Abstract
Prunus species play an important role in preserving the Nepal's unique identity; such as three endemic species of Prunus, Prunus topkegolensis, Prunus jajarkotensis, and Prunus taplejungnica, represent the country's local area. An ecological and social survey of P. topkegolensis and P. taplejungnica was conducted. To analyze these species' regeneration status and population structures, the density of seedlings, saplings, and adult trees were compared, and created a diameter size class diagram. The Wilcoxon signed-rank test was used to compare the average values of seedlings, saplings, and adult trees between two species. To determine the ethnobotanical importance of a species, the relative frequency of citation (RFC) was calculated for various use categories. We classified both species as trees as the average diameter at breast height (DBH) of P. topkegolensis was 19.55 ± 14.66 cm with an average height 5.05 ± 2.28 m, while that of P. taplejungnica was 14.5 ± 7.93 cm with an average height of 4.89 ± 1.66 m. P. topkegolensis had an average 32 ± 31 adult trees, 16 ± 15 saplings, and 21 ± 18 seedlings per 0.1 ha, while P. taplejungnica had 12 ± 5 adult trees, 2 saplings, and 6 ± 4 seedlings per 0.1 ha. The number of seedlings and saplings at the plot level differed significantly between species (W = 75.5, p- = 0.05, and W = 84, p = 0.01, respectively). Both had humped-shaped diameter at breast height size class distribution. However, P. taplejungnica had a more apparent hump-shaped diameter at breast height distribution than P. topkegolensis, indicating that P. taplejungnica has more unsustainable regeneration issues. P. taplejungnica was locally recognized as a separate species from P. rufa, whereas P. topkegolensis was not. P. topkegolensis have seven different use categories, with wood and fuel wood being the most frequently cited, with RFC value 1. Rapid vulnerability assessment (RVA) showed both species are having high conservation threats. In situ conservation measures are suggested to address the issue of poor regeneration and disturbances.
Keywords: Conservation, Ecology, Endemic, Ethnobotany, Population, Prunus topkegolensis, Prunus taplejungnica, Regeneration, Threats
1. Introduction
Himalayas are recognized globally as a biodiversity hotspot [1]. However, is rapidly deteriorating, increasing the likelihood of extinction of the endemic species [2]. Nepal is important part of Himalaya hosting over 6000 flowering plant species, including more than 300 endemic to the region [3]. Endemic species like Prunus jajarkotensis H. Hara, Prunus taplejungnica H. Ohba & S. Akiyama, and Prunus topkegolensis H. Ohba & S. Akiyama play a significant role in defining local biodiversity and cultural identity. However, such species sustainability is compromised due to anthropogenic and natural factor [4].
Endangered and endemic species are facing conservation threats throughout the world needing immediate action for conservation [5]. These species have ecological and cultural importance, however, such species in Nepal face severe threats from anthropogenic activities, particularly deforestation [6]. This threat jeopardizes the habitats and survival of many endemic plants, which often have restricted distributions and specialized ecological niches [7].
Understanding the regeneration dynamics of these endemic species is crucial for effective conservation strategies. Studies indicate that the availability of regeneration niches from seedling to sapling and adult stages is critical for sustaining populations [8]. The diameter at breast height (DBH) size class diagram is used to assess regeneration sustainability, where a reverse J-shaped curve signifies sustainable regeneration, while a hump-shaped curve indicates unsustainable regeneration [9].
The Nepal Himalayas are renowned for their rich floral diversity, including numerous endemic plant species essential for local livelihoods and biodiversity conservation. Among the endemic species distributed in Nepal, P. topkegolansis and P. taplejungnica are the poorly studied Prunus species that are crucial for understanding local biodiversity and cultural heritage [10].
Ethnobotanical studies highlight the importance of endemic plants like Prunus species in various aspects of local life, including animal husbandry, food, medicine, construction, and energy use [11]. Understanding these traditional uses is essential for integrating conservation efforts with local community practices.
Despite their ecological and cultural significance, endemic species face numerous threats, including habitat loss, climate change, and unsustainable harvesting practices [12]. These factors necessitate urgent conservation actions to ensure their survival. Therefore, this study has assessed the ecological status of P. topkegolansis and P. taplejungnica, their uses and conservation in their presence range Topke Gola and Olangchung Gola Taplejung Nepal. Studies on these endemic species provide conservation managers valuable information and help to protect their resources [13].
2. Materials and methods
2.1. Study area
This study was conducted in Mikwakhola rural municipality ward number five (Topke Gola) and Phaktanglung rural municipality ward number seven (Olangchung Gola) inTaplejung district, province one of Nepal, at elevations ranging from 3200 m to 3800 m (Fig. 1). P. topkegolensis and P. taplejungnica are recorded near streams, primarily in moist areas. Rhododendron spp., Betula utilis, Prunus rufa, and Pinus wallichiana are the dominating species of the region. P. topkegolansis and P. taplejungnica are endemic to Nepal distributed in Topke and Olangchung Gola respectively. The names P. topkegolansis represent Topke Gola and P. taplejungnica represent Taplejung district. Topke Gola had 28 households, and Olangchung Gola had 65 households. The Topke Gola community forest user group managed the forest of Topke Gola, and the Tiptala community group managed the forest of Olangchung Gola (Table 1). Households in Topke Gola are seasonal, whereas households in Olangchung Gola are year-round. Animal husbandry is the major occupation in these villages. Bhote people inhabit these areas. Most of the people follow Buddhism. Topke Gola and Olangchung Gola have a distinct culture and environment. Therefore, each year, approximately 1000 internal tourists visit these villages.
