Abstract
The 5-year survival rate for ovarian cancer has remained relatively static over the past number of years, which can be attributed in part to the lack of new therapeutic strategies to target this disease. Although numerous other cancer types have benefited from the success of immune checkpoint inhibitors, their use in clinical trials targeting ovarian cancer has shown limited efficacy. Most clinical trials have focused on PD-1/PD-L1 immune checkpoint blockade, either as a monotherapy or in combination with chemotherapies, however inhibiting other pathways may potentially be more efficacious in treating ovarian cancer. For example, drugs targeting some emerging immune checkpoints (such as LAG-3, TIM-3, TIGIT and PVRIG), are entering into clinical trials, which could show improved success for ovarian cancer patients. Similarly, predictive biomarkers that have been approved for use with immune checkpoint inhibitors, such as PD-L1 expression, are limited, as only the presence or absence of PD-L1 is assessed. However, the development of next generation predictive biomarkers, which assesses density and location of tumour infiltrating lymphocytes, could be more beneficial for this heterogenous cancer. In this review we discuss the use of immune checkpoint inhibitors in ovarian cancer, with a focus on high-grade serous disease, and delve into what the future may hold for immunotherapy in this cancer type.
Keywords: High-grade serous ovarian cancer, Immune checkpoint inhibitors, Tumour microenvironment
Highlights
-
•
Immune checkpoint inhibitors have shown limited efficacy in ovarian cancer – a summary of the clinical trials.
-
•
A summary of the evidence for and against the use of immune checkpoint inhibitors in ovarian cancer, with a specific focus on the high-grade serous subtype.
-
•
The possibility of emerging immune checkpoints; LAG-3, TIM-3, TIGIT, and PVRIG for use in high-grade serous ovarian cancer.
-
•
The emergence of next-generation biomarkers of immune checkpoint inhibitor response could improve on previous limitations.
1. Introduction
Ovarian cancer (OC) has a global incidence rate of 6.6 cases per 100,000 people and the highest mortality rate of all gynaecological cancers [1]. Globally the estimated number of new cases is expected to increase from 314,000 in 2020 to 446,000 in 2040, with the number of deaths also predicted to rise from 207,000 to 314,000 [2]. High-grade serous ovarian cancer (HGSOC) is the most prevalent OC subtype accounting for 75 % of all cases, and is characterised by genetic alterations in the TP53 gene, as well as genes involved in homologous recombination repair such as BRCA1, BRCA2 and PTEN [3]. HGSOC is also the most lethal OC subtype with ca. 70 % of patients being diagnosed at a late stage as a result of vague symptoms and lack of an appropriate screening test [3]. Elevated Cancer Antigen 125 (CA-125) serum levels remains the only biomarker for detecting and managing OC. However, high numbers of false positives occur when screening average-risk women due to the low incidence of OC [4]. The 5-year relative survival rate for late-stage disease is 32 % compared to 92 % for localised disease [5]. This poor survival rate for late-stage disease is due in part to the standard of care remaining relatively static for the past 20 years, consisting primarily of surgery combined with platinum-based chemotherapies [6]. In addition, up to 70 % of patients experience a recurrence following platinum-based chemotherapy highlighting the urgent need to find better treatment options for these women [6].
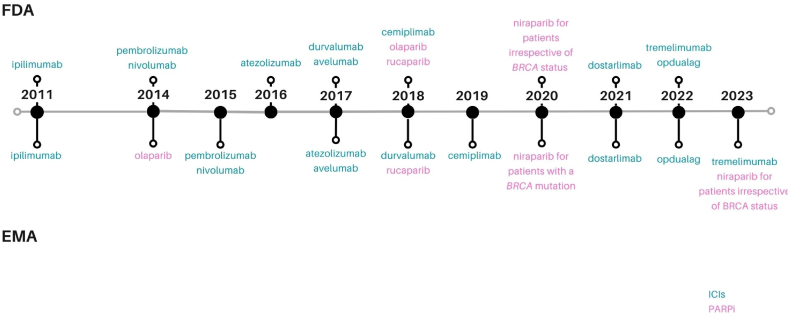
Some advances have been made in OC treatment. For example, three poly-ADP ribose polymerase inhibitors (PARPi), namely olaparib, rucaparib, and niraparib are approved for use in OC (Fig. 1) [[6], [7], [8], [9], [10]]. Numerous clinical trials have investigated the use of immunotherapies, specifically immune checkpoint inhibitors (ICIs) in OC, however, although approvals have occurred for other cancer types, ICIs are still not recommended for use in OC (Fig. 1). ICIs block immune checkpoint pathways, which regulate the immune response by managing the intensity and duration of immune reactions. This regulation helps avoid excessive and prolonged activation of the immune system, which might otherwise result in tissue damage and the onset of autoimmunity [11]. Tumours can exploit this system as a mechanism of immune evasion [11]. The goal of ICIs is to overcome this by blocking the checkpoint pathways, preventing a dampening of the immune reaction, and thereby revitalising the body's anti-tumour immune response.
Fig. 1.
ICIs – first approval, PARPi – first approval in ovarian cancer, by both the FDA and EMA. PARPi (PARP inhibitors), ICIs (Immune Checkpoint Inhibitors), FDA (United States Food and Drug Administration) EMA (European Medicines Agency).
The results so far for ICI monotherapy for OC have been disappointing (Table 1), which is why clinical trials have now focused on combining ICIs with other treatments such as chemotherapy, Vascular endothelial growth factor (VEGF) inhibitors (Table 2), PARPi (Table 3), or with different ICIs (Table 4) [12,13]. Several factors can influence ICI efficacy. These include intrinsic factors such as quantity and activation of immune subsets within the tumour microenvironment (TME), the presence or absence of immune checkpoints, as well as treatment regimens such as surgery and chemotherapy. Herein we discuss the evidence in relation to these factors and the use of ICIs in OC, with a specific focus on HGSOC.
Table 1.
Clinical trials investigating immune checkpoint inhibitor monotherapy for ovarian cancer.
| Trial Name | Phase | Disease stage | Drug | Status | Results | Ref |
|---|---|---|---|---|---|---|
| JAVELIN (NCT01772004) | Ib | recurrent or refractory OC | avelumab (anti-PD-L1) | completed with results | Demonstrated antitumor activity and acceptable safety in heavily pre-treated patients with recurrent or refractory ovarian cancer | [12] |
| KEYNOTE-028 (NCT02054806) | I | PD-L1-positive advanced OC | pembrolizumab (anti-PD-1) | completed | Pembrolizumab conferred durable antitumor activity with manageable safety and toxicity in patients with advanced PD-L1-positive ovarian cancer | [13] |
| KEYNOTE-100 (NCT02674061) | II | advanced recurrent ovarian cancer | pembrolizumab (anti-PD-1) | completed with results | Single agent pembrolizumab showed modest activity in patients with ROC. Higher PD-L1 expression was correlated with higher response | [14] |
Table 2.
Clinical trials investigating immune checkpoint inhibitor therapy in combination with chemotherapy and/or VEGF inhibitors for ovarian cancer.
| Trial Name | Phase | Disease stage | Drug | Combination | Status | Results | Ref |
|---|---|---|---|---|---|---|---|
| JAVELIN-200 (NCT02580058) | III | platinum-resistant or platinum-refractory ovarian cancer | avelumab (anti-PD-L1) | pegylated liposomal doxorubicin (PLD) | completed with results | Neither avelumab plus PLD nor avelumab alone significantly improved progression-free survival or overall survival versus PLD | [15] |
| IMagyn500 (NCT03038100) | III | newly diagnosed stage III or IV ovarian cancer | atezolizumab (anti-PD-L1) | paclitaxel, carboplatin and bevacizumab | completed | Current evidence does not support the use of immune checkpoint inhibitors in newly diagnosed OC. | [16] |
| NRG-GY009 (NCT02839707) | II/III | recurrent ovarian, fallopian tube, or primary peritoneal cancer | atezolizumab (anti-PD-L1) | pegylated liposomal doxorubicin and/or bevacizumab |
active, not recruiting | N/A | |
| IMagyn050/GOG 3015/ENGOT-OV39 (NCT03038100) | III | newly diagnosed stage III/IV ovarian cancer | atezolizumab (PD-L1) | platinum-based chemotherapy and bevacizumab | completed | Incorporation of atezolizumab into standard therapy for newly diagnosed ovarian cancer does not significantly improve efficacy or impose additional treatment burden for patients. | [17] |
| PemCiGem (NCT02608684) | II | recurrent platinum-resistant ovarian cancer | pembrolizumab (anti-PD-1) | cisplatin and gemcitabine | completed with results | The addition of pembrolizumab to cisplatin and gemcitabine did not appear to provide benefit beyond chemotherapy alone in patients with recurrent platinum-resistant ovarian cancer. | [18] |
| NCT02873962 | II | relapsed ovarian, fallopian tube or peritoneal cancer | nivolumab (anti-PD-1) | bevacizumab | active, not recruiting | Nivolumab plus bevacizumab appeared to show activity in patients with relapsed ovarian cancer, with greater activity in the platinum-sensitive setting. | [19] |
| NCT03353831 | III | recurrent ovarian-, fallopian tube, or primary peritoneal cancer | atezolizumab (anti-PD-L1) | bevacizumab chemotherapy |
active, not recruiting | N/A | |
| NCT02431559 | I and II | recurrent, platinum-resistant ovarian cancer | durvalumab (anti-PD-L1) | pegylated liposomal doxorubicin motolimod (small-molecule TLR8 agonist) |
completed with results | The combination of durvalumab and pegylated liposomal doxorubicin in women with platinum-resistant recurrent ovarian cancer appears to have a tolerable safety profile and promising efficacy. | [20] |
| TRU-D (KGOG3046) (NCT03899610) |
II | advanced-stage ovarian cancer | durvalumab (anti-PD-L1) tremelimumab (anti-CTLA-4) | neoadjuvant chemotherapy | unknown | N/A | |
| NRG GY003 (NCT02498600) | II | persistent or recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer | nivolumab (anti-PD-1) | ipilimumab (anti-CTLA-4) | active, not recruiting | Compared with nivolumab alone, the combination of nivolumab and ipilimumab in epithelial OC resulted in superior response rate and longer, albeit limited, PFS, with toxicity of the combination regimen comparable to prior reports. | [21] |
| ATALANTE (NCT02891824) | III | late relapse ovarian cancer | atezolizumab (anti-PD-L1) | platinum-based chemotherapy bevacizumab | active, not recruiting | N/A |
Table 3.
