Abstract
Objectives
Alopecia areata incognita is a non-scarring autoimmune hair loss condition primarily affecting women aged 20 to 40. It is often misdiagnosed due to its resemblance to other conditions. Diagnosis relies on clinical suspicion, trichoscopic findings, and histological features. Reflectance confocal microscopy (RCM) shows promise as a non-invasive diagnostic tool for alopecia areata incognita. In this study, we aimed to explore RCM’s diagnostic potential by investigating its association with trichoscopic and histopathological findings.
Methods
We conducted a prospective study with 12 female patients affected by alopecia areata incognita. Patient data, trichoscopy, and RCM were used for diagnosis. Biopsies were taken based on trichoscopic and RCM criteria. Agreement between RCM, trichoscopy, and histopathology was assessed.
Results
RCM showed substantial agreement with histopathology for fibrous tracts (92.9%). Other criteria, like infundibular ostia and inflammation, exhibited reasonable agreement (71.4% to 78.6%), with varying Kappa values. Miniaturized follicles had the lowest agreement (64.3%).
Conclusion
This study suggests that RCM holds promise as a diagnostic tool for alopecia areata incognita, offering advantages in non-invasiveness and real-time monitoring. It demonstrated substantial agreement with histopathology in identifying key features. While some discrepancies were noted, especially in detecting inflammatory infiltrates, further research may enhance RCM’s sensitivity. The non-invasive nature of RCM could improve patient experiences and offer dynamic disease tracking for better treatment decisions. This technology’s potential extends beyond alopecia areata incognita, presenting opportunities for more patient-friendly diagnostic procedures in trichology.
Keywords: alopecia areata incognita, reflectance confocal microscopy, trichoscopy, alopecia areata, histopathology
Introduction
Alopecia areata incognita (AAI) is non-scarring autoimmune alopecia first described by Rebora et al. in 1987. It is considered a subtype of alopecia areata (AA) [1]. This relatively common disease predominantly affects 20- to 40-year-old females [2]. The true prevalence, however, remains unclear as it is often misdiagnosed as telogen effluvium (TE) or androgenetic alopecia (AGA). Similar to other types of AA, a family history of autoimmune diseases and genetic susceptibility are known risk factors, but its etiopathogenesis is not fully understood. Peculiar to this condition is a favorable prognosis and higher therapy compliance.
AAI presents clinically as acute diffuse hair loss without the usual alopecic patches seen in other forms of AA. Trichoscopy is a crucial non-invasive tool for diagnosis and follow-up [3]. Key trichoscopic features of AAI include multiple diffusely distributed yellow dots, which can be empty or have thin hair shafts, observed in both the androgen-dependent and parietal areas, short hairs in the regrowth phase, and the pathognomonic pigtail hairs. When AAI is associated with AGA, anisotrichia–defined as a variability of more than 20% in the diameter of the hair shafts– in androgen-sensitive areas of the scalp is reported [4,5].
The diagnosis of AAI is established when high clinical suspicion, combined with the trichoscopic features mentioned earlier, is supported by a positive pull test with telogen roots and the following specific histological findings: a reduction in follicular density, a decreased number of terminal anagen follicles, and an increase in the number of catagen and telogen follicles exhibiting fibrous tracts (streamers) in the dermis and adipose tissue, which is often accompanied by peribulbar lymphocytic infiltrate. There may also be an increase in miniaturized follicles with dilated infundibular openings and small telogen follicles [6].
Reflectance confocal microscopy (RCM) has emerged as a valuable non-invasive alternative to conventional biopsy for diagnosing various skin diseases. This advanced imaging technique allows real-time assessment of the upper skin layers down to the superficial reticular dermis, thus providing cellular-level resolution comparable to conventional histopathology. Despite its potential, RCM remains underutilized, with few studies assessing its efficacy for alopecia [7,8]. Ardigò et al. studied RCM in the context of AA, but, to our knowledge, no studies have evaluated the concordance between RCM and histopathology in AAI [9].
The present study aimed to examine the main RCM features of scalp AAI and correlate them with trichoscopic and histopathological findings of biopsy specimens obtained from the same lesions. By doing so, we hoped to shed light on the potential diagnostic value of RCM and further expand its application in trichology.