Fig. 1.
Map of Study Area showing Topke Gola and Olangchung Gola.
Table 1.
General characteristics of studied sites (Field Survey, 2020/2022).
| Attributes | Topke Gola | Olangchung Gola |
|---|---|---|
| Local Authority | Mikwakhola rural municipality 5 | Phaktanglung rural municipality 7 |
| Geographic coordinates | 27° 37.845′ N and 87° 35.144′ E | 27° 46.0244′ N and 87° 48.9403′ E |
| Elevation range | 3300–3800m | 3300–3500m |
| Climatic Regime | Subalpine | Subalpine |
| Mean annual precipitation (mm) | 1920 | 1920 |
| Number of studied plot | 10 | 10 |
| Number of studied Household | 15 | 15 |
| Major tree species in Forest | Rhododendron spp., Betulautilis D.Don, P. rufa Wall. ex Hook.f. and Pinus wallichiana A.B.Jacks. | Rhododendron spp., Betula utilis D.Don and P. rufa Wall. ex Hook.f. |
| Characteristics of Habit | Moist near the stream | Moist near the stream |
2.2. Data collection
We visited Topke Gola and Olangnchung Gola in Nepal in October 2020/September 2021/August 2022 for vegetation and social survey. The ecological survey of P. taplejungnica was done in October 2020, and P. topkegolansis was done in September 2021 because the presence points of these species are far from each other.
2.2.1. Sampling methods for ecological data
The species were confirmed based on research on endemic Prunus species in the eastern Himalayas and Nepal flora [[10], [14]]. In Olangchung Gola, we walked from 2400 m to 3500 m (11 km); in Topke Gola, walked up to 3800 m (16 km). For vegetation surveys, a systematic sampling approach based on the presence point of the species was employed; a transect of 200 m was laid whenever possible and 100 m otherwise [8]. The width of the transect was maintained 10 m. A total of 4 line transects, three 200 m and one 100m were taken in Topkegola with gap 50–250 m between the transects. However, in Olangchung Gola 3 line transects 1 (200m) and 2 (100m) were taken with gap 20–1000 m. The difference in transect and gap taken is different as P. taplejungnica is distributed in a narrower range than P. topkegolansis as well as transect are chosen purposefully based on the presence of the species. In each transect 1–5 (10 m × 10m) plots were made based on the presence of the species. Hence, measurements were taken in 20, 10 m × 10 m rectangular plots (10 plots at each site). In each plot, seedlings (clearly visible and identifiable < 1m height), saplings (>1.3 m height and with 1–10 cm DBH), and adult trees (≥1.3 m height, 10 cm and > DBH) were counted, and DBH, the height of the Prunus species studied, crown cover by adult individual trees, slope, altitude, vegetation, and habitat character were recorded. For observation and measurements of leaf size of both species, collection of one-size-fits-all twigs from four different individuals for each species was done. Five leaves were randomly selected from each twig (a total of 20 leaves for each species) to count leaf veins and measure the length and breadth of blades and the length of the petiole [15]. A ruler was used to measure the size of 15 dry fruits and the peduncle of P. topkegolensis. In the field, two species' crown cover, leaf margin, leaf base, leaf shape, stem color, flower color, and fruit color were recorded, and referred to previous literature for the pedicel length [[10], [14]].
2.2.2. Sampling methods for ethnobotanical data
A total of 30 local respondents (15 at each site) were interviewed, to collect ethnobotanical data. Prior to the interview, verbal consent was taken from each respondent. This study followed the code of conduct established by the International Society of Ethnobiology [16] during the social data collecting process. The age of the respondents was between 20 and 80 years, in which 16 were men and 14 were women.
Each respondents were asked to provide demographic information (name, age, sex, and occupation), the species' vernacular name, and various uses. The recorded uses were divided into seven categories: wood, fuel wood, Havan (burned wood during religious ceremonies), Dhup (Inscent), Lapsing (stand used for keeping books by Buddhists), and Ghum (traditional umbrella).
2.3. Data analysis
2.3.1. Morphological character, regeneration, and population structure of the trees
The morphological characteristics of the field-collected species, such as DBH, height, leaf size, and fruit size were tabulated. The average value, standard deviation, minimum and maximum value, and skewness for seedling, sapling, adult tree data, DBH, height, leaf size, and fruit size were calculated using Microsoft Excel 2013.
The population density (per hectare) and frequency (%) of seedlings, saplings, and adult trees were calculated using the method of Kent and Coker [17] and presented them in bar graphs.
We used bar graphs to show the regeneration status of trees by displaying the number of seedlings, saplings, and adult trees per 0.1 hectare. The regeneration status of trees was determined as follows: “good,” if the number of seedlings > saplings > adult trees; “fair,” if the number of seedlings > saplings < adult trees; “poor,” if the number of adult trees > saplings > seedlings; “none,” if a species is absent in sapling and seedling stages; “mature and new,” if a species has only sapling or seedling stages [18]. To demonstrate population structure, we created a DBH size class diagram of trees for each species, in which the DBH of sapling and adult trees was grouped with a 10 cm gap, height was grouped with a 2 m gap, and displayed in a bar graph using Microsoft Excel 2013. We checked the distributions of the data of seedlings, saplings and adult trees using Shapiro test. As the data were count data, we used the Wilcoxon signed-rank test to compare the numbers of seedlings, saplings, and adult trees within and between species using R-program [19].
