y., et al. MDSCs in pregnancy and pregnancy-related complications: an update. Biol. Reprod. 2023;108(3):382–392. doi: 10.1093/biolre/ioac213. [DOI] [PubMed] [Google Scholar]
- 61.Song L., Zhu S., Liu C., Zhang Q., Liang X. Baicalin triggers apoptosis, inhibits migration, and enhances anti-tumor immunity in colorectal cancer via TLR4/NF-κB signaling pathway. J. Food Biochem. 2022;46(3) doi: 10.1111/jfbc.13703. [DOI] [PubMed] [Google Scholar]
- 62.Elsalam S.A., Mansor A.E., Sarhan M.H., Shalaby A.M., Gobran M.A., Alabiad M.A. Evaluation of apoptosis, proliferation, and adhesion molecule expression in trophoblastic tissue of women with recurrent spontaneous abortion and infected with Toxoplasma gondii. Int. J. Gynecol. Pathol. 2021;40(2):124–133. doi: 10.1097/pgp.0000000000000683. [DOI] [PubMed] [Google Scholar]
- 63.Su Y., Xu J., Gao R., Liu X., Liu T., Li C., et al. The Circ-CYP24A1-miR-224-PRLR Axis impairs cell proliferation and apoptosis in recurrent miscarriage. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.778116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Deftereou T.E., Tsolou A., Liberis A., Georgiadi K., Alexiadis T., Pagonopoulou O., et al. Differential apoptotic activity in trophoblast of spontaneous abortions and normal pregnancies. Folia Histochem. Cytobiol. 2022;60(1):24–30. doi: 10.5603/FHC.a2022.0003. [DOI] [PubMed] [Google Scholar]
- 65.Wang S., Zhu X., Xu Y., Zhang D., Li Y., Tao Y., et al. Programmed cell death-1 (PD-1) and T-cell immunoglobulin mucin-3 (Tim-3) regulate CD4+ T cells to induce Type 2 helper T cell (Th2) bias at the maternal-fetal interface. Hum. Reprod. 2016;31(4):700–711. doi: 10.1093/humrep/dew019. [DOI] [PubMed] [Google Scholar]
- 66.Qin X.Y., Shen H.H., Zhou W.J., Mei J., Lu H., Tan X.F., et al. Insight of autophagy in spontaneous miscarriage. Int. J. Biol. Sci. 2022;18(3):1150–1170. doi: 10.7150/ijbs.68335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fu Y., Shen J., Li Y., Liu F., Ning B., Zheng Y., et al. Inhibition of the PERK/TXNIP/NLRP3 Axis by baicalin reduces NLRP3 inflammasome-mediated pyroptosis in macrophages infected with Mycobacterium tuberculosis. Mediat. Inflamm. 2021 doi: 10.1155/2021/1805147. 2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robertson S.A., Moldenhauer L.M., Green E.S., Care A.S., Hull M.L. Immune determinants of endometrial receptivity: a biological perspective. Fertil. Steril. 2022;117(6):1107–1120. doi: 10.1016/j.fertnstert.2022.04.023. [DOI] [PubMed] [Google Scholar]
- 69.Fedorka C.E., Scoggin K.E., El-Sheikh Ali H., Loux S.C., Dini P., Troedsson M.H.T., et al. Interleukin-6 pathobiology in equine placental infection. Am. J. Reprod. Immunol. 2021;85(5) doi: 10.1111/aji.13363. [DOI] [PubMed] [Google Scholar]
- 70.Löb S., Ochmann B., Ma Z., Vilsmaier T., Kuhn C., Schmoeckel E., et al. The role of Interleukin-18 in recurrent early pregnancy loss. J. Reprod. Immunol. 2021;148 doi: 10.1016/j.jri.2021.103432. [DOI] [PubMed] [Google Scholar]
- 71.Lu J., Wang Z., Cao J., Chen Y., Dong Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2018;16(1):80. doi: 10.1186/s12958-018-0391-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jayasena C.N., Radia U.K., Figueiredo M., Revill L.F., Dimakopoulou A., Osagie M., et al. Reduced testicular steroidogenesis and increased semen oxidative stress in male partners as novel markers of recurrent miscarriage. Clin. Chem. 2019;65(1):161–169. doi: 10.1373/clinchem.2018.289348. [DOI] [PubMed] [Google Scholar]
- 73.Davies R., Jayasena C.N., Rai R., Minhas S. The role of seminal oxidative stress in recurrent pregnancy loss. Antioxidants. 2023;12(3) doi: 10.3390/antiox12030723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Luan X., Yan Y., Zheng Q., Wang M., Chen W., Yu J., et al. Excessive reactive oxygen species induce apoptosis via the APPL1-Nrf2/HO-1 antioxidant signalling pathway in trophoblasts with missed abortion. Life Sci. 2020;254 doi: 10.1016/j.lfs.2020.117781. [DOI] [PubMed] [Google Scholar]
- 75.Wu C.H., Zhang W.J., Tian J., Diao X.C., Zhen X.H. Interventional effect of baicalin on infectious preterm model rats and impact onAMPK/NF-kB pathways. Chin. J. Nosocomiol. 2023;33(22):3476–3480. [Google Scholar]