Patients and Methods
Patients
This prospective study adhered to the principles of the Declaration of Helsinki (1964) and its subsequent amendments. Ethics committee approval was not required. The main author (MS) enrolled a total of 12 exclusively female patients at the University of Modena and Reggio Emilia, Italy, who had never received local or systemic therapy. The mean age was 43.2 years (ranging from 28 to 66). Diagnosis of AAI was based on clinical, trichoscopic, and histopathological features. All patients signed informed consent, including permission to take photographs before and during the research study. The following clinical and demographic data were collected: age, sex, duration of disease, and clinical and medical history.
Methods
At the first visit, each patient underwent global photography, pull test, and trichoscopy examination to select the best site with the typical features (Figure 1). For the trichoscopic examination, we used a FotoFinder® dermatoscope (Teachscreen Software, Bad Birnbach, Germany), applying water as an interface. Different magnifications (20X, 40X, 70X) performed both on the affected and healthy areas of the scalp ensured better trichoscopic characterization. These images were then evaluated by two dermatologists with expertise in trichoscopy (MS and BMP) searching for the presence of four criteria: empty yellow dots, yellow dots with vellus hair, pigtail hair, and small hair in regrowth phase. Subsequently, RCM was executed on the anatomical sites identified for the upcoming trichoscopy-guided biopsy.
Figure 1.
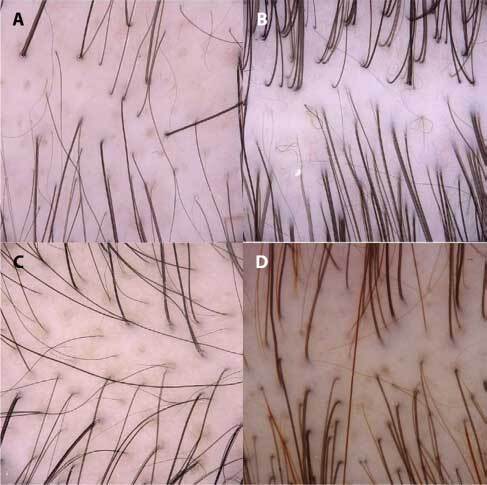
Trichoscopy (magnification ×20) showing empty yellow dots (A), pig-tail hairs (B), small hair in regrowth (C), and yellow dots with vellus hair (D).
Histopathology was performed on 4 mm punch biopsies that included a sample area of 12.6 mm2 containing subcutaneous fat and deep-rooted terminal hair bulbs. The specimens were fixed in 10% neutral buffered formalin for at least 24 hours and then cut into two identical parts for vertical and horizontal sections. This method provided qualitative and quantitative information about all hair units at various levels. Histopathological assessment was concomitantly performed by one of the authors (CM) on a high-resolution microscope. It required the presence of several features: a reduction in follicular density, characterized by the decreased number of terminal anagen follicles and increased number of catagen and telogen follicles, fibrous tracts (streamers) in the dermis, an increase in miniaturized follicles with dilated infundibular openings, and small telogen follicles ( Figures 2–3). [6]. Here we used the same histological criteria listed by Alessandrini et al. [4]. The areas selected for biopsy corresponded to active disease based on trichoscopy and the RCM imaging.
Figure 2.
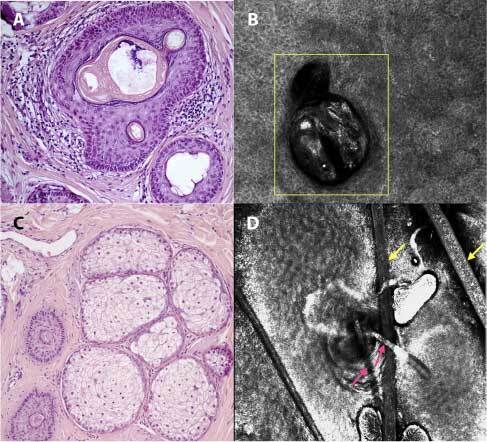
RCM evaluation and related histopathological section of alopecia areata incognita: infundibular ostia empty or full of sebum and keratin (A), visualized at RCM as an infundibulum fulfilled with bright material which corresponds to keratin (yellow square - dermis) (B), miniaturized anagen-vellus hair follicles (“nanogen”) (C), seen in RCM as miniaturized follicles, represented by a reduction of the caliber of the hair shaft (pink arrows) in comparison to normal hair (yellow arrows - corneal layer) (D) (H&E, ×8).
Figure 3.