2.3.2. Ethnobotanical use
The relative frequency of citation (RFC) was calculated according to Tardio and Pardo-de-Santayana [20] for each use category: wood, fuel wood, Havan (holy fireworks), Dhup (Inscent), Lapsing (book stand), and Ghum (traditional umbrella).
where,
FC = Number of respondents who mentioned the specific use of species.
N = Total number of respondents who participated in a survey.
The value of RFC remains between 0 and 1, with 0 indicating that none of the respondents use the species in mentioned used catagories and 1 indicating that all the respondents use the species in mentioned used cataogories. The RFC value near 1 indicates that the more people uses the species in mentioned categories, while the RFC value near 0 indicates that the fewer people uses the species in the specific use categories.
2.3.3. Conservation
This study used rapid vulnerability assessment (RVA) proposed by Cunningham [21] similar to Bhattarai et al. [22], Pyakurel et al. [23] and Wagner et al. [24] for assessing conservation and threats on P. taplejungnica and P. topkegolansis. RVA has 15 attributes and uses a 1–4 score level to indicate vulnerability levels, ranging from minimum to maximum (Table 2).
Table 2.
Attributes and scoring for rapid vulnerability assessment of P. taplejungnica and P. topkegolansis.
| Attributes | Score |
Hypothesis | |||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| Life form | Annual or biennial herb | Perennial herb, perennial climbing herb | Shrub, woody climber | Tree | Trees are more vulnerable than annual herbs |
| Geographical distribution | Pluri-regional | Pan-Himalaya and two other phytogeographical domains within Asia | Pan-Himalaya | Endemic | Endemic species are vulnerable |
| Elevational range (m) | >2400 | >1600 to 2400 | >800 to 1600 | <800 | Narrow elevation span species are more vulnerable |
| Resource origin | Cultivation | Wild and commercial cultivation | Wild and trial cultivation | Wild | Wild species are more vulnerable |
| Habitat specificity | Habitat generalist 2 (occurring in more than two habitats, including farmlands and wastelands) | Habitat generalist 1 (occurring in more than 2 habitats excluding farmlands and wastelands) | Habitat specialist 2 (occurring in more than one specialized habitat) | Habitat specialist 1 (occurring in a single, very specific habitat) | Plant growing in specific habitat possess more vulnerability |
| Habitat destruction | None | Low | Medium | High | High habitat destruction cause more vulnerability |
| Local population size | Mostly large | Mostly large, somewhere small | Mostly small, somewhere large | Everywhere small | Species with small population are more vulnerable |
| Frequency | Very frequent | Frequent | Moderate | Rare | Rare species are more vulnerable |
| Abundance | Abundant | Profusely distributed | Common | Sparse | Sparsely abundant species are more vulnerable |
| Regeneration status | High | Medium | Low | None | Species with low regeneration are more vulnerable |
| Harvest phase | After fruit ripe | At fruiting time | At flowering | Before flowering and fruiting | Species harvested before flowering and fruiting are more vulnerable |
| Parts used | Leaf | Fruits, seeds, flowers, resin | Bark, stem, wood | Root, rhizome, tuber, whole plant | Root and whole plant harvesting plants are more vulnurable |
| Commercial demand | None | Low | Medium | High | Commercially high demand species are more vulnerable |
| Availability of substitutes | Available and affordable | Available but unaffordable | Seldom available | Not available | Species without alternative substitutes are more vulnerable |
| Traditional conservation practices | Strong | Medium | Low | None | Species with no traditional conservation practices are more vulnerable |
3. Results
3.1. Habitat and ecology
The average DBH of P. topkegolensis was 19.55 ± 14.66 cm, with a maximum DBH of 66 cm and an average height 5.05 ± 2.28 m, reaching up to 12 m. In contrast, P. taplejungnica had an average DBH of 14.5 ± 7.93 cm, with a maximum DBH of 26 cm and an average height 4.89 ± 1.66 m, reaching up to 8 m. Both species had elliptic double-serrated leaf margins, however, P. topkegolansis has a sharper leaf margin and ovate leaf base, making it clearly distinguishable from P. taplejungnica. Additionally, compared to P. taplejungnica, P. topkegolansis has a larger lamina with average length of 6.02 ± 1.601 cm and breadth of 2.62 ± 0.64 cm, a longer petiole with a length average of 0.93 ± 0.27, and more number of veins (7-13) (Table 3).
Table 3.
