Abstract
Heterotrigona itama stingless bee propolis extract is known for its diverse bioactive compounds, making it a potential natural shield against UV radiation. This research assesses the photoprotective potential of crude ethanol extract from H. itama propolis collected from four structures (involucrum, pillar, pot, and entrance) of five bee hives (H1-H5), totalling 20 samples. Initially, the samples underwent testing for SPF value and UV absorption spectra. The crude ethanol extract (E) from the involucrum (H4) with the highest SPF value and broadest spectrum was selected for fractionation using hexane and water. Subsequently, the extract (E) and its hexane (H) and water (W) fractions were subjected to SPF analysis, UVA/UVB absorption assessment, determination of total phenolic and flavonoid content, free radical scavenging capacity, anti-collagenase effects, and cytotoxicity assessment. Additionally, LC-MS/MS analysis was performed to identify chemical constituents in the active fraction (W). The extract E demonstrated an SPF of 8.23 ± 0.09 and UV absorption. Notably, its fraction W exhibited the highest SPF (16.55 ± 0.24) at 100 μg/mL, surpassing the H fraction (SPF 5.7 ± 0.45). Phenolic content was highest in the H fraction (388.95 ± 4.54 mg/g GAE DW), followed by the W fraction (286.76 ± 6.48 mg/g GAE DW) and crude E (91.83 ± 4.12 mg/g GAE DW) from the involucrum. Regarding flavonoids, the fraction W led with 79.82 ± 6.21 mg/g QE DW, followed by the H fraction (45.56 ± 0.05 mg/g QE DW) and E (34.57 ± 1.11 mg/g QE DW). The extract E also exhibited modest DPPH scavenging (EC50 = 120 μg/mL), while the H fraction demonstrated stronger activity (EC50 = 4.37 μg/mL), and the W fraction displayed moderate effects (EC50 = 17.55 μg/mL). Notably, the W fraction showed remarkable anti-collagenase activity, outperforming the positive control, EG. HaCaT cell cytotoxicity revealed that the extract E was cytotoxic, whereas the H and W fractions showed no toxicity. LC-MS/MS analysis identified bioactive flavonoids (e.g., pratensein, quercetin) in the W fraction. These findings highlight the superior photoprotective properties of the water fraction from the involucrum of H. itama stingless bee propolis extract, suggesting its potential as a natural and effective ingredient for sunscreen and skincare formulations.
Keywords: H. itama, Propolis, UV, HaCaT, Flavonoids, Sunscreen
1. Introduction
Sun exposure is essential for daily life as it facilitates the synthesis of vitamin D, yet it also carries significant risks due to exposure to ultraviolet (UV) radiation [1]. UV radiation spans three types: UVA (400–320 nm), UVB (290–320 nm), and UVC (290-200 nm), each with distinct wavelengths and varying degrees of penetration into the atmosphere [2]. The rise in global temperatures, intensifying heat waves, and environmental factors such as ozone depletion and climate change have heightened the risks associated with UV exposure. UV radiation poses substantial risks to both human health and the environment [3,4]. Exposure to UV radiation can lead to diverse skin issues like sunburn, accelerated skin ageing, and heightened susceptibility to skin cancer. Prolonged exposure may also result in eye problems such as cataracts and photokeratitis [5]. Additionally, UV radiation can weaken the immune system, making individuals more susceptible to infections. It can also damage DNA, potentially leading to mutations and the development of skin cancers [6].
Beyond human health, UV radiation also affects ecosystems, disrupting marine life, coral reefs, plants, and phytoplankton, which threatens biodiversity and ecological balance [7]. Effective photoprotection strategies are crucial for reducing the harmful impacts of UV radiation on both public health and ecological systems. Current photoprotection methods mainly involve the use of synthetic creams containing chemical UV filters, such as oxybenzone, oxalate, and avobenzone, which absorb or reflect UV radiation [8]. While effective, these synthetic compounds can have environmental implications, such as coral reef bleaching and potential endocrine-disrupting effects [9]. In response to these concerns, there has been growing interest in exploring natural sources for photoprotection.
Propolis, a natural resinous substance gathered by honey bees and stingless bees, has attracted significant attention due to its diverse pharmacological properties, including immune-boosting, wound-healing, antiproliferation and antiviral effects [[10], [11]]. Within propolis, compounds like flavonoids, phenolic acids, and polyphenols exhibit UV-absorbing and antioxidant capabilities, making it a promising candidate for photoprotection [12,13]. Research shows that propolis extracts can neutralize free radicals generated by UV radiation, reducing oxidative stress and preventing skin damage [13,14]. These antioxidant and anti-inflammatory properties are crucial for maintaining skin health and preventing photoaging, a process characterized by wrinkles, loss of elasticity, and other ageing signs due to prolonged UV exposure.