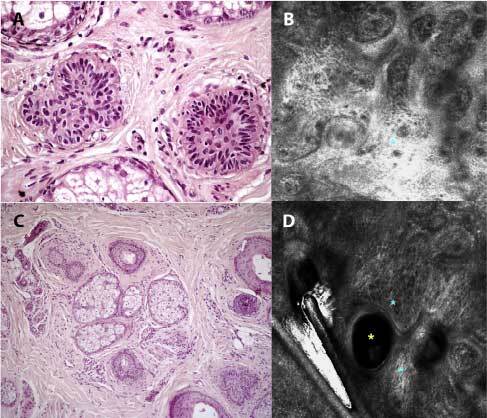
RCM evaluation and histopathological section of alopecia areata incognita: early anagen follicles (A) visualized at RCM with dense bright fibrillar structures which correspond to fibrous tracts (blue asterisk - papillary dermis) (B), vellus follicles-regrowing hairs-dilated infundibula (C) seen at RCM as dilated infundibular unit (yellow asterisk) associated to fibrotic tissue (blue asterisks) (D). The combination of these two findings represents a follicle in telogen phase (H&E, ×8).
RCM was performed with a VivaScope® 3000 (Mavig GmbH, Munich, Germany), capable of scanning an area of 1 x 1 mm down to a depth of 200 μm. Details about the instrument and acquisition procedures have been described in previous works [10, 11]. The Vertical VivaStack software processed a sequence of horizonal sections from the same area spaced 5μm apart, thus allowing evaluation of the adnexal structures from the ostium to the maximum depth. The images were simultaneously analyzed on a high-resolution monitor, enabling identification of relevant AAI features. We evaluated the RCM images focusing on the following criteria: 1) perinfundibular and dermal inflammation (round-to-polygonal mildly refractive cells around the infundibulum at the level of the superficial dermis); 2) miniaturized follicles (reduction in the caliber of the hair shaft in comparison to normal hair as well as reduction in the caliber of the adnexal infundibulum); 3) content of infundibular ostia empty or full of sebum and keratin (dark infundibular unit (empty) or highly refractive material inside the infundibulum (full of sebum and keratin)); 4) follicles in telogen phase (dilated infundibular unit associated to fibrotic tissue at the level of the papillary dermis); 5) presence of fibrous tracts (pale, ill defined, sometimes compact fibers at the level of the superficial dermis) [9]. The visualization of follicle in telogen is better understood by the visualization of a sequence of images through Vivostack®, in which we noted the presence of pale fibers (corresponding to fibrosis) displayed around a dilated infundibular unit at the level of the superficial dermis.
Table 1 summarizes the correlation between these RCM and the histopathological features. The RCM images are presented in Figures 2–3. Concordance between RCM and histopathology was tested by comparing data findings from evaluations independently performed by one expert dermopathologist and two dermatologists with expertise in RCM.
Table 1.
Description of Correlation between HP and RCM Criteria in AAI.
| Histopathological criteria | RCM features |
|---|---|
| Peribulbar and dermal mild inflammatory infiltrate | Round-to-polygonal, mildly refractive cells around the hair bulb at the level of the superficial dermis |
| Miniaturized follicles | Reduction of the caliber of the hair shaft in comparison to normal hair as well as reduction of the caliber of the adnexal infundibulum |
| Infundibular ostia empty or full of sebum and keratin | Dark infundibula unit (empty) or highly refractive material inside the infundibula (full of sebum and keratin) |
| Catagen and telogen germinal units | Catagen: infundibular units connected to the hair shaft Telogen: dilated infundibular unit associated to fibrotic tissue at the level of the superficial dermis Increased catagen: 2 to 3 (then, repeat the chosen description) Increased telogen: 1 to 2 (then, repeat the chosen description) |
| Follicles in telogen | Dilated infundibular unit associated to fibrotic tissue at the level of the papillary dermis |
| Fibrous tracts or streamers | Pale, ill defined, sometimes compact fibers at the level of the superficial dermis |
Abbreviations: AAI, alopecia areata incognita; HP, histopathology; RCM, Reflectance confocal microscopy.
Statistical Analysis
The inter-methods agreement between RCM imaging, trichoscopy, and horizontal histopathology sections (HHS) was assessed using Cohen’s Kappa (k) statistics. Cohen’s Kappa values were classified as: poor (<0.21); fair ( 0.21–0.40); moderate (0.41–0.60); good (0.61–0.80); very good (0.81–1.00) agreement. Additionally, p-values were reported, and statistical significance was considered at P < 0.05. To quantify the agreement between methods, incidence percentages based on positive results for each method, percent agreements, and the kappa statistic were calculated using Stata/SE 12.0 for Mac software for statistical analysis.