Morphological features of Prunus topkegolensis and Prunus taplejungnica (Source: [10]; and field survey, 2020/2022).
| Morphological features | P. topkegolensis | P. taplejungnica |
|---|---|---|
| Average DBH ± S.D (max.), skewness in cm | 19.55 ± 14.66 (66), 1.02 | 15.22 ± 6.78 (26), −0.62 |
| Average height ± S.D (max) in m | 5.05 ± 2.28 (12), 0.62 | 4.93 ± 1.562 (8), −0.9 |
| Maximum Crown observed (m2) | 36 | 30 |
| Leaf margin | Sharp double serrated | Double serrated |
| Leaf base | Ovate | Rounded to cuneate |
| Leaf shape | Elliptic | Elliptic |
| Stem color | Redish brown | Redish brown |
| Average fruit diameter ± S.D (min-max), skewness in cm | 0.59 ± 0.103 (0.4–0.8), 0.15 | Unknown |
| Average length of Peduncle ± S.D (min-max), skewness in cm | 4.04 ± 0.582 (3–5), −0.24 | Unknown |
| Flower color | White fate pink | White |
| Fruit color | Red when mature | Not recorded |
| Average length of lamina ±S.D (min-max), skewnessin cm | 6.02 ± 1.601 (3.4–9.1), 0.21 | 4.77 ± 1.6 (2.1–7.9), 0.05 |
| Average breadth of lamina ±S.D (min-max), skewness in cm | 2.62 ± 0.64 (1.7–3.9), 0.46 | 2.31 ± 0.51 (1.5–3.2), −0.26 |
| Length of the petiole ± S.D (min-max), skewness in cm | 0.93 ± 0.27 (0.5–1.4), −0.27 | 0.89 ± 0.24 (0.6–1.3), 0.17 |
| Length of the pedicel in cm | 1.3 | 0.5–2.8 |
| Average number of veins (min-max) | 11 (7–13) | 9 (6–13) |
Both species were recorded near streams. P. topkegolensis was recorded 3300–3800 m along the Mikwakhola, while P. taplejungnica was recorded 3300–3500 m along the Tamor River. The neighboring tree species for P. topkegolensis were Rhododendron spp., Pinus wallichiana, and P. rufa (Table 4).
Table 4.
General characteristics of the species' habitat.
| Species | Slope (degree) | Habitat | Neighboring species | Observed Elevation range | Distance to village from nearest species presence point (Approx) |
|---|---|---|---|---|---|
| P. topkegolensis | 15–35 | Near the stream |
Rhododendron spp., Betula utilis D.Don, P. rufa Wall. ex Hook.f. and Pinuswallichiana A.B.Jacks. |
3300–3800m | 300m |
| P. taplejungnica | 15–45 | Near the stream | Rhododendron spp., B. utilis D.Don and P. rufa Wall. ex Hook.f. | 3300m–3500m | 600m |
3.2. Regeneration status
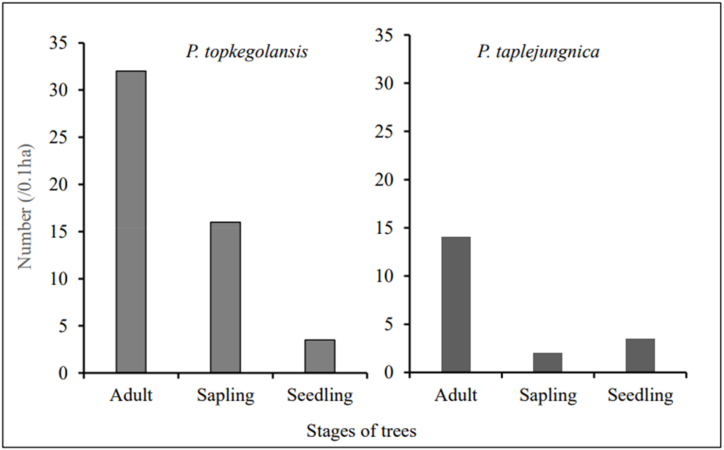
In the Topke Gola community forest, we counted 21 seedlings, 16 saplings, and 32 adult trees of P. topkegolensis in 10 plots of 10 × 10 m each. Similarly, in Tiptala community forest, Olangchung Gola, we counted six seedlings, two saplings, and 14 adult trees of P. taplejungnica in 10 plots of 10 × 10 m each. The plot level data of seedlings, saplings and adult trees of P. topkegolansis were normally distributed as the p-value obtained from the Shapiro test was greater than (>) 0.05. However, the plot level data of seedlings, saplings and adult trees of P. taplejugnica were not normally distributed as the p-value obtained from the Shapiro test was smaller than (<) 0.05. For P. topkegolensis, we recorded 32 ± 31 adult trees with a frequency of 70 %, 16 ± 15 saplings with a frequency of 80 %, and 21 ± 18 seedlings with a frequency of 80 % in total 0.1 ha area. The skewness of seedlings of P. topkegolansis was less followed by 0.39 followed by 0.59 adult trees, and 1.35 saplings. Similarly, we recorded 12 ± 5 adult trees with a frequency of 100 %, two saplings with a frequency of 20 %, and 6 ± 4 seedlings with a frequency of 50 % of P. taplejungnica in total 0.1 ha area (Fig. 2). The skewness of adult trees of P. taplejungnica was less 0.48 followed by seedling 2.23 and not applicable for sapling as they were measured only in two plots. The number of seedlings and saplings at the plot level varied significantly between species (W = 75.5, p = 0.05, and W = 84, p = 0.01, respectively). However, the adult tree numbers did not differ significantly (W = 61, p = 0.41) between species. For both species, seedling and sapling numbers were lower than adult tree numbers, indicating poor regeneration.
Fig. 2.
Regeneration status of P. topkegolensis and P. taplejungnica.