- 76.Li M., Haixia Y., Kang M., An P., Wu X., Dang H., et al. The arachidonic acid metabolism mechanism based on UPLC-MS/MS metabolomics in recurrent spontaneous abortion rats. Front. Endocrinol. 2021;12 doi: 10.3389/fendo.2021.652807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jing G., Yao J., Dang Y., Liang W., Xie L., Chen J., et al. The role of β-HCG and VEGF-MEK/ERK signaling pathway in villi angiogenesis in patients with missed abortion. Placenta. 2021;103:16–23. doi: 10.1016/j.placenta.2020.10.005. [DOI] [PubMed] [Google Scholar]
- 78.Zhi Z., Yang W., Liu L., Jiang X., Pang L. Early missed abortion is associated with villous angiogenesis via the HIF-1α/VEGF signaling pathway. Arch. Gynecol. Obstet. 2018;298(3):537–543. doi: 10.1007/s00404-018-4802-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu Z., Geng Y., Huang Y., Hu R., Li F., Ding J., et al. Bushen Antai recipe alleviates embryo absorption by enhancing immune tolerance and angiogenesis at the maternal-fetal interface via mobilizing MDSCs in abortion-prone mice. Phytomedicine. 2024;123 doi: 10.1016/j.phymed.2023.155164. [DOI] [PubMed] [Google Scholar]
- 80.Depoix C.L., Colson A., Hubinont C., Debieve F. Impaired vascular endothelial growth factor expression and secretion during in vitro differentiation of human primary term cytotrophoblasts. Angiogenesis. 2020;23(2):221–230. doi: 10.1007/s10456-019-09702-z. [DOI] [PubMed] [Google Scholar]
- 81.Chunsheng Z., Jinni L., Jia L. Safety evaluation of baicalin micropowder additive. Feed Res. 2020;43(10):113–116. doi: 10.13557/j.cnki.issn1002-2813.2020.10.028. [DOI] [Google Scholar]
- 82.Expert C.C.B.L.D. Consensus of use of Baicalin for treatment of liver diseases. Chin. J. Liver Dis. 2021;13(4):5–8. [Google Scholar]
- 83.Li L., Gao H., Lou K., Luo H., Hao S., Yuan J., et al. Safety, tolerability, and pharmacokinetics of oral baicalein tablets in healthy Chinese subjects: a single-center, randomized, double-blind, placebo-controlled multiple-ascending-dose study. Clinical and Translational Sciencei. 2021;14(5):2017–2024. doi: 10.1111/cts.13063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang M., Dong Y., Wu J., Li H., Zhang Y., Fan S., et al. Baicalein ameliorates ionizing radiation-induced injuries by rebalancing gut microbiota and inhibiting apoptosis. Life Sci. 2020;261 doi: 10.1016/j.lfs.2020.118463. [DOI] [PubMed] [Google Scholar]
- 85.Liao H., Ye J., Gao L., Liu Y. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: a comprehensive review. Biomed. Pharmacother. 2021;133 doi: 10.1016/j.biopha.2020.110917. [DOI] [PubMed] [Google Scholar]
- 86.Yang S., Liang J.Q., Chen H., X. Huang J., Yuan C.Y., Liu B.H., et al. Exploratory study of Baicalein capsule combined with entecavir to reduce pregenomic RNA level of hepatitis B virus in patients with chronic hepatitis B. Chin. J. Liver Dis. 2021;13(3):42–47. doi: 10.3969/j.issn.1674-7380.2021.03.007. [DOI] [Google Scholar]
- 87.Deng J., Wang D.X., Liang A.L., Tang J., Xiang D.K., He Z.L. Treatment effect of baicalin capsule in patients with hospital-acquired pneumonia. Proc. Clin. Med. 2017;26(6):423–427. doi: 10.16047/j.cnki.cn14-1300/r.2017.06.008. [DOI] [Google Scholar]
- 88.Xu W., Niu Y., Ai X., Xia C., Geng P., Zhu H., et al. Liver-targeted nanoparticles facilitate the bioavailability and anti-HBV efficacy of baicalin in vitro and in vivo. Biomedicines. 2022;10(4) doi: 10.3390/biomedicines10040900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deng L.L., Song D.R., Guo J.M., Wang Y.F. Study on ingredients of Scutellaria Radix extract penetrable through placental barrier ofpregnant rat. China J. Chin. Mater. Med. 2012;37(3):327–330. CNKI:SUN:ZGZY.0.2012-03-018. [PubMed] [Google Scholar]
- 90.Lu D., Zhang W., Guo J., Wang Y.N., Du W.X., Song D.R. Study on ingredients of Scutellaria baicalensis through human placental barrier. The Chinese Journal of Clinical Pharmacology. 2021;37(6):676–680. doi: 10.13699/j.cnki.1001-6821.2021.06.008. [DOI] [Google Scholar]
- 91.Wang J. Huazhong University of Science and Technology; 2013. Baicalin Suppresses the Proliferation of ESCs by Regulating the Expression of Proto-Oncogenesvia miR-294. [Google Scholar]
- 92.Wang J., Masika J., Zhou J., Wang J., Zhu M., Luo H., et al. Traditional Chinese medicine baicalin suppresses mESCs proliferation through inhibition of miR-294 expression. Cell. Physiol. Biochem. 2015;35(5):1868–1876. doi: 10.1159/000373997. [DOI] [PubMed] [Google Scholar]