Results
The presence of RCM features and the comparison with histopathology is shown on a case-by-case basis in Table 2.
Table 2.
Comparison of Inter-Methods Agreement (HP and RCM) for Six Criteria Evaluated in 14 Patients.
| Patient | Peribulbar and dermal mild inflammatory infiltrate | Increased miniaturized follicles | Infundibular ostia empty or full of sebum and keratin | Increased number of catagen and telogen germinal units | Follicles in telogen | Fibrous tracts or | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDHP | PDRCM | IMHP | IMRCM | IOHP | IORCM | INHP | INRCM | FTHP | FTRCM | FTHP | FTRCM | |
| 01 | X | X | X | X | X | X | X | X | X | X | X | X |
| 02 | X | X | X | X | X | X | X | X | X | X | ||
| 03 | X | X | X | X | X | X | X | X | X | X | X | X |
| 04 | X | X | X | X | X | X | X | X | X | X | X | |
| 05 | X | X | X | X | X | X | X | X | X | |||
| 06 | X | X | X | X | X | X | X | X | X | X | X | |
| 07 | X | X | X | X | X | X | X | X | ||||
| 08 | X | X | X | X | X | X | X | X | X | |||
| 09 | X | X | X | X | X | X | X | X | X | X | X | |
| 10 | X | X | X | X | X | X | X | X | ||||
| 11 | X | X | X | X | X | X | X | X | X | X | X | X |
| 12 | X | X | X | X | X | X | X | X | ||||
| 13 | X | X | X | X | X | |||||||
| 14 | X | X | X | X | X | X | X | X | X | X | X | X |
| Incidence (%) | 11/14 (78.6) | 7/14 (50.0) | 12/14 (85.7) | 11/14 (78.6) | 12/14 (85.7) | 13/14 (92.9) | 10/14 (71.4) | 13/14 (92.9) | 11/14 (78.6) | 14/14 (100.0) | 12/14 (85.7) | 13/14 (92.9) |
| Agreement(%) | 10/14 (71.4) | 9/14 (64.3) | 11/14 (78.6) | 11/14 (78.6) | 10/14 (71.4) | 13/14 (92.9) | ||||||
| Cohen’s Kappa (p-value) | 0.429 (P=0.025) | −0.207 (0.788) | −0.105 (0.664) | 0.323 (0.050) | 0.000 (0.500) | 0.632 (0.006) | ||||||
Abbreviations: HP, histopathology; RCM, Reflectance confocal microscopy; X, presence of condition.
Fibrous tracts or streamers showed the highest correlation and Cohen’s Kappa (k) level (92.9% k = .632; P = .006). Increased number of germinal units in catagen and telogen phases and empty- or sebum/keratin-filled infundibular ostia both had percent agreements of 78.6%, but different levels of Kappa (k = 0.323; P = 0.050 and k = − 0.105; P = 0.664, respectively). Peribulbar and dermal mild inflammatory infiltrate and follicles in telogen had a percent agreement of 71.4% but also different Kappa levels (k = 0.429; P = 0.025 and k = 0.000; P = 0.500). An increase in miniaturized follicles had the lowest percent agreement and the lowest level of Kappa (64.3%; k = −0.207; P = 0.788).
As shown in Table 3, the percent agreement varied from 71.4% to 85.7%. However, because Kappa statistics were not higher than 0.20, the agreement was deemed poor. An exception was the agreement between vellus follicle-regrowing hair-dilated infundibula and yellow dots with vellus hair, which was considered fair (k = 0.323; P = 0.050).
Table 3.
Comparison of Inter-Methods Agreement (HP and RCM + TSC) for Four Criteria Evaluated in 14 Patients.