3.3. Population structure
The DBH size class diagram of trees revealed distinct characteristics in P. topkegolensis and P. taplejungnica. P. topkegolensis was divided into seven DBH size classes based on the measured DBH, while P. taplejungnica was divided into three DBH size classes. P. topkegolensis, more trees were found in DBH size classes 0–10 cm (n = 16) and 20–30 cm (n = 16), followed by 10–20 cm (n = 7), 30–40 cm (n = 3), 40–50 cm (n = 4), 50–60 cm (n = 1), and 60–70 cm (n = 1). Fewer trees were observed in size classes 10–20 cm than in 0–10 cm and 20–30 cm DBH, resulting in a hump-type distribution in the DBH size class diagram. Similarly, in P. taplejungnica, more trees were observed in the DBH size class 10–20 cm (n = 9) was followed by 20–30 cm (n = 5) and 0–10 cm (n = 2), indicating a hump-type distribution (Fig. 3).
Fig. 4.
Height class diagram of the P. topkegolansis and P. taplejungnica dashes in the figure represent average height.
P. topkegolansis has more height classes than P. taplejungnica. For both of the species more height of the tree was between 4 and 6m range. However, the average height of the P. topkegolansis was more 5.05m and P. taplejungnica was 4.93m (Fig. 4).
Fig. 3.
DBH size class diagram of P. topkegolensis and P. taplejungnica and dashes in the figure represent average DBH.
3.4. Ethnobotany
3.4.1. Vernacular name
None of the respondents were aware that both P. topkegolensis and P. taplejungnica are endemic species to Nepal. P. taplejungnica was recognized as a separate species from P. rufa in Olangchung Gola as the local name (in Bhote language) Chematagpa. Meanwhile, P. topkegolensis was known as Tagpa/Maamuk, similar to P. rufa in Topke Gola.
3.4.2. Uses
The perspectives of respondents regarding the ethnobotanical use of both species in the study area were gathered. P. topkegolensis has a higher number of use categories compared to P. taplejungnica. With a RFC value of 1, P. topkegolensis was perceived to be used for wood, fuel wood, and fodder, followed by lapsing, inscent, and traditional instruments such as umbrellas (Ghum). On the other hand, P. taplejungnica was used for three purposes. The highest RFC value of 1 was attributed to its use as fodder, followed by an RFC value of 0.6 for incense and an RFC value of 0.27 for fuel wood (Table 5).
Table 5.
Relative Frequency of citations for different use catagories of P. taplejungnica and P. topkegolensis in their respective available area.
| Use Reports | Parts used | Relative Frequency of Citation (RFC) |
|
|---|---|---|---|
| P. topkegolensis | P. taplejungnica | ||
| Wood | Stem | 1 | 0 |
| Fuelwood | Stem | 1 | 0.27 |
| Fodder | Leaves | 1 | 1 |
| Holy fire works (Havan) | Stem | 0.27 | 0 |
| Inscent (Dhup) | Leaves | 0.4 | 0.6 |
| Stand for keeping book (Lapsing) | Stem | 0.6 | 0 |
| Traditional instruments work as umbrella (Ghum) | Bark | 0.13 | 0 |
3.5. Conservation
Through our investigation, we found that P. topkegolensis faces more threats compared to P. taplejungnica. The RVA score showed that both species are highly vulnerable, however the vulnerable score for P. topkegolansis was higher (49/60) than that of P. taplejungnica (45/60). Among the 15 attributes assesed, only the availability of substitutes had a low RVA score (1) for both species. Additionally, P. taplejungnica had low score (1) for commercial demand and habitat destruction (Fig. 5).
Fig. 5.
RVA score for different attributes of P. taplejungnica and P. topkegolansis.
4. Discussions
4.1. Habitat and ecology
The stems of both species P. topkegolansis and P. taplejungnica were with DBH greater than 10 cm, indicating that they are trees This finding contradicts previous research that classified these species as shrubs [10]. This study recorded fruits of P. topkegolensis that had never been reported before. This new information sheds more light on the characteristics of P. topkegolansis species. Both species were found in moist areas near rivers at elevations above 3000 m, indicating that hydrophilic plants can withstand cold stress and are essential for climate change research in the Himalayas.
4.2. Regeneration status and population structure
The lower number of seedlings and saplings compared to adult trees in P. topkegolansis and P. taplejungnica indicates the poor regeneration status of these trees [25]. P. taplejungnica has a weaker regeneration status than P. topkegolensis because it has significantly fewer seedlings and saplings. However, there was no significant difference in the number of adult trees between the species. It could be because environmental and plant characteristics are more significant in defining tree population structure and regeneration. Therefore, P. taplejungnica species require more attention to increase seedling and sapling numbers than P. topkegolensis.
The species' DBH size class diagram revealed a humped-shaped distribution, indicating unsustainable regeneration [26,27]. These findings are consistent with previous studies of high-altitude regions in the Nepal Himalayas. They may be attributed to climate change [28] or anthropogenic disturbances in the Himalayas, such as species use for fodder, firewood, and timber, as well as grazing pressure [8,29,30]. Seed ecology and germination research are critical for promoting these species' regeneration potential in their natural habitat [31].
