Abstract
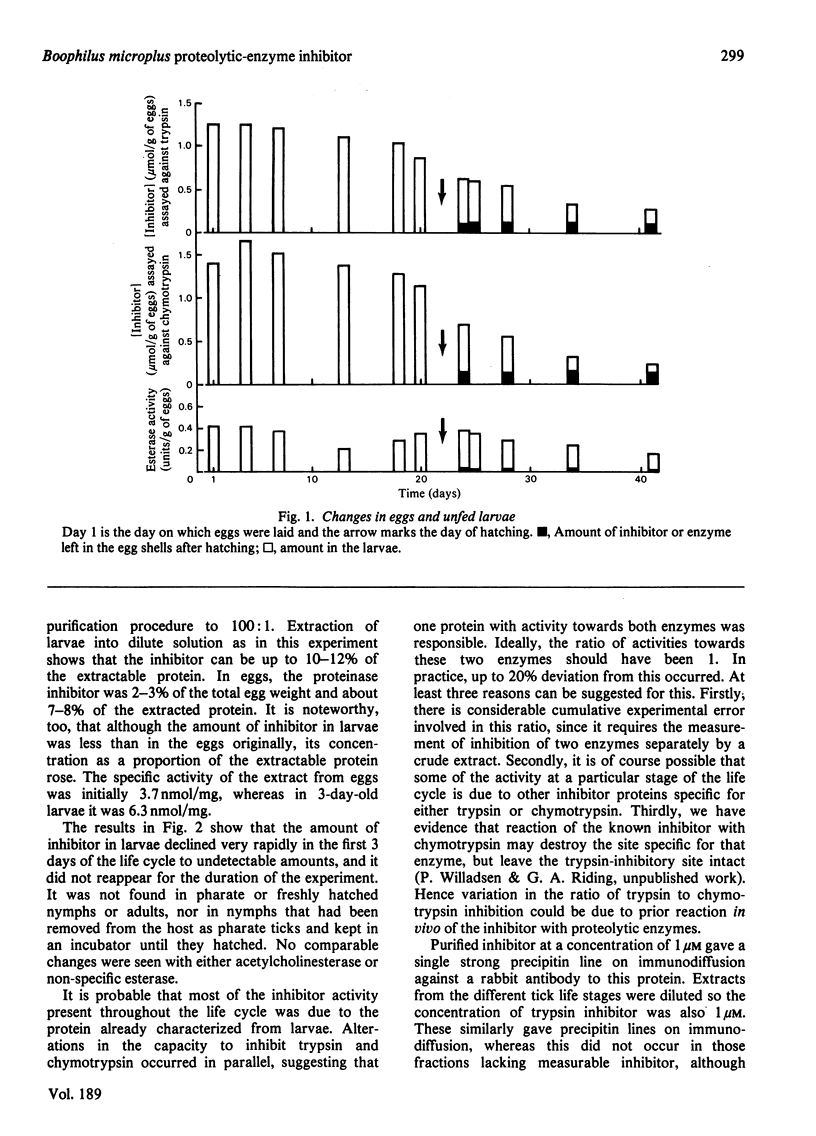
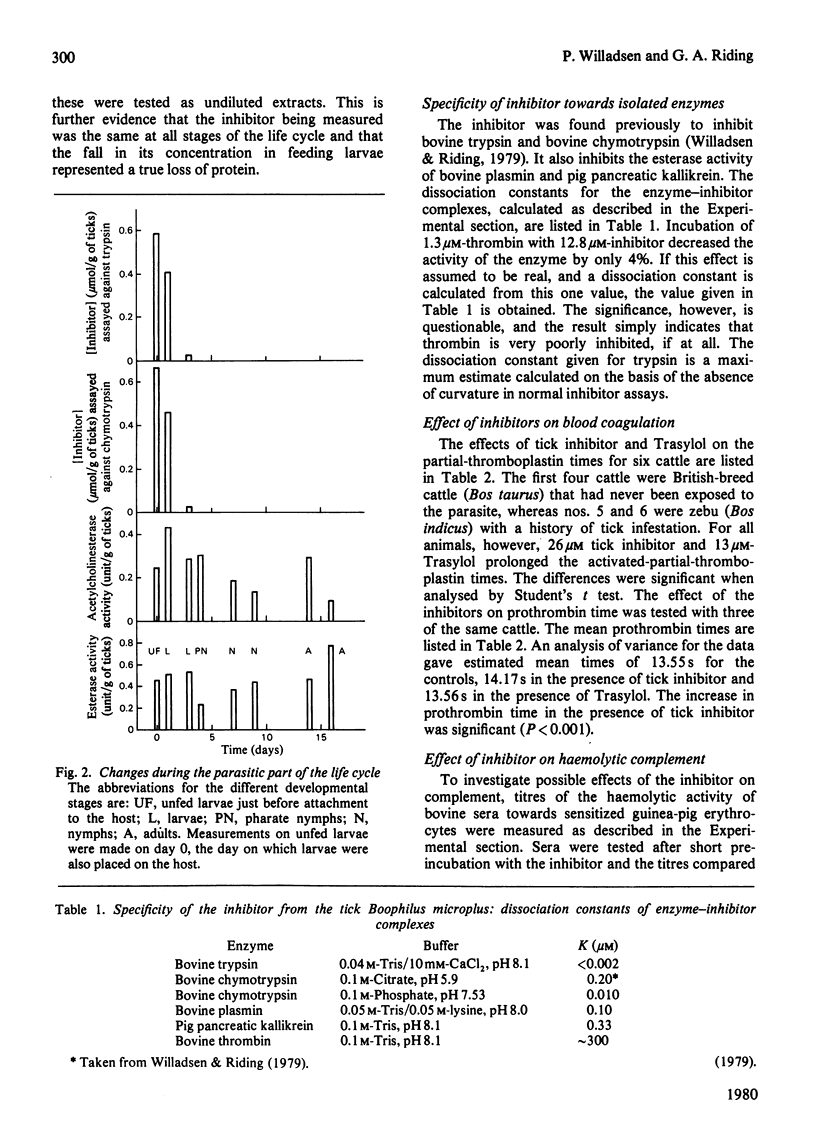
The tick Boophilus microplus contains a protein that inhibits a range of proteolytic enzymes. Variations in the concentration of this protein throughtout the life cycle were followed by measuring simultaneously the inhibition of trypsin and chymotrypsin and reaction with an antiserum to the purified inhibitor. The protein is present in large amounts in eggs and in unfed larvae, but its concentration falls very rapidly after the start of the parasitic stage of the life cycle. This, together with previous evidence, suggests that the inhibitor is important both in eggs and in the initial establishment of the parasite on its host. The activity of the protein towards several enzymes has been measured as an indication of its possible function. Bovine trypsin, chymotrypsin and plasmin and pig pancreatic kallikrein are all inhibited. The protein also affects the blood-coagulation system at several points, since it prolongs both activated-partial-thromboplastin time and prothrombin time. It inhibits the complement-dependent lysis of erythrocytes, but is without significant effect on mitogen-induced lymphocyte stimulation. Thus the inhibitor could have several effects on the host that would be beneficial to the parasite.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barta O., Barta V. Haemolytic assay of bovine serum complement. J Immunol Methods. 1972 Aug;1(4):363–374. doi: 10.1016/0022-1759(72)90029-4. [DOI] [PubMed] [Google Scholar]
- Castellino F. J., Sodetz J. M. Rabbit plasminogen and plasmin isozymes. Methods Enzymol. 1976;45:273–286. doi: 10.1016/s0076-6879(76)45026-7. [DOI] [PubMed] [Google Scholar]
- Fritz H., Kruck J., Rüsse I., Liebich H. G. Immunofluorescence studies indicate that the basic trypsin-kallikrein-inhibitor of bovine organs (Trasylol) originates from mast cells. Hoppe Seylers Z Physiol Chem. 1979 Mar;360(3):437–444. doi: 10.1515/bchm2.1979.360.1.437. [DOI] [PubMed] [Google Scholar]
- GREEN N. M., WORK E. Pancreatic trypsin inhibitor. II. Reaction with trypsin. Biochem J. 1953 May;54(2):347–352. doi: 10.1042/bj0540347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutner A., Simmler M. C., Tapon J., Rosenfeld C. Modulation by alpha-2 macroglobulin of human lymphocyte proliferation in response to mitogens and antigen. Differentiation. 1976 Jun 4;5(2-3):171–173. doi: 10.1111/j.1432-0436.1976.tb00910.x. [DOI] [PubMed] [Google Scholar]