A major factor in photoaging is the breakdown of collagen, a protein that gives skin its structure and elasticity. UV damage accelerates collagen degradation through the activation of collagenase enzymes [13]. Thus, the ability of propolis to inhibit collagenase activity is a significant aspect of its photoprotective potential. By preserving collagen integrity, propolis helps reduce the effects of photoaging and enhances skin resilience against UV damage [13,15]. This makes propolis not only a potential natural sunscreen but also a protective agent against long-term UV damage.
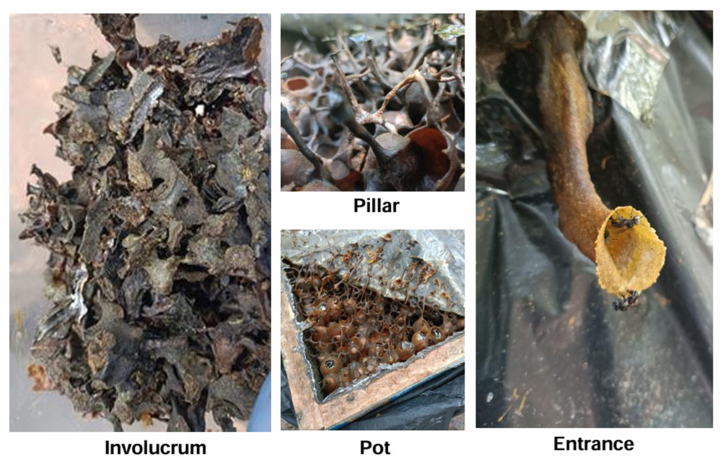
Furthermore, propolis-based formulations have demonstrated compatibility with the skin, making them suitable for daily use as natural sunscreens or as components in sun protection products [16,17]. Incorporating propolis into photoprotective formulations meets the increasing demand for sustainable and eco-friendly skincare solutions. Although studies have reported the photoprotective properties of propolis from various origins, the photoprotective and chemical constituents present in Malaysian stingless bee H. itama propolis remain underexplored. Moreover, there is an impact of different structure selection of propolis in the stingless beehive. Each hive has four basic structures, namely, involucrum, pillar, pot, and entrance, as shown in Fig. 1. Based on the structure selection, the chemical constituents will vary, and their biological properties will also change [18]. Thus, identifying the suitable beehive structure to collect the propolis and its photoprotective properties is essential.
Fig. 1.
Various beehive structures of H. itama stingless bees, Involucrum, Pillar, Pot, and Entrance.
In this study, the propolis was collected from four structures within H. itama beehive across five bee hives to assess their photoprotective efficacy. Subsequently, the most active extract was fractionated using hexane and water solvents, and the resulting fractions were analyzed for their chemical composition and photoprotective properties. The identification of chemical constituents was conducted using Liquid Chromatography-Mass Spectrometry/Mass Spectrometry (LC-MS/MS), a powerful analytical technique that allows for the precise characterization of complex mixtures. Our goal is to clearly demonstrate the photoprotective potential of H. itama propolis, ascertain its optimal extraction structure selection, and identify the chemical constituents responsible for its efficacy.
2. Methodology
2.1. Chemicals and reagents
The extraction solvents, hexane (CAS No. 110-54-3), methanol (CAS No. 67-56-1) and ethanol (CAS No. 64-17-5), were of analytical grade and sourced from Merck Millipore (Darmstadt, Germany). Various chemicals and reagents including collagenase from Clostridium histolyticum (CAS No. 9001-12-1), 2,2-diphenyl-1-picrylhydrazyl (DPPH, CAS No. 1898-66-4), gelatin (CAS No. 9000-70-8), Coomassie blue R-250 (CAS No. 6104-59-2), ascorbic acid (CAS No. 50-81-7), Folin-Ciocalteu's phenol reagent (CAS No. 9005-38-3), gallic acid (CAS No. 149-91-7), quercetin (CAS No. 117-39-5), and aluminium chloride (CAS No. 7446-70-0), epigallocatechin gallate (EG, CAS No. 989-51-5), Tris-HCl (CAS No. 1185-53-1), agarose (CAS No. 9012-36-6), acetic acid (CAS No. 64-19-7), dimethyl sulfoxide (DMSO, CAS No. 67-68-5), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, CAS No. 57360-69-7) were obtained from Sigma-Aldrich Chemicals (St. Louis, MO, USA). Sodium carbonate (CAS No. 497-19-8) was obtained from Merck, Germany. In addition, Dulbecco's modified Eagle's medium (DMEM, CAT No. 11-995-073), TrypLE Express (CAT No. 12604–013), fetal bovine serum (FBS, CAT No. 26-140-079), and penicillin/streptomycin (CAT No. 15140–122) were procured from Gibco (Life Technologies, California, USA).