| Patient | HP Infundibular ostia empty or full of sebum and keratin | (RCM(1) + TSC (Y)) Empty Yellow dots | HP Miniaturized anagen-vellus hair follicles (nanogen) | (RCM + TSC) Small hair in regrowth | HP early anagen | (RCM + TSC) Pigtail hair | HP Vellus follicles-regrowing hairs-dilated infundibula | (RCM + TSC) Yellow dots with vellus hair |
|---|---|---|---|---|---|---|---|---|
| 01 | X | X | X | X | X | X | X | X |
| 02 | X | X | X | X | X | X | X | X |
| 03 | X | X | X | X | X | X | X | X |
| 04 | X | X | X | X | X | X | X | X |
| 05 | X | X | X | X | X | X | X | X |
| 06 | X | X | X | X | X | X | X | |
| 07 | X | X | X | X | X | X | ||
| 08 | X | X | X | X | X | X | X | |
| 09 | X | X | X | X | X | X | X | |
| 10 | X | X | X | X | X | X | ||
| 11 | X | X | X | X | X | X | X | X |
| 12 | X | X | X | X | X | |||
| 13 | X | X | X | X | ||||
| 14 | X | X | X | X | X | X | X | X |
| Incidence (%) | 12/14 (85.7) | 14/14 (100.0) | 12/14 (85.7) | 12/14 (85.7) | 12/14 (85.7) | 13/14 (92.9) | 10/14 (71.4) | 13/14 (92.4) |
| Agreement(%) | 12/14 (85.7) | 10/14 (71.4) | 11/14 (78.6) | 11/14 (78.6) | ||||
| Cohen’s Kappa (p-value) | 0.000 (P=0.500) | −0.167 (P=0.734) | −0.105 (P=0.664) | 0.323 (P=0.050) | ||||
Abbreviations: HP, histopathology; RCM, Reflectance confocal microscopy; TSC, Trichoscopy; X, presence of condition.
The RCM findings consistently correlated with histopathology in cases where increased numbers of catagen/telogen follicles were observed along with fibrous tracts. Among the samples, one displayed both RCM and histopathology findings, while RCM identified both features in two cases where histopathology revealed fibrous tracts without an elevated number of follicles in catagen/telogen. Furthermore, in one case lacking the mentioned histopathological features, RCM detected an increased number of catagen/telogen follicles without fibrous tracts. However, discrepancies emerged between RCM and histopathology in identifying peribulbar and dermal inflammation, an additional diagnostic criterion for AAI. While histopathology identified this finding in four out of the 14 cases, RCM failed to detect it in any of them. Regarding the increase in miniaturized follicles, both RCM and histopathology yielded a specific criterion for evaluation. The two methods fully agreed in four cases, with RCM solely identifying it in two cases and histopathology in three. Notably, in all cases, RCM provided sufficient criteria to support the diagnosis of AAI when combined with clinical/trichoscopic evaluations and a positive pull test.
Histopathologically, all patients exhibited a preserved number of follicular units but a reduced number of terminal follicles, particularly those in anagen. The most frequent histopathological feature in our series was the presence of dilated infundibular openings (0.02 mm–0.05 mm), empty or filled with keratin. These openings were visible in both horizontal and vertical sections and corresponded to the yellow dots described by trichoscopy and RCM. Hair count analysis revealed a significant reduction in terminal anagen follicles associated with an increase in small telogen follicles, catagen and telogen germinal units, mainly located at the bulge level. There was also an upsurge in the number of vellus and miniaturized follicles. Furthermore, a mild peribulbar and upper dermal inflammatory infiltrate was observed, especially around the bulb of miniaturized follicles. In patients with acute hair loss and a positive pull test, a mild inflammatory infiltrate surrounding the hair follicle in the upper dermis and remnants of fibrous tracts or streamers in the dermis and adipose tissue were noted, indicative of the rapid shortening of the hair cycle.
Conclusion
In the present study, we aimed to investigate the potential diagnostic value of RCM in the context of AAI, a form of non-scarring autoimmune alopecia. AAI often poses diagnostic challenges due to its clinical presentation, which lacks the typical alopecic patches observed in other forms of AA [1,4]. RCM, with its non-invasive capabilities and cellular-level resolution, has emerged as a promising alternative to traditional biopsy methods for diagnosing various skin diseases. However, it has remained underused in the context of alopecia, with few studies assessing its efficacy in this field [7–9].
Our study enrolled 12 exclusively female patients, and the diagnosis of AAI was established based on clinical, trichoscopic, and histopathological features. The mean age of the participants was 43.2 years, with a range of from 28 to 66 years. The clinical and demographic data were meticulously collected to ensure the homogeneity of the study population. Trichoscopy was used in conjunction with RCM to select the most representative sites for imaging. Trichoscopic evaluations included the identification of specific criteria such as empty yellow dots, yellow dots with vellus hair, pigtail hair, and small hairs in the regrowth phase [4].
The results of our study revealed several key findings. RCM demonstrated a remarkable 92.9% agreement with histopathology in identifying fibrous tracts or streamers, a diagnostic feature associated with AAI. Additionally, both RCM and histopathology exhibited a 78.6% agreement in detecting an increased number of germinal units in catagen and telogen phases, and the status of infundibular ostia (empty or filled with sebum and keratin). This is particularly significant because it corresponds to the yellow dots observed in trichoscopy and RCM.