4.3. Ethnobotany and conservation
Both species were used for a variety of livelihood purposes. However, P. topkegolensis was perceived to be used in more categories than P. taplejungnica. This difference could be attributed to P. topkegolensis's greater availability [32]. Furthermore, the proximity of of these species to the village might have influenced their usage patterns; species located closer to the village tend to be used more frequently [33]. The use of wood and bark of P. topkegolensis poses significant conservation threats that must be addressed. Sustainable harvesting practices for fuel wood and fodder usage are critical for both species [34], including selective harvesting of branches and leaves. It is worth noting that other abundant species could be used as substitutes for these species. Local communities should be made aware of these alternatives and encouraged to adopt improved cooking stoves through subsidies for cooking and heating purposes. P. taplejungnicahas a local common name, while P. topkegolansis is regarded locally as the same species as P. rufa. than P. rufa aThe vernacular name is given based on the species' morphological characteristics, which can provide valuable information to researchers and stakeholders while aiding in species conservation [35]. People should be aware about these species as a separate species from P. rufa and are endemic species as local people perspectives and involvement are critical in species conservation [36]. Implementing a species literacy program can help to foster public support for species conservation [37].
The RVA score showed both species are highly vulnerable, the understanding ethnobotanical knowledge is important for involving local communities in species conservation and management [38]. Improper collection and overuse of endemic species endanger their survival and limit their distribution [39]. The community forest Topkeg Gola and Tiptala should recognize these species and protect these species.
Olangchung Gola is inside the protected area Kanchanjunga conservation area which should also monitor the P. taplejungnica and promote action to promote it. People are not permitted to cut trees haphazardly, and there are other trees with better wood and fuelwood. However, in Topke Gola people were using P. topkegolansis for fuelwood and timber due to easy availability in the area and lack of knowledge about the importance of this plant. People occasionally cut twigs of these plants as fodder. These species' bark is used to make ghum (traditional umbrella), an alternative to Betula utilis.
There is a systematic local sharing method of natural resources. For example, people in the Olangchung Gola community set yearly dates to cut grass from the forest and shrubland. However, effective resource management is still lacking, particularly for P. topkegolensis of Topke Gola. Therefore, it is necessary to implement sustainable utilization practices and effective conservation measures [40]. Human encroachment, land use change, and climate-related hazards such as floods are identified as threats to these species, which is consistent with previous Himalayan studies [41].
There was previously no information on these species' population, regeneration and conservation status. These species' populations are concentrated in a narrow area; P. topkegolensis is distributed in Topkegola, and P. taplejungnica is distributed in Olangchung Gola. More research on their genetic resources is needed to conserve these species. There is a need for implementation of conservation strategies at the local and national levels, like the approach taken for other Prunus species in the Himalayas [42]. The conservation of these species is a shared responsibility that benefits global biodiversity [43]. Raising awareness, managing trails, and collecting seeds to preserve genetic resources for future use and conservation should all be part of conservation efforts. Researchers should also investigate the process of endemism in these species. It is important to note that climate change threatens Himalayan species [44]. Therefore, the effects of climate change on these areas should be investigated, and a climate adaptation program should be implemented.
5. Conclusions
P. topkegolensis and P. taplejungnica are endemic trees in Nepal's subalpine zone. Both species were recorded near the stream. The regeneration status of these species were poor and unsustainable. These species have ethnobotanical value. Both species are highly vulnerable to threats of conservation. Threats to the conservation of these species include species used for wood/fuel wood and lack of awareness. These species require both in-situ and ex-situ conservation.
Funding
This research was funded by the Science and Technology Projects in Guangzhou, grant number 202102021016.
Institutional review board statement
Not applicable.
Data availability statement
All the data used in this study are inside the manuscript.
CRediT authorship contribution statement
Dipak Khadka: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. BaoHuan Wu: Writing – review & editing, Validation, Supervision, Data curation. Sijar Bhatta: Writing – review & editing, Validation, Resources, Investigation. Hem Raj Paudel: Writing – review & editing, Visualization, Validation. Keyi Fu: Writing – review & editing, Visualization, Validation. Dafang Cui: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Funding acquisition, Conceptualization. Shi Shi: Writing – review & editing, Visualization, Validation, Supervision, Resources, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We want to thank all the respondents who participated in this survey, Tenzing Walung, chairman of Mikwakhola rural municipality ward number five (Topke Gola) and Phaktanglung rural municipality ward number seven (Olangchung Gola) of Taplejung district for their support in fieldwork. We thank Prakash Chandra Aryal and Bharat Gotame for their technical support in this work. We thank Satyam Kumar Chaudhari for helping with data management. We would also like to thank Department of Forest and Soil Conservation for permitting to do this work and, and the District forest office Taplejung for their support in field.
Contributor Information
Dafang Cui, Email: cuidf@scau.edu.cn.
Shi Shi, Email: shis@scau.edu.cn.