- 93.Zhang W., Song D.R., Wang Y.N., Zhu Z. Evaluation of embryotoxicity of baicalin based on embryonic stem cell test system. Chin. J. Pharmacol. Toxicol. 2012;26(6):864–869. doi: 10.3867/j.issn.1000-3002.2012.06.015. [DOI] [Google Scholar]
- 94.Li M.X. Clinical study of 39 cases of severe preeclampsia treated by Baicalin capsule combined with Labetalol hydrochloride injection. J. North Pharm. 2019;16(4):143–144. CNKI:SUN:BFYX.0.2019-04-111. [Google Scholar]
- 95.Xin L., Gao J., Lin H., Qu Y., Shang C., Wang Y., et al. Regulatory mechanisms of baicalin in cardiovascular diseases: a review. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.583200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Han X. Nanjing University Of Chinese Medicine; 2020. Antibacterial Research on Baicalin-loaded Nano-Polydopamine Combined with Photothermal Therapy. [Google Scholar]
- 97.Ibrahim A., Abdel Gaber S.A., Fawzi Kabil M., Ahmed-Farid O.A.H., Hirsch A.K.H., El-Sherbiny I.M., et al. Baicalin lipid nanocapsules for treatment of glioma: characterization, mechanistic cytotoxicity, and pharmacokinetic evaluation. Expet Opin. Drug Deliv. 2022;19(11):1549–1560. doi: 10.1080/17425247.2022.2139370. [DOI] [PubMed] [Google Scholar]
- 98.Qi D., Jia B., Peng H., He J., Pi J., Guo P., et al. Baicalin/ambroxol hydrochloride combined dry powder inhalation formulation targeting lung delivery for treatment of idiopathic pulmonary fibrosis: fabrication, characterization, pharmacokinetics, and pharmacodynamics. Eur. J. Pharm. Biopharm. 2023;188:243–253. doi: 10.1016/j.ejpb.2023.05.017. [DOI] [PubMed] [Google Scholar]
- 99.Mi X., Hu M., Dong M., Yang Z., Zhan X., Chang X., et al. Folic acid decorated zeolitic imidazolate framework (ZIF-8) loaded with baicalin as a nano-drug delivery system for breast cancer therapy. Int. J. Nanomed. 2021;16:8337–8352. doi: 10.2147/ijn.S340764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gu Y., Zheng Q., Fan G., Liu R. Advances in anti-cancer activities of flavonoids in Scutellariae radix: perspectives on mechanism. Int. J. Mol. Sci. 2022;23(19) doi: 10.3390/ijms231911042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bock C., Boutros M., Camp J.G., Clarke L., Clevers H., Knoblich J.A., et al. The organoid cell atlas. Nat. Biotechnol. 2021;39(1):13–17. doi: 10.1038/s41587-020-00762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abbas Y., Turco M.Y., Burton G.J., Moffett A. Investigation of human trophoblast invasion in vitro. Hum. Reprod. Update. 2020;26(4):501–513. doi: 10.1093/humupd/dmaa017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bai S., Huang B., Fu S., Zhu M., Hu L., Zhu L., et al. Changes in the distribution of intrauterine microbiota may attribute to immune imbalance in the CBA/J×DBA/2 abortion-prone mice model. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.641281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shen J., Cheng J., Zhu S., Zhao J., Ye Q., Xu Y., et al. Regulating effect of baicalin on IKK/IKB/NF-kB signaling pathway and apoptosis-related proteins in rats with ulcerative colitis. Int. Immunopharm. 2019;73:193–200. doi: 10.1016/j.intimp.2019.04.052. [DOI] [PubMed] [Google Scholar]
- 105.Zhou H., Yang Y., Deng L., Yao Y., Liao X. A potential mechanism of kidney-tonifying herbs treating unexplained recurrent spontaneous abortion: clinical evidence from the homogeneity of embryo implantation and tumor invasion. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.775245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Christgen S., Tweedell R.E., Kanneganti T.D. Programming inflammatory cell death for therapy. Pharmacol. Ther. 2022;232 doi: 10.1016/j.pharmthera.2021.108010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Onoda N., Kawabata A., Hasegawa K., Sakakura M., Urakawa I., Seki M., et al. Spatial and single-cell transcriptome analysis reveals changes in gene expression in response to drug perturbation in rat kidney. DNA Res. 2022;29(2) doi: 10.1093/dnares/dsac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article. No data associated in this article has been deposited into a publicly available repository.