Clinical trials investigating immune checkpoint inhibitor therapy in combination with PARP inhibitors for ovarian cancer.
| Trial Name | Phase | Disease stage | Drug | Combination | Status | Results | Ref |
|---|---|---|---|---|---|---|---|
| TOPACIO/KEYNOTE-162 (NCT02657889) | I and II | advanced or metastatic TNBC or recurrent OC | pembrolizumab (anti-PD-1) | niraparib | completed with results | Tolerable, with promising antitumor activity for patients who have limited treatment options regardless of platinum, biomarker status, or prior treatment with bevacizumab. | [22] |
| DUO-O (NCT03737643) | III | advanced ovarian cancer | durvalumab (anti-PD-L1) | chemotherapy and bevacizumab followed by maintenance durvalumab, bevacizumab and olaparib |
recruiting | Statistically significant and clinically meaningful improvement in Progression Free Survival vs paclitaxel/carboplatin + bevacizumab followed by maintenance bevacizumab | [23] |
| NCT02484404 | I and II | advanced or recurrent ovarian cancer | durvalumab (anti-PD-L1) | olaparib and/or cediranib | recruiting | Treatment was tolerable and active in OC and patients without germline BRCA mutation. Phase II expansion studies are now open to accrual. | [24] |
| NCT02873962 | II | relapsed ovarian, fallopian tube or peritoneal cancer | nivolumab (anti-PD-1) | bevacizumab or bevacizumab and rucaparib |
recruiting | Nivolumab/bevacizumab demonstrated clinical activity in women with recurrent OC, with an overall confirmed response rate of 21 % and a median PFS of 9.4 months. | [25] |
| NCT02571725 | I and II | BRCA1 and BRCA2 Mutation Carriers with Recurrent Ovarian Cancer | tremelimumab (anti-CTLA-4) | olaparib | active, not recruiting | The combination is tolerable in heavily pre-treated women with recurrent BRCA-associated OC. Preliminary results demonstrate evidence of therapeutic effect, supporting ongoing evaluation of this regimen in Phase II trials. | [26] |
| OPAL (NCT03574779) | I and II | newly diagnosed and recurrent ovarian cancer | TSR-042 (anti-PD-1) | niraparib bevacizumab carboplatin paclitaxel |
recruiting | Combination therapy with niraparib, dostarlimab, and bevacizumab is tolerable and demonstrated clinical activity in patients with platinum-resistant ovarian cancer, most of which were BRCA or Homologous Recombination Repair wild type. | [27] |
| ATHENA (NCT03522246) | III | newly diagnosed ovarian cancer patients | nivolumab (anti-PD-1) | rucaparib | active, not recruiting | Rucaparib monotherapy is effective as first-line maintenance, conferring significant benefit versus placebo in patients with advanced ovarian cancer with and without HRD. | [28] |
Table 4.
Emerging Immune Checkpoint Inhibitor clinical trials under investigation for use in ovarian cancer.
| Trial Name | Phase | Disease stage | Drug | Combination | Status | Results | Ref |
|---|---|---|---|---|---|---|---|
| NCT02465060 | II | patients with advanced refractory solid tumours, lymphomas, or multiple myeloma (inc. ovarian cancer) | nivolumab (anti-PD-1) relatlimab (anti-LAG-3) included in a list of 40 other drugs | active, not recruiting | There was an overall response rate of 16 % with copanlisib showing clinical activity in select tumours with PIK3CA mutation in the refractory setting. | [29] | |
| NCT03219268 | I | advanced solid tumours (inc. ovarian cancer) | tebotelimab (bi-specific LAG-3 and PD-1) | margetuximab (in HER2+ advanced solid tumours) | completed | Tebotelimab is safe as a monotherapy and in combination with margetuximab. Antitumor activity was observed tumours not typically responsive to anti–PD-1. | [30] |
| DUET-4 (NCT03849469) | I | advanced solid tumours (inc. ovarian cancer) | XmAb22841 (bi-specific LAG-3 and CTLA-4) | pembrolizumab (anti-PD-1) | completed | No results posted yet | |
| CITRINO (NCT03250832) | I | advanced solid tumours in a broad range of solid tumours | TSR-033 (anti-LAG-3) | dostarlimab (anti-PD-1) mFOLFOX6 mFOLFOX6 bevacizumab |
completed | No results posted yet | |
| NCT03538028 | I | aelect advanced malignancies (inc. ovarian cancer) | INCAGN02385 (anti-LAG-3) | N/A | completed | INCAGN02385 monotherapy was generally well tolerated. Phase 1b/2 studies are underway in melanoma and head and neck cancer to assess response in combinations with other immunotherapies (NCT04370704, NCT05287113) | [31] |
| NCT03652077 | I | select advanced malignancies (inc. ovarian cancer) | INCAGN02390 (anti-TIM-3) | N/A | completed | Anti-TIM-3 monotherapy was well tolerated | [32] |
| NCT03365791 | II | Patients with advanced solid and hematologic malignancies (inc. ovarian cancer) | spartalizumab (anti-PD-1) | lieramilimab (anti-LAG-3) | completed | Spartalizumab and LAG525 showed promising activity in neuroendocrine tumours, small cell lung cancer and diffuse large B-cell lymphoma. The Gastroesophageal cohort was declared futile. Remaining cohorts are paused pending further analysis | [33] |
| NCT04354246 | I | advanced malignancies (inc. ovarian cancer) | COM902 (anti-TIGIT) | COM701 (anti-PVRIG) | recruiting | COM902 has an acceptable safety, tolerability, and PK profiles. | [34] |
| NCT04254107 | I | advanced cancer | SEA-TGT (anti-TIGIT) | sasanlimab (anti-PD-1) brentuximab vedotin (anti-CD30) | active, not recruiting | N/A | |
| NCT04570839 | I and II | advanced solid tumours (inc. ovarian cancer) | COM701 (anti-PVRIG) | BMS-986207 (anti-TIGIT) nivolumab (anti-PD-1) | active, not recruiting | This combination demonstrates a favourable safety, tolerability, and PK profiles. | [35] |
| NCT03667716 | I | advanced solid tumours (inc. ovarian cancer) | COM701 (anti-PVRIG) | nivolumab (anti-PD-1) | active, not recruiting | COM701 with or without nivolumab is well tolerated with no new safety signals. Encouraging signal of antitumor activity including in pts with prior treatment with ICI or prior treatment refractory disease. | [36] |
| NCT05746897 | I | advanced solid tumours (inc. ovarian cancer) | NM1F (anti-PVRIG) | pembrolizumab (anti-PD-1) | recruiting | N/A |
2. CTLA-4 and PD-1 in HGSOC
Numerous ICIs against the most widely studied immune checkpoints, CTLA-4 and PD-1, have proven beneficial in a number of solid tumours. However, this success has not been seen in OC where ICI use is only approved for patients with high microsatellite instability (MSI), which is a feature of ovarian clear cell carcinoma but is rare in HGSOC [57].