However, discrepancies were noted in some areas, especially in the detection of peribulbar and dermal inflammatory infiltrates, which were identified in more cases by histopathology then by RCM. Further research may be needed to improve RCM’s sensitivity in detecting inflammation. The detection of miniaturized follicles also exhibited varying levels of agreement, with a 64.3% agreement and a relatively lower Kappa statistic.
In conclusion, our study highlights the potential of RCM as a valuable diagnostic tool for AAI, offering significant advantages such as its non-invasiveness. RCM’s real-time assessment capabilities provide a novel means of monitoring the progression of AAI. This could lead to a shift from static diagnostic assessments to dynamic disease tracking, enabling clinicians to make more informed decisions about treatment strategies and the timing of interventions. Furthermore, the non-invasive nature of RCM is a major advantage, significantly reducing patient discomfort during the diagnostic process. As this technology continues to improve, it may pave the way for less invasive and more patient-friendly diagnostic procedures in other trichology conditions, improving overall patient experiences.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Rebora A. Alopecia areata incognita: a hypothesis. Dermatologica. 1987;174(5):214–218. doi: 10.1159/000249182. [DOI] [PubMed] [Google Scholar]
- 2.Molina L, Donati A, Valente NS, Romiti R. Alopecia areata incognita. Clinics (Sao Paulo) 2011;66(3):513–515. doi: 10.1590/s1807-59322011000300027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alessandrini A, Bruni F, Piraccini BM, Starace M. Common causes of hair loss - clinical manifestations, trichoscopy and therapy. J Eur Acad Dermatol Venereol. 2021;35(3):629–640. doi: 10.1111/jdv.17079. [DOI] [PubMed] [Google Scholar]
- 4.Alessandrini A, Starace M, Bruni F, et al. Alopecia Areata Incognita and Diffuse Alopecia Areata: Clinical, Trichoscopic, Histopathological, and Therapeutic Features of a 5-Year Study [published correction appears in Dermatol Pract Concept 2019 Dec 31;10(1):e2020027 10.5826/dpc.1001a27] Dermatol Pract Concept. 2019;9(4):272–277. doi: 10.5826/dpc.0904a05. Published 2019 Oct 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tosti A, Whiting D, Iorizzo M, et al. The role of scalp dermoscopy in the diagnosis of alopecia areata incognita. J Am Acad Dermatol. 2008;59(1):64–67. doi: 10.1016/j.jaad.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 6.Miteva M, Misciali C, Fanti PA, Tosti A. Histopathologic features of alopecia areata incognito: a review of 46 cases. J Cutan Pathol. 2012;39(6):596–602. doi: 10.1111/j.1600-0560.2012.01896.x. [DOI] [PubMed] [Google Scholar]
- 7.Ardigò M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy for scarring and non-scarring alopecia real-time assessment. Arch Dermatol Res. 2016;308(5):309–318. doi: 10.1007/s00403-016-1657-4. [DOI] [PubMed] [Google Scholar]
- 8.Rudnicka L, Olszewska M, Rakowska A. In vivo reflectance confocal microscopy: usefulness for diagnosing hair diseases. J Dermatol Case Rep. 2008 Dec 27;2(4):55–9. doi: 10.3315/jdcr.2008.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ardigò M, Tosti A, Cameli N, Vincenzi C, Misciali C, Berardesca E. Reflectance confocal microscopy of the yellow dot pattern in alopecia areata. Arch Dermatol. 2011;147(1):61–64. doi: 10.1001/archdermatol.2010.288. [DOI] [PubMed] [Google Scholar]
- 10.Mandel VD, Cinotti E, Benati E, et al. Reflectance confocal microscopy and optical coherence tomography for the diagnosis of bullous pemphigoid and pemphigus and surrounding subclinical lesions. J Eur Acad Dermatol Venereol. 2018;32(9):1562–1569. doi: 10.1111/jdv.14795. [DOI] [PubMed] [Google Scholar]
- 11.Melo DF, De Carvalho N, Ardigò M, et al. Concordance among in vivo reflectance confocal microscopy, trichoscopy, and histopathology in the evaluation of scalp discoid lupus. Skin Res Technol. 2020;26(5):675–682. doi: 10.1111/srt.12852. [DOI] [PubMed] [Google Scholar]