References
- 1.Manish K., Pandit M.K. Geophysical upheavals and evolutionary diversification of plant species in the Himalaya. PeerJ. 2018;6 doi: 10.7717/peerj.5919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dhyani S. Are Himalayan ecosystems facing hidden collapse? Assessing the drivers and impacts of change to aid conservation, restoration and conflict resolution challenges. Biodivers. Conserv. 2023;32(12):3731–3764. [Google Scholar]
- 3.Rajbhandari K.R., Rai S.K., Chhetri R. vol. 1. 2017. Handbook of the flowering plants of Nepal. (Department of Plant Resources). [Google Scholar]
- 4.Birhane E., Gidey T., Abrha H., Brhan A., Zenebe A., Gebresamuel G., Noulèkoun F. Impact of land-use and climate change on the population structure and distribution range of the rare and endangered Dracaena ombet and Dobera glabra in northern Ethiopia. J. Nat. Conserv. 2023;76 [Google Scholar]
- 5.Biaou S., Gouwakinnou G.N., Noulèkoun F., Salako K.V., Noumagnan N.B.A., Ahouandjinou E.B.O., Houehanou T.D. Insights from analyzing local ecological knowledge and stand structure for guiding conservation actions for the endangered tropical tree Pterocarpus erinaceus. Poir. Trees, Forests and People. 2023;14 [Google Scholar]
- 6.Tiwari A., Uprety Y., Rana S.K. Plant endemism in the Nepal Himalayas and phytogeographical implications. Plant Diversity. 2019;41(3):174–182. doi: 10.1016/j.pld.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shrestha N., Tiwari A., Paudel P.K. Assessing conservation priorities of endemic seed plants in the central Himalaya (Nepal): a complementarity and phylogenetic diversity approach. Biol. Conserv. 2021;261 [Google Scholar]
- 8.Dhamala M.K., Aryal P.C., Suwal M.K., Bhatta S., Bhuju D.R. Population structure and regeneration of Himalayan endemic Larix species in three high-altitude valleys in Nepal Himalaya. Journal of Ecology and Environment. 2020;44(1):1–11. [Google Scholar]
- 9.Rijal B., Sharma M. Modelling diameter at breast height distribution for eight commercial species in natural-origin mixed forests of ontario, Canada. Forests. 2024;15(6):977. doi: 10.3390/f15060977. [DOI] [Google Scholar]
- 10.Ohba H., Akiyama S., others Four new species of himalayan Prunus subgenus cerasus (Rosaceae-Prunoideae) Bulletin of the National Museum of Nature and Science, Series B. 2010;36(36):133–140. [Google Scholar]
- 11.Bhattarai S., Chaudhary R.P., Quave C.L., Taylor R.S.L. The use of medicinal plants in the trans-himalayan arid zone of Mustang district, Nepal. J. Ethnobiol. Ethnomed. 2010;6:1–11. doi: 10.1186/1746-4269-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pei S., Alan H., Wang Y. Vital roles for ethnobotany in conservation and sustainable development. Plant Diversity. 2020;42(6):399. doi: 10.1016/j.pld.2020.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coelho N., Gonçalves S., Romano A. Endemic plant species conservation: biotechnological approaches. Plants. 2020;9(3):345. doi: 10.3390/plants9030345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohba H., Pendry C.A., Rajbhandary S. In: Flora of Nepal. Watson M.F., Akiyama H., Ikeda C.A., Pendry C.A., Rajbhandari K.R., Shrestha K.K., editors. Royal Botanic Garden Edinburgh; Edinburgh, UK: 2012. Rosaceae, Prunus L. [Google Scholar]
- 15.Li Y., Kang X., Zhou J., Zhao Z., Zhang S., Bu H., Qi W. Geographic variation in the petiole--lamina relationship of 325 Eastern Qinghai--Tibetan woody species: analysis in three dimensions. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.748125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ethnobiology Is. ISO; 2006. International Society of Ethnobiology Code of Ethics (With 2008 Additions) [Google Scholar]
- 17.Kent M., Coker P. John Wiley \& Sons; 1992. Vegetation Description and Data Analysis: A Practical Approach. [Google Scholar]
- 18.Mekonnen A.B., Mohammed A.S., Demissew A., others Species diversity, structure, and regeneration status of woody plants in saleda yohans church forest, South Wollo, Ethiopia. Sci. Tech. Rep. 2023;2023 doi: 10.1155/2023/3853463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.R Core Team, R. R Foundation for statistical computing; Vienna: 2023. R: A Language and Environment for Statistical Computing. R Core Team. [Google Scholar]
- 20.Tardio J., Pardo-de-Santayana M. Cultural importance indices: a comparative analysis based on the useful wild plants of Southern Cantabria (Northern Spain) Econ. Bot. 2008;62:24–39. [Google Scholar]
- 21.Cunningham A.B. Working towards a" top 50" listing. Medicinal Plant Conservation. 1996;2 [Google Scholar]
- 22.Bhattarai S., Bhatta B., Shrestha A.K., Kunwar R.M. Ecology, economic botany and conservation of diploknema butyracea in Nepal. Global Ecology and Conservation. 2024 [Google Scholar]
- 23.Pyakurel D., Sharma I.B., Ghimire S.K. Trade and conservation of medicinal and aromatic plants in western Nepal. Bot. Orient. J. Plant Sci. 2017;11:27–37. [Google Scholar]