2.1. CTLA-4
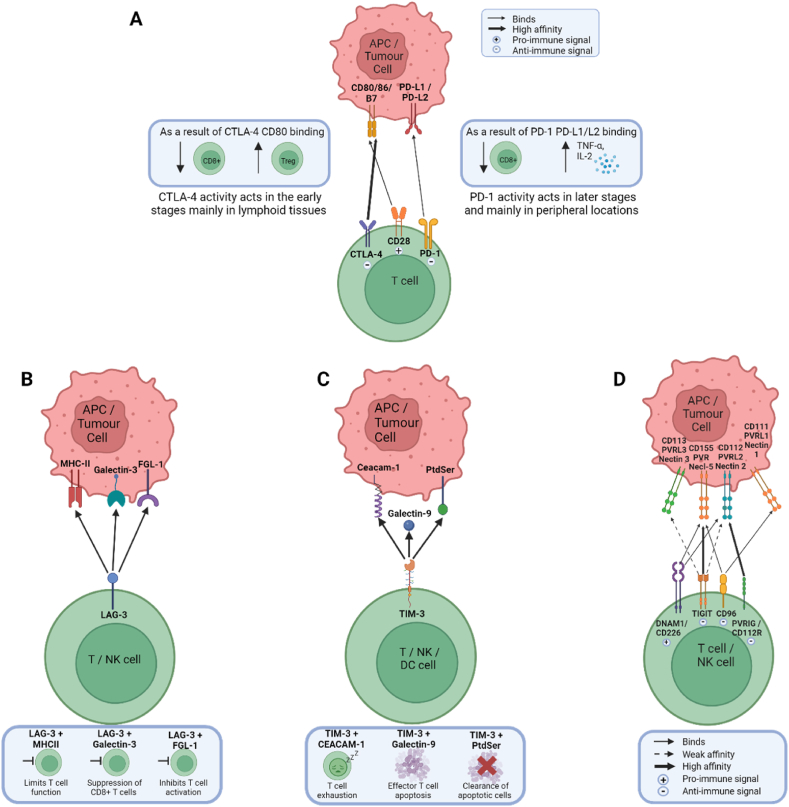
In 1996, Cytotoxic T lymphocyte antigen 4 (CTLA-4) was reported as a potential target for cancer treatment when Leach et al. described how colon tumours were rejected in mice following in vivo administration of antibodies targeting CTLA-4, resulting in immunity to subsequent cancer cell exposure [58]. CTLA-4 regulates T cells in the lymph nodes during the early stages of an immune response (Fig. 2A) [59].
Fig. 2.
Immune checkpoint receptors and binding ligands, and their immunologic effect. A) The B7-CD28 family: The binding interactions of three of the receptors in this family; CTLA-4, CD28 and PD-1 to the ligands CD80, CD86, PD-L1 and PD-L2. B) LAG3 receptor binding ligands and their immunologic effect. C) TIM-3 receptor binding ligands and their immunological effect. D) TIGIT family receptors: The binding interactions and affinity between receptors and their corresponding ligands.
There are limited data available for CTLA-4 in OC/HGSOC, but strong expression has been seen in residual HGSOC tumours after cytoreductive surgery, suggesting that CTLA-4 blockade could be beneficial post-surgery [60]. There are also very few clinical trials investigating the use of anti-CTLA-4 ICIs in this disease (Table 3, Table 4). One such trial found that the infusion of a CTLA-4 blocking antibody reduced or stabilised CA-125 levels in two metastatic OC patients, suggesting that this blockade improved disease status [61]. Most research has concentrated on combination treatments, incorporating anti-CTLA-4 and anti-PD-1 ICIs, as this combination has had success in other cancers.
2.2. PD-1
In 1996, Programmed cell death 1 (PD-1) was found to be involved in inhibiting immune responses [62]. PD-1 is an immunoinhibitory receptor, which binds to two ligands, PD-1 ligand 1 (PD-L1) and 2 (PD-L2) [63,64] (Fig.2A). PD-1 regulates T cells in the later stages of an immune response at the tissue site [59]. PD-1 inhibitors, nivolumab and pembrolizumab, were approved in 2014 for patients with unresectable or metastatic melanoma (Fig.1) [65].
In contrast to CTLA-4, there has been extensive research into the PD-1 immune checkpoint pathway in OC with studies indicating that the expression of PD-L1, and to a lesser extent PD-L2, is associated with a poorer prognosis [64]. A significant inverse correlation between PD-L1 expression and CD8+ TILs has been observed, suggesting that PD-L1 could suppress CD8+ TILs [64]. HGSOC tumours with positive PD-1 expression, showed significantly higher numbers of stromal CD3+ TILs and intraepithelial CD8+ TILs compared to PD-1 negative tumours, however PD-1 was not associated with survival [66]. HGSOC tumours with a BRCA1/2 mutation have higher expression of PD-1 and PD-L1 compared to BRCA wildtype, suggesting that these patients may respond better to PD-1/PD-L1 blockade [67]. The blockade of this pathway with a bispecific antibody targeting both PD-1 and PD-L1 caused both Natural Killer (NK) cells and a subset of T cells to shift to a more active cytotoxic state, suggesting that a dual blockade could improve efficacy in the PD-1 immune checkpoint pathway [68].
Most clinical trials investigating the use of ICIs in OC have focused on the PD-1 pathway but despite initial results indicating a tolerable safety profile for PD-1 or PD-L1 monotherapy (Table 1), response rates were modest for multiple trials with progression-free survival ranging from 1.9 to 3.5 months [[12], [13], [14]]. This was not improved when combined with various chemotherapies or VEGF inhibitors (Table 2) [15,16].
3. The tumour microenvironment (TME) and external factors that can impact immune checkpoint inhibitor efficacy
The efficacy of ICIs is impacted by the TME. A ‘cold’ TME has lower numbers of tumour infiltrating lymphocytes (TILs) and a poorer response to immune checkpoint blockade, as there is no immune response to reinvigorate. By contrast, a ‘hot’ TME has a higher number of TILs and can thus benefit from ICIs, which can reinvigorate the immune response, and which in turn is associated with improved clinical outcome, survival, prognosis and therapeutic efficacy [[37], [38], [39], [40], [41], [42], [43]]. OC is generally considered to have a cold TME and HGSOC is a complex and heterogeneous disease where multiple distinct TMEs can co-exist between different tumours within the one patient making its treatment challenging [[37], [38], [39], [40], [41], [42], [43], [44]].
While the presence of TILs is a good prognostic indicator, the type of TIL is also important. For example, increased infiltration of cytotoxic CD8+ T cells and decreased CD4+ regulatory T cells indicates a more favourable prognosis and improved therapeutic efficacy [43]. The presence of CD103 is a common feature on TILs in the epithelium of primary OC, and CD103+ TILs are abundantly present in HGSOC [45]. Tumour infiltration by these TILs is associated with better survival, so much so that patients with CD8+ TILs negative for CD103 have a prognosis similar to that of patients completely lacking CD8+ TILs [45]. CD103+ TILs mostly consist of activated, cytolytic CD8+ T cells, which suggests that CD103 could be a biomarker for highly activated cytolytic TILs [45]. PD-1 expression on this TIL subset could also indicate functional exhaustion due to chronic stimulation [45]. Immune cell populations in metastatic HGSOCs have higher immune infiltration (CD8+ T cells, CD20+ B cells, and NKp46+ NK cells), compared to matched primary samples. However these cells are functionally impaired by high levels of immunosuppressive M2-like tumour associated macrophages (TAMs), which limits a clinically relevant immune response [46].
Surgery remains one of the most important treatment options for HGSOC patients [47]. However, surgery may also impact the immune response, creating a pro-tumour environment as the chemokines and cytokines released to promote surgical wound healing also promote tumour growth, invasion and angiogenesis [54]. A small number of studies have investigated the impact of cytoreductive surgery on the immune response in HGSOC patients. A post-surgical increase in circulatory anti-inflammatory cytokines (interleukin-6 and 10) and a decrease in the number and function of NK cells, indicates a dampening down of the immune response post-surgery [55]. The ICONIC clinical trial (NCT03959761) combined surgery with hyperthermic intraperitoneal chemotherapy (HIPEC) and an intraperitoneal infusion of the PD-1 ICI nivolumab, to investigate a synergistic effect through an increase in tumour-antigen expression and mutational load. Phase I trial results appear promising, indicating that intraperitoneal nivolumab is feasible and well tolerated [56].
4. Emerging immune checkpoint inhibitors in HGSOC
Innate or acquired resistance in patients receiving anti-PD1 or CTLA-4 treatments, combined with modest results, has fuelled research into other immune checkpoints such as LAG-3, TIM-3, TIGIT, and PVRIG [69]. The number of phase I clinical trials investigating the use of these emerging ICIs are limited (Table 4), however preliminary results indicate tolerability, and activity has been observed in tumours that typically do not respond to anti-PD-1 therapy [30,83].