- Moreau P., Dornand J., Kaplan J. G. Inhibition of lymphocyte transformation: effect of soy bean trypsin inhibitor and synthetic anti-proteases. Can J Biochem. 1975 Dec;53(12):1337–1341. doi: 10.1139/o75-182. [DOI] [PubMed] [Google Scholar]
- Movat H. Z. The kinin system: its relation to blood coagulation, fibrinolysis and the formed elements of the blood. Rev Physiol Biochem Pharmacol. 1978;84:143–202. doi: 10.1007/BFb0030492. [DOI] [PubMed] [Google Scholar]
- Riddles P. W., Blakeley R. L., Zerner B. Ellman's reagent: 5,5'-dithiobis(2-nitrobenzoic acid)--a reexamination. Anal Biochem. 1979 Apr 1;94(1):75–81. doi: 10.1016/0003-2697(79)90792-9. [DOI] [PubMed] [Google Scholar]
- Roberts J. A. Resistance of cattle to the tick boophilus microplus (canestrini). II. Stages of the life cycle of the parasite against which resistance is manifest. J Parasitol. 1968 Aug;54(4):667–673. [PubMed] [Google Scholar]
- Sodetz J. M., Brockway W. J., Castellino F. J. Multiplicity of rabbit plasminogen. Physical characterization. Biochemistry. 1972 Nov 21;11(24):4451–4458. doi: 10.1021/bi00774a005. [DOI] [PubMed] [Google Scholar]
- Summaria L., Arzadon L., Bernabe P., Robbins K. C. Isolation, characterization, and comparison of the S-carboxymethyl heavy (A) and light (B) chain derivatives of cat, dog, rabbit, and bovine plasmins. J Biol Chem. 1973 Sep 25;248(18):6522–6527. [PubMed] [Google Scholar]
- Thomson A. W., Pugh-Humphreys R. G., Tweedie D. J., Horne C. H. Effects of the antiprotease Trasylol on peripheral blood leucocytes. Experientia. 1978 Apr 15;34(4):528–530. doi: 10.1007/BF01935971. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]
- Willadsen P., Riding G. A. Characterization of a proteolytic-enzyme inhibitor with allergenic activity. Multiple functions of a parasite-derived protein. Biochem J. 1979 Jan 1;177(1):41–47. doi: 10.1042/bj1770041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willadsen P., Williams P. G. Isolation and partial characterization of an antigen from the cattle tick, Boophilus microplus. Immunochemistry. 1976 Jul;13(7):591–597. doi: 10.1016/0019-2791(76)90171-3. [DOI] [PubMed] [Google Scholar]
- Willadsen P., Williams P. G., Roberts J. A., Kerr J. D. Responses of cattle to allergens from Boophilus microplus. Int J Parasitol. 1978 Apr;8(2):89–95. doi: 10.1016/0020-7519(78)90003-6. [DOI] [PubMed] [Google Scholar]