- 24.Wagner A., Kriechbaum M., Koch M.A. Applied vulnerability assessment of useful plants: a case study of Tibetan medicinal plants from Nepal. Botanische Jahrbücher Für Systematik. 2008:359–387. Pflanzengeschichte Und Pflanzengeographie. [Google Scholar]
- 25.Wani Z.A., Pant S., others Tree diversity and regeneration dynamics in gulmarg wildlife sanctuary, kashmir Himalaya. Acta Ecol. Sin. 2023;43(2):375–381. [Google Scholar]
- 26.Bagri A.S., Singh A., Rawat D.S., Dhingra G.K., Wani Z.A. Population structure and regeneration dynamics of tree species in banj oak forests of tehri garhwal, western Himalaya. Acta Bot. Hung. 2023;65(3–4):229–246. [Google Scholar]
- 27.West D.C., Shugart H.H., Ranney J.W. Population structure of forests over a large area. For. Sci. 1981;27(4):701–710. [Google Scholar]
- 28.Gaire N.P., Fan Z.-X., Chhetri P.K., Shah S.K., Bhuju D.R., Wang J., Sharma B., Shi P., Dhakal Y.R. Ecology of Himalayan Treeline Ecotone. Springer; 2023. Treeline dynamics in Nepal Himalaya in a response to complexity of factors; pp. 519–563. [Google Scholar]
- 29.Malik Z.A., Pandey R., Bhatt A.B. Anthropogenic disturbances and their impact on vegetation in Western Himalaya, India. J. Mt. Sci. 2016;13(1):69–82. [Google Scholar]
- 30.Vetaas O.R. The effect of environmental factors on the regeneration of Quercus semecarpifolia Sm. in central Himalaya, Nepal. Plant Ecol. 2000;146:137–144. [Google Scholar]
- 31.Rashid S., Rashid K., Ganie A.H., Nawchoo I.A., Khuroo A.A. Seed ecology enlightens restoration of endemic species: a case study of Actaea kashmiriana from the Himalaya. Ecol. Eng. 2023;187 [Google Scholar]
- 32.Panyadee P., Wangpakapattanawong P., Inta A., Balslev H. Very high food plant diversity among ethnic groups in Northern Thailand. Diversity. 2023;15(1):120. [Google Scholar]
- 33.Billong Fils P.E., Afiong Nana N., Betti J.L., Farick Njimbam O., Tientcheu Womeni S., Ávila Martin E., Ros Brull G., Okale R., Fa J.E., Funk S.M. Ethnobotanical survey of wild edible plants used by Baka people in southeastern Cameroon. J. Ethnobiol. Ethnomed. 2020;16:1–15. doi: 10.1186/s13002-020-00413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma N., Kala C.P. Harvesting and management of medicinal and aromatic plants in the Himalaya. Journal of Applied Research on Medicinal and Aromatic Plants. 2018;8:1–9. [Google Scholar]
- 35.Addi Y.-W., Zhang Y., Ding X.-Y., Guo C.-A., Wang Y.-H. A study of the plant folk nomenclature of the Yi people in Xiaoliangshan, Yunnan Province, China, and the implications for protecting biodiversity. J. Ethnobiol. Ethnomed. 2022;18(1):18. doi: 10.1186/s13002-022-00504-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma H., Papworth S.K., Ge T., Wu X., Yu C., Zhang H., Turvey S.T. Local awareness and interpretations of species extinction in a rural Chinese biodiversity hotspot. Frontiers in Conservation Science. 2021;2 [Google Scholar]
- 37.Hooykaas M.J.D., Schilthuizen M., Aten C., Hemelaar E.M., Albers C.J., Smeets I. Identification skills in biodiversity professionals and laypeople: a gap in species literacy. Biol. Conserv. 2019;238 [Google Scholar]
- 38.Zerbo I., Salako K.V., Hounkpèvi A., Zozoda D., Kaka i R.G., Thiombiano A. Ethnobotanical knowledge and conservation of Bombax costatum Pellegr. and Vuillet: an overexploited savanna tree species. Trees, Forests and People. 2022;10 [Google Scholar]
- 39.Majid A., Ahmad H., Saqib Z., Rahman I.U., Khan U., Alam J., Shah A.H., Jan S.A., Ali N. Exploring threatened traditional knowledge; ethnomedicinal studies of rare endemic flora from Lesser Himalayan region of Pakistan. Revista Brasileira de Farmacognosia. 2020;29:785–792. [Google Scholar]
- 40.Chauhan H.K., Oli S., Bisht A.K., Meredith C., Leaman D. Review of the biology, uses and conservation of the critically endangered endemic Himalayan species Nardostachys jatamansi (Caprifoliaceae) Biodivers. Conserv. 2021;30(12):3315–3333. [Google Scholar]
- 41.Ganie A.H., Butt T.A., Khuroo A.A., Rasool N., Ahmad R., Basharat S., Reshi Z.A. Taxonomy and threat assessment of Lagotis kunawurensis Rupr.(Plantaginaceae), an endemic medicinal plant species of the Himalaya, India. J. Threat. Taxa. 2022;14(6):21239–21245. [Google Scholar]
- 42.Zhang L., Huang S., Yuan Y., Wu X., Tan Z., Yao L., Hong Z., Cai Q., Wang Y., Xiang H. Geographical distribution and predict potential distribution of Cerasus serrulata. Environ. Sci. Pollut. Control Ser. 2023;30(15):43369–43376. doi: 10.1007/s11356-023-25282-4. [DOI] [PubMed] [Google Scholar]
- 43.Kraus D., Enns A., Hebb A., Murphy S., Drake D.A.R., Bennett B. Prioritizing nationally endemic species for conservation. Conservation Science and Practice. 2023;5(1) [Google Scholar]
- 44.Sarma K., Roy S.J., Kalita B., Regon P., Bawri A., Sahariah D., Saikia A., Tanti B. Impact of climate change on potential distribution and altitudinal shift of critically endangered Amentotaxus assamica DK Ferguson in Arunachal Pradesh Himalaya, India. Theor. Appl. Climatol. 2024;155(1):261–271. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data used in this study are inside the manuscript.