4.1. LAG-3
Lymphocyte activating gene 3 (LAG-3) is an inhibitory receptor with a similar structure to CD4 and has an affinity for binding with MHC-II, galectin-3 and fibrinogen-like protein-1 (Fig. 2B) [69]. In 2022, the FDA approved opdualag, a relatlimab (anti-LAG-3)/nivolumab (anti-PD-1) combination drug for the treatment of unresectable or metastatic melanoma [70].The success of this dual blockade, which shows efficacy similar to anti-PD-1/anti-CTLA-4 but with lower adverse events led to a number of studies investigating its use in OC [71]. Dual blockade of LAG-3 and PD-1 in murine OC models increased the anti-tumour immune response, evidenced by an increase in the number of CD8+ TILs and suppression of regulatory T cells in the TME [72]. Single blockade of PD-1 caused an increase in the level of LAG-3 and CTLA-4, and similarly when LAG-3 was blocked, use of the PD-1 pathway increased [73]. This highlights the potential tumours have to adapt to single blockades using compensatory immune checkpoint pathways, supporting a dual blockade approach. While PD-L1 and LAG-3 expression is reportedly low in most HGSOC, their expression is positively correlated [74]. LAG-3 and PD-1 expressing CD8+ T cells exhibit less IFN-γ/TNF-α production compared to CD8+ TILs expressing only LAG-3 or neither [75]. Dual blockade of LAG-3 and PD-1 restored the frequency and effect of CD8+ TILs that specifically target the immunogenic tumour antigen NY-ESO-1 [75].
4.2. TIM-3
T-cell immunoglobulin and mucin domain 3 (TIM-3) has been shown to be expressed on the surface of T cells, NK cells, B cells, Dendritic Cells (DCs), macrophages and monocytes, and binds to the ligands galectin-9, carcinoembryonic antigen cell adhesion molecule 1 (Ceacam-1), and the phosphatidyl serine (PtdSer) (Fig. 2C) [76].
TIM-3 is actually the most abundant immune checkpoint in epithelial OC, with more than 75 % of cases being TIM-3 positive [50]. TIM-3 is expressed intratumorally at high levels on CD8+ and CD4+ T cells at both the gene and protein level [89]. In HGSOC tumours, immunosuppression was found to be dictated by TIM-3 with one third of CD8+ TILs in this cohort expressing TIM-3, which was almost exclusively expressed together with PD-1 [77]. Cells positive for TIM-3 and PD-1 are characteristically exhausted, distinguished by a state of T cell dysfunction, low levels of cytokine production, inability to kill and hypo-proliferation compared to cells positive for other immune checkpoints such as CTLA-4 and LAG-3. These checkpoints did not indicate T-cell exhaustion, rather a continuing immune response dependent on IFN-γ with positive outcomes. Furthermore CD8+ TILs with high TIM-3 expression are sensitive to TIM-3 blockade in combination with PD-1 but not CTLA-4 blockade, whereas low TIM-3 expressing cells are not sensitive [77]. These findings provide support for the use of TIM-3 blockade in HGSOC.
4.3. TIGIT family
The TIGIT family of receptors, also known as the nectin and nectin-like family, consists of four receptors; CD226 (DNAM-1), TIGIT, CD96 (TACTILE), PVRIG (CD112R) and five ligands; CD155 (PVR, Necl5), CD111 (Nectin-1, PVRL1), CD112 (Nectin-2, PVRL2), CD113 (Nectin-3, PVRL3) and Nectin-4 (PVRL4) (Fig.2D) [78]. This family of proteins represent a co-stimulatory axis that could be therapeutically targeted for the treatment of cancer. TIGIT (T-cell immunoglobulin and ITIM domain) is the best characterised of the TIGIT family of receptors [78]. TIGIT binds to PVR, PVRL2 and PVRL3 and counteracts positive co-stimulatory signalling [78]. TIGIT indirectly inhibits T cell activity by manipulating DC activity [79]. Both TIGIT and PD-1 limit CD8+ T cell responses through distinct mechanisms, and therefore an anti-TIGIT/anti-PD-1 dual blockade has been suggested as a potential therapeutic showing promising results in colon and breast cancer mouse models, where only a co-blockade of TIGIT and PD-L1, and neither alone, resulted in tumour rejection [80].
In OC, TIGIT shows elevated expression on CD4+ regulatory T cells compared to effector CD4+ and CD8+ T cells, and NK cells. An anti-TIGIT blockade reduces the number of regulatory T cells but effector CD4+ and CD8+ T cells and NK cells are not affected. This reduction in CD4+ regulatory T cells resulted in a lowering of immunosuppression [81]. When specifically looking at HGSOC, TIGIT levels are enhanced in recurrent tumours compared to matched primary samples [82].
Poliovirus receptor-related immunoglobulin domain-containing (PVRIG), also known as CD112R is a lesser-characterised member of the TIGIT family receptors (Fig. 2D) [78]. PVRIG expression has been examined on TILs for a range of different cancer types and it was found that OC had among the highest expression of PVRIG on CD4+ and CD8+ T cells in blood and tissues [78]. Furthermore its ligand, PVRL2, also had the highest expression in OC samples on CD14+ (monocytes and tumour-associated macrophages), and CD45− cells (tumour epithelial and other non-immune cells), and when compared to normal tissue, PVRL2 expression is higher in a number of cancer types, including OC [78]. OC tissue also showed the highest percentage of PVR−PVRL2+ cells and had the highest ratio of PVRL2 to PVR. This indicates that the PVRIG-PVRL2 pathway, rather than the TIGIT-PVR pathway, plays a more important role in regulating the immune response in OC [78].
5. Combination therapy
Clinical trials evaluating ICIs as a monotherapy for OC have yielded disappointing outcomes (Table 1), which is why research is focused on combining ICIs with other treatment options. However, the combination of ICIs with chemotherapy and/or VEGF inhibitors has also yielded disappointing results, with trials concluding that the addition of ICIs did not provide any additional benefit compared to chemotherapy alone (Table 2). For this reason, studies have progressed to combining multiple ICIs together or ICIs with other treatment options such as PARPi.
5.1. Chemotherapy
Standard of care for OC involves cytoreductive surgery to illicit local disease control and systemic chemotherapy for the treatment of metastatic disease [47]. The timing of cytoreductive surgery in relation to systemic chemotherapy is still debated with most patients undergoing surgery first, followed by chemotherapy [47]. However, neoadjuvant chemotherapy followed by surgery is becoming more widely accepted and is offered to patients if they have disease that is unlikely to be optimally cytoreduced or if they present at an advanced age or with comorbidities [47]. The introduction of immunotherapies stands to make this an even more complex topic. Evidence suggests that the timing and dose of chemotherapy can impact the immune response and increase the effectiveness of immunotherapies [48].
The standard first-line chemotherapy for OC consists of 3-weekly platinum and paclitaxel doses [47,49]. Carboplatin is the preferred platinum-based agent, as cisplatin is associated with higher levels of toxicity [47]. Docetaxel or pegylated liposomal doxorubicin can be used as alternatives to paclitaxel in the combination regimen for those patients who do not tolerate or develop an allergy to paclitaxel [49]. For patients who become platinum-resistant or refractory, paclitaxel, topotecan, pegylated liposomal doxorubicin and gemcitabine can be used to manage symptoms [49].
Chemotherapy can potentially convert a ‘cold’ TME to a ‘hot’ TME, more favourable for ICI treatment. Following neoadjuvant chemotherapy, immune checkpoint expression (IDO, PD-L1, LAG3, TIM3) was impacted in over 70 % of epithelial OC samples, where expression either increased or decreased [50]. HGSOC tumours are heterogeneous; prior to chemotherapy both ‘hot’ and ‘cold’ TMEs can often coexist within the same patient and even within the same tumour [44]. Neoadjuvant chemotherapy has been found to increase the number of NK and T cells, indicating that chemotherapy increases the immune response and could improve response rates to subsequent ICIs [44]. Indeed, neoadjuvant chemotherapy (carboplatin and taxane) increased TIL infiltration and PD-L1 levels in the TME of HGSOC tumour samples, specifically on macrophages [51,52]. An increase in the ratio of effector to regulatory T cells, with retention of immune checkpoints PD-1 and LAG3, and increased T-cell activation has also been observed in HGSOC omental biopsies [52]. However, the significant increase of immune checkpoints PD-1, PD-L1 and CTLA-4 on T cells could dampen the immune response, or perhaps provides an opportunity for sequential treatment with chemotherapy and ICIs [53].
5.2. Immune checkpoint inhibitor combinations
The rationale for ICI combination therapies comes from the findings that more than 50 % of epithelial OC tumours are positive for two or more immune checkpoints [50]. As CTLA-4 typically operates in the early stages of an immune response, with PD-1 operating in later stages, a dual blockade of these non-redundant pathways could improve patient response. This concept was proven in preclinical OC mouse models, in which tumours were rejected when both PD-1 and CTLA-4 were blocked, leading to a reversal in CD8+ T cell dysfunction [84]. To date, two clinical trials have investigated the use of this dual blockade in OC. The NRG GY003 trial concluded that response rates were higher in epithelial OC patients (subtypes not specified) treated with a combination of nivolumab and ipilimumab, compared to nivolumab alone [21]. The objective tumour response occurred within 6 months for 31.4 % of patients on the combination treatment and was significantly improved compared to only 12.2 % of patients on nivolumab alone. A longer, albeit limited, progression-free survival (PFS) of 3.9 months was observed for the combination treatment compared to 2 months for nivolumab alone. Although there were significant improvements with the combination treatment, there was still a lack of benefit for most patients. The KGOG 3046 trial investigated the use of neoadjuvant chemotherapy in combination with anti-PD-1 (durvalumab) and anti-CTLA-4 (tremelimumab) in the treatment of newly diagnosed advanced-stage HGSOC. Promising results were indicated with this treatment regimen with 12-month, 24-month, and 30 month PFS rates of 63.6 %, 45.0 %, and 40.0 %, respectively [85]. However, this study was limited by a small sample size of only 23 patients, and lack of a control arm, instead comparing to a historical control. The small number of clinical trials investigating this combination in OC and the fact that 55 % of patients experience adverse events of grade 3 or 4 indicates that this may not be a promising treatment approach [86]. However, results from NRG GY003 and KGOG 3046 indicate that additional studies with further combinations are warranted to enhance durability of the dual regimen.
PVRIG-PVRL2 and TIGIT-PVR represent another two non-redundant T cell inhibitory checkpoints [78]. Blockade of both PVRIG and PVR together resulted in higher production of IFN-γ when compared to the co-blockade of PVRIG and PVRL2 [78]. This indicates the potential therapeutic strategy of a co-blockade of both TIGIT and PVRIG. This is further supported by experiments showing minimal single agent activity in OC TILs following blockade with anti-TIGIT, anti-PVRIG or anti-PD-1. There are a limited number of clinical trials investigating the combination of anti-TIGIT and anti-PVRIG blockade for advanced cancers including OC (Table 4).
5.3. Immune checkpoint inhibitors combined with PARPi
PARPi have been a breakthrough treatment for OC and are another potential option for combination treatment with ICIs, especially considering that there are already three PARPi approved for use, and BRCA mutational status may be a reliable biomarker for response to ICIs (Fig. 1) [10]. There are a number of trials ongoing investigating this combination (Table 3), with initial results indicating tolerability, promising anti-tumour activity, and even better than expected responses in patients without a BRCA mutation [22]. It is noteworthy that specific signatures have been identified as determinants of response to the combination treatment of ICI and PARPi [87]. These include a defective homologous recombination repair pathway and the presence of IFN-primed exhausted CD8+ T cells [87]. The presence of either feature alone, or in combination, has been associated with improved outcome, while no response was seen if both were absent [87].
6. Predictive biomarkers of ICI response
It may be that ICIs are not effective for all OC patients, instead a specific TME may indicate whether a patient will respond. The FDA and EMA have approved the use of MSI, PD-L1 expression, as well as tumour mutational burden (TMB), as tissue biomarkers for predicting response to ICIs [88,89].
6.1. Microsatellite instability (MSI)
In 2017, pembrolizumab was approved by the FDA for all advanced cancer patients with high MSI where no other treatment is available, making this the first tissue-agnostic approval for any cancer treatment [90]. This paved the way for tumour type-agnostic therapy, where treatments could be approved based on biomarker analysis rather than tumour site [90]. MSI is typically seen in only a small subset of OC patients [91].
6.2. PD-L1 expression
Currently the FDA and EMA have approved four assays for measuring the expression of PD-L1 (PD-L1 IHC 22C3 pharmaDx, PD-L1 IHC 28–8 pharmaDx assay, PD-L1 IHC SP 142, PD-L1 IHC SP263) for use in non-small cell lung cancer, urothelial cancer, head and neck cancer, oesophageal cancer and triple-negative breast cancer [88,92]. However, this biomarker is limited because its predictive value is impacted by cell type, tumour heterogeneity, and whether the assessment has been performed on the primary or metastatic site [63]. Improved survival has also been seen in patients with low or negative PD-L1 expression [93]. The assays use different antibodies, scoring systems and thresholds, resulting in practical challenges for incorporating these tests clinically [88]. The development of the Combined Positive Score (PD-L1 scoring method) has tried to overcome some of these limitations, resulting in greater correspondence between PD-L1 presence and effective treatment response [94].
6.3. Tumour mutational burden (TMB)
The KEYNOTE-158 study employed the FoundationOne CDx assay, a targeted cancer gene panel to detect TMB, and found that in patients with advanced solid tumours, a subset with high TMB had a durable response to pembrolizumab monotherapy [57]. TMB alone was found to be predictive of response regardless of MSI, PD-L1 expression or tumour type. The FoundationOne CDx assay was approved alongside pembrolizumab as a companion diagnostic for patients with unresectable or metastatic solid tumours with high TMB, defined as ≥10 mutations/megabase [57]. A further study, which investigated the value of TMB in 12 trials using pembrolizumab as a monotherapy identified a clinically meaningful improvement in efficacy of pembrolizumab monotherapy with a TMB ≥175 mutations/exome [95]. In this study, OC was found in general to have a low TMB, which may explain why PD-1 monotherapy has not been beneficial for many HGSOC patients.
6.4. BRCA mutation
A biomarker more relevant for HGSOC is the presence of a BCRA mutation. HGSOC patients with a BRCA1/2 mutation have significantly increased expression of PD-1 and PD-L1 as well as significantly increased numbers of CD3+ and CD8+ TILs [67]. These patients may respond better to PD-1/PD-L1 ICIs compared to patients without a mutation due to the higher number of tumour-specific neoantigens associated with higher expression of the pro-inflammatory cytokine IFN-γ [67]. BRCA status and number of TILs have been identified as independent indicators of prognosis with two distinct groups of patients; BRCA mutated with high number of TILs had good prognosis, while patients without a BRCA mutation and low number of TILs had very poor prognosis [67].
6.5. Next generation biomarkers
The next generation of biomarkers for predicting response to ICIs has recently been reviewed [92]. The Immunoscore assesses the density and location of cytotoxic and memory TILs and has shown prognostic ability in colorectal cancer and is now being investigated for use in other cancer types. Tumour Gene Expression Profiles assess immunologic transcriptomic patterns and has been shown to correlate with improved survival across nine cancer types [96]. Multiplex immunohistochemistry/immunofluorescence has shown improved accuracy at predicting response to anti-PD-1/PD-L1 blockade [97]. HLA testing looks at the presence and functionality of the antigen-presenting machinery, and has shown to be a reliable biomarker of response to anti-CTLA4 [98]. Peripheral blood biomarkers are an attractive approach since they are less invasive. Cellular peripheral blood biomarkers, such as a rise in Ki-67+ PD1+ CD8+ T cells, have correlated with clinical benefit in non-small lung cancer patients receiving anti-PD-1. Soluble peripheral blood biomarkers, such as circulating tumour DNA, has been associated with response and improved survival in non-small cell lung cancer patients [99,100]. Further work is needed to assess the functionality and potential benefit these biomarkers could provide for HGSOC.
7. Conclusions
ICI treatment has dominated the field of immunotherapy for solid malignancies and has improved patient outcomes. However, this effect is yet to be reported for HGSOC. The heterogeneity observed, not just between patients but between metastatic tumours from the same patient and even within the same tumour could impact ICI efficacy in this cancer. There are multiple factors at play when it comes to ICI efficacy, including both intrinsic and external components, but by understanding the TME, the impact of chemotherapy, and surgery on the immune response we can begin to overcome ineffective treatments. The development of biomarkers has resulted in the approval of companion diagnostics for certain ICIs in some cancer types but so far these have not proven beneficial for HGSOC patients, but the next generation of biomarkers could change this. Lastly there has been extensive work investigating the use of ICIs against PD-1 and PD-L1, with little success so far, but it may be that success could come from investigating new emerging checkpoints such as LAG-3, TIM-3, PVRIG, and TIGIT and even combining ICIs against a number of these pathways. Additionally, investigations into combinational therapy regimes with PARPi could strengthen responses by simultaneously targeting multiple hallmarks of cancer.
CRediT authorship contribution statement
A.E. Connor: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. P.M. Lyons: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft. A.M. Kilgallon: Supervision. J.C. Simpson: Funding acquisition, Supervision, Writing – review & editing. A.S. Perry: Funding acquisition, Supervision, Visualization, Writing – original draft, Writing – review & editing. J. Lysaght: Supervision, Visualization, Writing – original draft, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Irish Cancer Society [CRS20CON]. This funding body had no role in the design of the study or collection, analysis, and interpretation of data or in writing the manuscript. This work was also supported through the M.Sc in Translational Oncology, Trinity College Dublin.
Abbreviations:
- DC
Dendritic cell
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- HGSOC
High-grade serous ovarian cancer
- HIPEC
Hyperthermic intraperitoneal chemotherapy
- ICIs
Immune checkpoint inhibitors
- IHC
Immunohistochemistry
- MSI
Microsatellite instability
- NICE
National Institute for Health and Care
- NK
Natural killer cell
- OC
Ovarian cancer
- OS
Overall survival
- PARPi
PARP inhibitors
- PFS
Progression free survival
- TILs
Tumour infiltrating lymphocytes
- TMB
Tumour mutational burden
- TME
Tumour microenvironment
- VEGF
Vascular endothelial growth factor
References
- 1.Siegel R.L., Giaquinto A.N., Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 2.CANCER TOMORROW[Internet]. 2021 [cited 01-11-2021]. Available from: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?sexes=2&single_unit=10000&cancers=25.
- 3.Labidi-Galy S.I., Papp E., Hallberg D., Niknafs N., Adleff V., Noe M., et al. High grade serous ovarian carcinomas originate in the fallopian tube. Nat. Commun. 2017;8(1):1093. doi: 10.1038/s41467-017-00962-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charkhchi P., Cybulski C., Gronwald J., Wong F.O., Narod S.A., Akbari M.R. CA125 and ovarian cancer: a comprehensive review. Cancers. 2020;12(12) doi: 10.3390/cancers12123730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ovary SEER 5-Year Relative Survival Rates. 2013 https://seer.cancer.gov/statistics-network/explorer/application.html?site=61&data_type=4&graph_type=5&compareBy=race&chk_race_1=1&chk_race_6=6&chk_race_5=5&chk_race_4=4&chk_race_9=9&chk_race_8=8&series=9&hdn_sex=3&age_range=1&stage=104&advopt_precision=1&advopt_show_ci=on&hdn_view=0 2019 [Internet]. 2023 [cited 16/11/2023]. Available from: [Google Scholar]
- 6.Foo T., George A., Banerjee S. PARP inhibitors in ovarian cancer: an overview of the practice-changing trials. Genes Chromosomes Cancer. 2021;60(5):385–397. doi: 10.1002/gcc.22935. [DOI] [PubMed] [Google Scholar]
- 7.European Commission approves Zejula (niraparib) as first-line monotherapy maintenance treatment in advanced ovarian cancer. 2020 [press release] [Google Scholar]
- 8.Rucaparib Receives EC Approval for Advanced Ovarian Cancer. 2023 [press release] [Google Scholar]
- 9.Zejula (Niraparib . 2020. First PARP Inhibitor Approved for First-Line Maintenance Therapy in All Women with Advanced Ovarian Cancer, Regardless of Biomarker Status. [press release] [Google Scholar]
- 10.Smith M., Pothuri B. Appropriate selection of PARP inhibitors in ovarian cancer. Curr. Treat. Options Oncol. 2022;23(6):887–903. doi: 10.1007/s11864-022-00938-4. [DOI] [PubMed] [Google Scholar]
- 11.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Disis M.L., Taylor M.H., Kelly K., Beck J.T., Gordon M., Moore K.M., et al. Efficacy and safety of avelumab for patients with recurrent or refractory ovarian cancer: phase 1b results from the JAVELIN solid tumor trial. JAMA Oncol. 2019;5(3):393–401. doi: 10.1001/jamaoncol.2018.6258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Varga A., Piha-Paul S., Ott P.A., Mehnert J.M., Berton-Rigaud D., Morosky A., et al. Pembrolizumab in patients with programmed death ligand 1-positive advanced ovarian cancer: analysis of KEYNOTE-028. Gynecol. Oncol. 2019;152(2):243–250. doi: 10.1016/j.ygyno.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Matulonis U.A., Shapira-Frommer R., Santin A.D., Lisyanskaya A.S., Pignata S., Vergote I., et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann. Oncol. 2019;30(7):1080–1087. doi: 10.1093/annonc/mdz135. [DOI] [PubMed] [Google Scholar]
- 15.Pujade-Lauraine E., Fujiwara K., Ledermann J.A., Oza A.M., Kristeleit R., Ray-Coquard I.L., et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study. Lancet Oncol. 2021;22(7):1034–1046. doi: 10.1016/S1470-2045(21)00216-3. [DOI] [PubMed] [Google Scholar]
- 16.Moore K.N., Bookman M., Sehouli J., Miller A., Anderson C., Scambia G., et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39) J. Clin. Oncol. 2021;39(17):1842–1855. doi: 10.1200/JCO.21.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pignata S., Bookman M., Sehouli J., Miller A., Penson R.T., Taskiran C., et al. Overall survival and patient-reported outcome results from the placebo-controlled randomized phase III IMagyn050/GOG 3015/ENGOT-OV39 trial of atezolizumab for newly diagnosed stage III/IV ovarian cancer. Gynecol. Oncol. 2023;177:20–31. doi: 10.1016/j.ygyno.2023.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh C.S., Kamrava M., Rogatko A., Kim S., Li A., Cass I., et al. Phase II trial of cisplatin, gemcitabine and pembrolizumab for platinum-resistant ovarian cancer. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0252665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J.F., Herold C., Gray K.P., Penson R.T., Horowitz N., Konstantinopoulos P.A., et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: a phase 2 clinical trial. JAMA Oncol. 2019;5(12):1731–1738. doi: 10.1001/jamaoncol.2019.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Cearbhaill R.E., Wolfer A., Disilvestro P., O'Malley D.M., Sabbatini P., Shohara L., et al. A phase I/II study of chemo-immunotherapy with durvalumab (durva) and pegylated liposomal doxorubicin (PLD) in platinum-resistant recurrent ovarian cancer (PROC) Ann. Oncol. 2018;29 [Google Scholar]
- 21.Zamarin D., Burger R.A., Sill M.W., Powell D.J., Jr., Lankes H.A., Feldman M.D., et al. Randomized phase II trial of nivolumab versus nivolumab and ipilimumab for recurrent or persistent ovarian cancer: an NRG oncology study. J. Clin. Oncol. 2020;38(16):1814–1823. doi: 10.1200/JCO.19.02059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Konstantinopoulos P.A., Waggoner S., Vidal G.A., Mita M., Moroney J.W., Holloway R., et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma. JAMA Oncol. 2019;5(8):1141–1149. doi: 10.1001/jamaoncol.2019.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harter P., Bidziński M., Colombo N., Floquet A., Pérez M.J.R., Kim J.-W., et al. DUO-O: a randomized phase III trial of durvalumab (durva) in combination with chemotherapy and bevacizumab (bev), followed by maintenance durva, bev and olaparib (olap), in newly diagnosed advanced ovarian cancer patients. J. Clin. Oncol. 2019;37(15_suppl):TPS5598–TPS. [Google Scholar]
- 24.Lee J-m, Zimmer A.D.S., Lipkowitz S., Annunziata C.M., Ho T.W., Chiou V.L., et al. Phase I study of the PD-L1 inhibitor, durvalumab (MEDI4736; D) in combination with a PARP inhibitor, olaparib (O) or a VEGFR inhibitor, cediranib (C) in women's cancers ( NCT02484404) J. Clin. Oncol. 2016;34(15_suppl):3015. [Google Scholar]
- 25.Liu J.F., Herold C., Luo W., Penson R., Horowitz N., Konstantinopoulos P., et al. A phase II trial of combination nivolumab and bevacizumab in recurrent ovarian cancer. Ann. Oncol. 2018;29:viii334–v335. [Google Scholar]
- 26.Adams S.F., Rixe O., Lee J.-H., McCance D.J., Westgate S., Eberhardt S.C., et al. Phase I study combining olaparib and tremelimumab for the treatment of women with BRCA-deficient recurrent ovarian cancer. J. Clin. Oncol. 2017;35(15_suppl) e. [Google Scholar]
- 27.Liu J., Gaillard S., Hendrickson A.W., Moroney J., Yeku O., Diver E., et al. An open-label phase II study of dostarlimab (TSR-042), bevacizumab (bev), and niraparib combination in patients (pts) with platinum-resistant ovarian cancer (PROC): cohort A of the OPAL trial. Gynecol. Oncol. 2021;162:S17–S18. [Google Scholar]
- 28.Monk B.J., Parkinson C., Lim M.C., O'Malley D.M., Oaknin A., Wilson M.K., et al. A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45) J. Clin. Oncol. 2022;40(34):3952–3964. doi: 10.1200/JCO.22.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damodaran S., Zhao F., Deming D.A., Mitchell E.P., Wright J.J., Gray R.J., et al. Phase II study of copanlisib in patients with tumors with PIK3CA mutations: results from the NCI-match ECOG-ACRIN trial (EAY131) subprotocol Z1F. J. Clin. Oncol. 2022;40(14):1552–1561. doi: 10.1200/JCO.21.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tebotelimab is safe and effective across multiple cancer types. Cancer Discov. 2023 doi: 10.1158/2159-8290.CD-RW2023-174. [DOI] [PubMed] [Google Scholar]
- 31.Powderly J.D., Hamid O., Gutierrez M.E., Balmanoukian A.S., Janik J., Hoyle P., et al. 742P First-in-human phase I study of INCAGN02385, a LAG-3 monoclonal antibody antagonist in patients with advanced malignancies. Ann. Oncol. 2022;33:S883. [Google Scholar]
- 32.Gutierrez ST M., Powderly J.D., Balmanoukian A.S., Janik J., Hoyle P., Wei W., Gong X., Hamid O. 730MO - first-in-human phase I study of INCAGN02390, a TIM-3 monoclonal antibody antagonist in patients with advanced malignancies. Ann. Oncol. 2022;33:S331–S355. [Google Scholar]
- 33.Uboha N.V., Milhem M.M., Kovacs C., Amin A., Magley A., Purkayastha D.D., et al. Phase II study of spartalizumab (PDR001) and LAG525 in advanced solid tumors and hematologic malignancies. J. Clin. Oncol. 2019;37(15_suppl):2553. [Google Scholar]
- 34.Dumbrava E., Rasco D., Patnaik A., Vaena D., Papadopoulos K., ElNaggar A., et al. 477 COM902 (Anti-TIGIT antibody) monotherapy – preliminary evaluation of safety, tolerability, pharmacokinetics and receptor occupancy in patients with advanced solid tumors ( NCT04354246) Journal for ImmunoTherapy of Cancer. 2021;9(Suppl 2):A507–A. [Google Scholar]
- 35.Dumbrava E., Sharma M., Fleming G., Papadopoulos K., Sullivan R., Vaena D., et al. 478 COM701 in combination with BMS-986207 (anti-TIGIT antibody) and nivolumab – preliminary results of safety, tolerability and pharmacokinetics in patients with advanced solid tumors ( NCT04570839) Journal for ImmunoTherapy of Cancer. 2021;9(Suppl 2):A508–A. [Google Scholar]
- 36.Vaena D.A., Fleming G.F., Chmielowski B., Sharma M., Hamilton E.P., Sullivan R.J., et al. COM701 with or without nivolumab: results of an ongoing phase 1 study of safety, tolerability and preliminary antitumor activity in patients with advanced solid malignancies ( NCT03667716) J. Clin. Oncol. 2021;39(15_suppl):2504. [Google Scholar]
- 37.Yang Y., Zhao T., Chen Q., Li Y., Xiao Z., Xiang Y., et al. Nanomedicine strategies for heating "cold" ovarian cancer (OC): next evolution in immunotherapy of OC. Adv. Sci. 2022;9(28) doi: 10.1002/advs.202202797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milne K., Köbel M., Kalloger S.E., Barnes R.O., Gao D., Gilks C.B., et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4(7) doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leffers N., Gooden M.J., de Jong R.A., Hoogeboom B.N., ten Hoor K.A., Hollema H., et al. Prognostic significance of tumor-infiltrating T-lymphocytes in primary and metastatic lesions of advanced stage ovarian cancer. Cancer Immunol. Immunother. 2009;58(3):449–459. doi: 10.1007/s00262-008-0583-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z., Huang J., Zhang C., Yang H., Qiu H., Li J., et al. Infiltration of dendritic cells and T lymphocytes predicts favorable outcome in epithelial ovarian cancer. Cancer Gene Ther. 2015;22(4):198–206. doi: 10.1038/cgt.2015.7. [DOI] [PubMed] [Google Scholar]
- 41.Zhang L., Conejo-Garcia J.R., Katsaros D., Gimotty P.A., Massobrio M., Regnani G., et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N. Engl. J. Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 42.Hwang W.T., Adams S.F., Tahirovic E., Hagemann I.S., Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol. Oncol. 2012;124(2):192–198. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun J., Yan C., Xu D., Zhang Z., Li K., Li X., et al. Immuno-genomic characterisation of high-grade serous ovarian cancer reveals immune evasion mechanisms and identifies an immunological subtype with a favourable prognosis and improved therapeutic efficacy. Br. J. Cancer. 2022;126(11):1570–1580. doi: 10.1038/s41416-021-01692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiménez-Sánchez A., Cybulska P., Mager K.L., Koplev S., Cast O., Couturier D.L., et al. Unraveling tumor-immune heterogeneity in advanced ovarian cancer uncovers immunogenic effect of chemotherapy. Nat. Genet. 2020;52(6):582–593. doi: 10.1038/s41588-020-0630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Webb J.R., Milne K., Watson P., Deleeuw R.J., Nelson B.H. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin. Cancer Res. 2014;20(2):434–444. doi: 10.1158/1078-0432.CCR-13-1877. [DOI] [PubMed] [Google Scholar]
- 46.Hensler M., Kasikova L., Fiser K., Rakova J., Skapa P., Laco J., et al. M2-like macrophages dictate clinically relevant immunosuppression in metastatic ovarian cancer. J Immunother Cancer. 2020;8(2) doi: 10.1136/jitc-2020-000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong D.K., Alvarez R.D., Bakkum-Gamez J.N., Barroilhet L., Behbakht K., Berchuck A., et al. Ovarian cancer, version 2.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(2):191–226. doi: 10.6004/jnccn.2021.0007. [DOI] [PubMed] [Google Scholar]
- 48.Song W., Shen L., Wang Y., Liu Q., Goodwin T.J., Li J., et al. Synergistic and low adverse effect cancer immunotherapy by immunogenic chemotherapy and locally expressed PD-L1 trap. Nat. Commun. 2018;9(1):2237. doi: 10.1038/s41467-018-04605-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ledermann J.A., Raja F.A., Fotopoulou C., Gonzalez-Martin A., Colombo N., Sessa C. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013;24(Suppl 6):vi24–32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 50.Blanc-Durand F., Genestie C., Galende E.Y., Gouy S., Morice P., Pautier P., et al. Distribution of novel immune-checkpoint targets in ovarian cancer tumor microenvironment: a dynamic landscape. Gynecol. Oncol. 2021;160(1):279–284. doi: 10.1016/j.ygyno.2020.09.045. [DOI] [PubMed] [Google Scholar]
- 51.Lee Y.J., Woo H.Y., Kim Y.N., Park J., Nam E.J., Kim S.W., et al. Dynamics of the tumor immune microenvironment during neoadjuvant chemotherapy of high-grade serous ovarian cancer. Cancers. 2022;14(9) doi: 10.3390/cancers14092308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vanguri R., Benhamida J., Young J.H., Li Y., Zivanovic O., Chi D., et al. Understanding the impact of chemotherapy on the immune landscape of high-grade serous ovarian cancer. Gynecol Oncol Rep. 2022;39 doi: 10.1016/j.gore.2022.100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Böhm S., Montfort A., Pearce O.M., Topping J., Chakravarty P., Everitt G.L., et al. Neoadjuvant chemotherapy modulates the immune microenvironment in metastases of tubo-ovarian high-grade serous carcinoma. Clin. Cancer Res. 2016;22(12):3025–3036. doi: 10.1158/1078-0432.CCR-15-2657. [DOI] [PubMed] [Google Scholar]
- 54.Ceelen W., Pattyn P., Mareel M. Surgery, wound healing, and metastasis: recent insights and clinical implications. Crit. Rev. Oncol. Hematol. 2014;89(1):16–26. doi: 10.1016/j.critrevonc.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 55.Brøchner A.C., Mikkelsen S., Hegelund I., Hokland M., Mogensen O., Toft P. The immune response is affected for at least three weeks after extensive surgery for ovarian cancer. Dan Med J. 2016;63(6) [PubMed] [Google Scholar]
- 56.Corbaux P., You B., Kepenekian V., Bakrin N., Gelot A., Dayde D., et al. 614P Tolerance and preliminary efficacy of intraperitoneal (IP) nivolumab after cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients (pts) with advanced ovarian carcinoma: A phase I study with expansion cohort (ICONIC) Ann. Oncol. 2022;33:S825. [Google Scholar]
- 57.Marabelle A., Fakih M., Lopez J., Shah M., Shapira-Frommer R., Nakagawa K., et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 58.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 59.Fife B.T., Bluestone J.A. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol. Rev. 2008;224:166–182. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 60.Abdalla M., El-Arabey A.A., Gai Z. CTLA-4 and ovarian cancer residual tumors: the dark side of debulking surgery. Hum. Cell. 2023;36(6):2281–2283. doi: 10.1007/s13577-023-00976-6. [DOI] [PubMed] [Google Scholar]
- 61.Hodi F.S., Mihm M.C., Soiffer R.J., Haluska F.G., Butler M., Seiden M.V., et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100(8):4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agata Y., Kawasaki A., Nishimura H., Ishida Y., Tsubata T., Yagita H., et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int. Immunol. 1996;8(5):765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 63.Borella F., Ghisoni E., Giannone G., Cosma S., Benedetto C., Valabrega G., et al. Immune checkpoint inhibitors in epithelial ovarian cancer: an overview on efficacy and future perspectives. Diagnostics. 2020;10(3) doi: 10.3390/diagnostics10030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hamanishi J., Mandai M., Iwasaki M., Okazaki T., Tanaka Y., Yamaguchi K., et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.FDA approves nivolumab for advanced melanoma [press release] The ASCO Post. 2014 [Google Scholar]
- 66.Ölmez F., Oğlak S.C., Ölmez Ö.F., Akbayır Ö., Yılmaz E., Akgöl S., et al. High expression of CD8 in the tumor microenvironment is associated with PD-1 expression and patient survival in high-grade serous ovarian cancer. Turk J Obstet Gynecol. 2022;19(3):246–256. doi: 10.4274/tjod.galenos.2022.59558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Strickland K.C., Howitt B.E., Shukla S.A., Rodig S., Ritterhouse L.L., Liu J.F., et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7(12):13587–13598. doi: 10.18632/oncotarget.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wan C., Keany M.P., Dong H., Al-Alem L.F., Pandya U.M., Lazo S., et al. Enhanced efficacy of simultaneous PD-1 and PD-L1 immune checkpoint blockade in high-grade serous ovarian cancer. Cancer Res. 2021;81(1):158–173. doi: 10.1158/0008-5472.CAN-20-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qin S., Xu L., Yi M., Yu S., Wu K., Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol. Cancer. 2019;18(1):155. doi: 10.1186/s12943-019-1091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.FDA FDA approves Opdualag for unresectable or metastatic melanoma. 2022 https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-opdualag-unresectable-or-metastatic-melanoma [cited 2023 24-11-2023]. Available from: [Google Scholar]
- 71.Hodi F.S., Chiarion-Sileni V., Gonzalez R., Grob J.J., Rutkowski P., Cowey C.L., et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1480–1492. doi: 10.1016/S1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 72.Huang R.Y., Eppolito C., Lele S., Shrikant P., Matsuzaki J., Odunsi K. LAG3 and PD1 co-inhibitory molecules collaborate to limit CD8+ T cell signaling and dampen antitumor immunity in a murine ovarian cancer model. Oncotarget. 2015;6(29):27359–27377. doi: 10.18632/oncotarget.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang R.Y., Francois A., McGray A.R., Miliotto A., Odunsi K. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. OncoImmunology. 2017;6(1) doi: 10.1080/2162402X.2016.1249561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Whitehair R., Peres L.C., Mills A.M. Expression of the immune checkpoints LAG-3 and PD-L1 in high-grade serous ovarian carcinoma: relationship to tumor-associated lymphocytes and germline BRCA status. Int. J. Gynecol. Pathol. 2020;39(6):558–566. doi: 10.1097/PGP.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 75.Matsuzaki J., Gnjatic S., Mhawech-Fauceglia P., Beck A., Miller A., Tsuji T., et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A. 2010;107(17):7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu F., Liu Y., Chen Z. Tim-3 expression and its role in hepatocellular carcinoma. J. Hematol. Oncol. 2018;11(1):126. doi: 10.1186/s13045-018-0667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fucikova J., Rakova J., Hensler M., Kasikova L., Belicova L., Hladikova K., et al. TIM-3 dictates functional orientation of the immune infiltrate in ovarian cancer. Clin. Cancer Res. 2019;25(15):4820–4831. doi: 10.1158/1078-0432.CCR-18-4175. [DOI] [PubMed] [Google Scholar]
- 78.Whelan S., Ophir E., Kotturi M.F., Levy O., Ganguly S., Leung L., et al. PVRIG and PVRL2 are induced in cancer and inhibit CD8(+) T-cell function. Cancer Immunol. Res. 2019;7(2):257–268. doi: 10.1158/2326-6066.CIR-18-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu X., Harden K., Gonzalez L.C., Francesco M., Chiang E., Irving B., et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009;10(1):48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 80.Johnston R.J., Yu X., Grogan J.L. The checkpoint inhibitor TIGIT limits antitumor and antiviral CD8(+) T cell responses. OncoImmunology. 2015;4(9) doi: 10.1080/2162402X.2015.1036214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen F., Xu Y., Chen Y., Shan S. TIGIT enhances CD4(+) regulatory T-cell response and mediates immune suppression in a murine ovarian cancer model. Cancer Med. 2020;9(10):3584–3591. doi: 10.1002/cam4.2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Westergaard M.C.W., Milne K., Pedersen M., Hasselager T., Olsen L.R., Anglesio M.S., et al. Changes in the tumor immune microenvironment during disease progression in patients with ovarian cancer. Cancers. 2020;12(12) doi: 10.3390/cancers12123828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.ESMO 730MO - First-in-human phase I study of INCAGN02390, a TIM-3 monoclonal antibody antagonist in patients with advanced malignancies. 2022 https://oncologypro.esmo.org/meeting-resources/esmo-congress-2022/first-in-human-phase-i-study-of-incagn02390-a-tim-3-monoclonal-antibody-antagonist-in-patients-with-advanced-malignancies [Available from: [Google Scholar]
- 84.Duraiswamy J., Kaluza K.M., Freeman G.J., Coukos G. Dual blockade of PD-1 and CTLA-4 combined with tumor vaccine effectively restores T-cell rejection function in tumors. Cancer Res. 2013;73(12):3591–3603. doi: 10.1158/0008-5472.CAN-12-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park J., Lee J.B., Lim M.C., Kim B.G., Kim J.W., Kim S., et al. Phase II study of durvalumab and tremelimumab with front-line neoadjuvant chemotherapy in patients with advanced-stage ovarian cancer: primary analysis in the original cohort of KGOG3046/TRU-D. J Immunother Cancer. 2023;11(10) doi: 10.1136/jitc-2023-007444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valsecchi M.E. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373(13):1270. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 87.Färkkilä A., Gulhan D.C., Casado J., Jacobson C.A., Nguyen H., Kochupurakkal B., et al. Immunogenomic profiling determines responses to combined PARP and PD-1 inhibition in ovarian cancer. Nat. Commun. 2020;11(1):1459. doi: 10.1038/s41467-020-15315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y., Tong Z., Zhang W., Zhang W., Buzdin A., Mu X., et al. FDA-approved and emerging next generation predictive biomarkers for immune checkpoint inhibitors in cancer patients. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.683419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Orellana García L.P., Ehmann F., Hines P.A., Ritzhaupt A., Brand A. Biomarker and companion diagnostics-A review of medicinal products approved by the European Medicines agency. Front. Med. 2021;8 doi: 10.3389/fmed.2021.753187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yan L., Zhang W. Precision medicine becomes reality-tumor type-agnostic therapy. Cancer Commun. 2018;38(1):6. doi: 10.1186/s40880-018-0274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fraune C., Rosebrock J., Simon R., Hube-Magg C., Makrypidi-Fraune G., Kluth M., et al. High homogeneity of MMR deficiency in ovarian cancer. Gynecol. Oncol. 2020;156(3):669–675. doi: 10.1016/j.ygyno.2019.12.031. [DOI] [PubMed] [Google Scholar]
- 92.Sankar K., Ye J.C., Li Z., Zheng L., Song W., Hu-Lieskovan S. The role of biomarkers in personalized immunotherapy. Biomark. Res. 2022;10(1):32. doi: 10.1186/s40364-022-00378-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brahmer J., Reckamp K.L., Baas P., Crinò L., Eberhardt W.E., Poddubskaya E., et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N. Engl. J. Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kulangara K., Zhang N., Corigliano E., Guerrero L., Waldroup S., Jaiswal D., et al. Clinical utility of the combined positive Score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch. Pathol. Lab Med. 2019;143(3):330–337. doi: 10.5858/arpa.2018-0043-OA. [DOI] [PubMed] [Google Scholar]
- 95.Cristescu R., Aurora-Garg D., Albright A., Xu L., Liu X.Q., Loboda A., et al. Tumor mutational burden predicts the efficacy of pembrolizumab monotherapy: a pan-tumor retrospective analysis of participants with advanced solid tumors. J Immunother Cancer. 2022;10(1) doi: 10.1136/jitc-2021-003091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ayers M., Lunceford J., Nebozhyn M., Murphy E., Loboda A., Kaufman D.R., et al. IFN-γ-related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Invest. 2017;127(8):2930–2940. doi: 10.1172/JCI91190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu S., Stein J.E., Rimm D.L., Wang D.W., Bell J.M., Johnson D.B., et al. Comparison of biomarker modalities for predicting response to PD-1/PD-L1 checkpoint blockade: a systematic review and meta-analysis. JAMA Oncol. 2019;5(8):1195–1204. doi: 10.1001/jamaoncol.2019.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rodig S.J., Gusenleitner D., Jackson D.G., Gjini E., Giobbie-Hurder A., Jin C., et al. MHC proteins confer differential sensitivity to CTLA-4 and PD-1 blockade in untreated metastatic melanoma. Sci. Transl. Med. 2018;10(450) doi: 10.1126/scitranslmed.aar3342. [DOI] [PubMed] [Google Scholar]
- 99.Kamphorst A.O., Pillai R.N., Yang S., Nasti T.H., Akondy R.S., Wieland A., et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. 2017;114(19):4993–4998. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goldberg S.B., Narayan A., Kole A.J., Decker R.H., Teysir J., Carriero N.J., et al. Early assessment of lung cancer immunotherapy response via circulating tumor DNA. Clin. Cancer Res. 2018;24(8):1872–1880. doi: 10.1158/1078-0432.CCR-17-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]