2.2. Collection of H. itama propolis
The propolis samples were collected from four beehive structures of H. itama stingless bees, specifically the involucrum, pillar, pot, and entrance, as shown in Fig. 1. These samples were collected in April 2023 from the Big Bee Honey Farm located in Marang, Terengganu, Malaysia. The hives from which the samples were collected were coded as H1, H2, H3, H4, and H5, representing five different hives. A total of 20 samples were collected, with four samples obtained from each hive.
2.3. Extraction procedure
Approximately 500g of each propolis sample was mixed with ethanol (95 %), and the extraction was done using an ultrasound-assisted extraction technique at room temperature. Briefly, the samples were sonicated for 1 h at 100Khz and left overnight. Then, the mixture was filtered, and the filtrate was concentrated using a rotary vacuum evaporator (Buchi R-300 Rotavapor, Switzerland). Then the resultant extracts were dried under vacuum, lyophilized, and stored at −20 °C for further use [2].
2.4. Selection of the optimum propolis sample
-
i
Sunscreen protection factor (SPF) value determination and UV absorption spectra
This assessment followed the methodology outlined in Santhanam et al. (2022) [2], which included dissolving propolis extracts in methanol at a concentration of 100 μg/mL. The SPF values were determined using the Mansur equation (1) on a UV–visible spectrophotometer (UV-1900i Shimadzu, Japan), scanning at 5 nm intervals within the 290–320 nm range.
| (1) |
Where: EE (λ) is the erythemal effect spectrum I (λ) is the solar intensity spectrum EE (λ) x I(λ) are constants. Abs (λ) is the absorbance of the test sample CF is the correction factor (=10)
-
ii
Analysis of UV absorption spectra
The UV absorption spectrum of the propolis extracts was analyzed at a concentration of 100 μg/mL in methanol, covering a wavelength range from 200 to 400 nm. This analysis was performed using a quartz cell with a 1 cm path length on the UV–visible spectrophotometer (UV-1900i Shimadzu, Japan) [2].
2.5. Fractionation using solvent-solvent extraction
Based on the optimum test results, the ethanol extract (E) from the involucrum layer of H4, weighing 12.3 g, was selected for further fractionation due to its high SPF value and broad UV absorption spectra. The fractionation was performed using solvent-solvent extraction with hexane and water. Specifically, 200 mL of distilled water was added to the ethanol extract, followed by an equal volume of hexane (200 mL). The mixture was placed in a separating funnel and allowed to stand, resulting in two distinct layers: the upper hexane layer containing lipophilic compounds and the lower aqueous layer containing hydrophilic compounds. This extraction process was repeated three times to ensure thorough separation. The hexane and aqueous layers were then collected, and each solvent fraction was concentrated using a rotary evaporator before being subjected to freeze-drying. The hexane fraction (H) was obtained with a yield of 6.8 g, which corresponds to approximately 55.3 % of the total ethanol extract. In comparison, the water fraction (W) yielded 4.9 g, accounting for about 39.8 % of the total extract. These yields were calculated using equation (2):
| (2) |
2.6. SPF value determination and UV absorption spectra
The SPF value and UV absorption spectra of the extract (E) and solvent fractions (H and W) obtained from H. itama propolis from Hive-4 were calculated following the method described in Section 2.4.
2.7. Total phenolic and flavonoid content of the active propolis extract and its fractions
The total phenolic and flavonoid content of extract (E) and solvent fractions (H and W) obtained from H4 of H. itama propolis were assessed using the Folin-Ciocalteu method and the Aluminium chloride method, respectively.
2.7.1. ii. Total phenolic content (TPC)
A volume of 50 μL of the extract (E) (1 mg/mL) or fractions H or W was mixed with 50 μL of distilled water, 50 μL of 10 % Folin-Ciocalteu's phenol reagent, and 50 μL of 1 M sodium carbonate solution in a 96-well microtitre plate. Methanol was used as a blank. The reaction mixtures were incubated for 1 h at room temperature and protected from light. The absorbance of the reaction mixture was measured at 750 nm using a microplate reader (Infinite F200 Pro, Tecan Group Ltd., Männedorf, Switzerland). The total phenolic content was determined using a standard curve created with gallic acid (0–200 μg/mL) as the standard [2,19]. Results were expressed as milligrams of Gallic Acid Equivalents (GAE) per gram of dry propolis extract or fractions.
2.7.2. ii. Total flavonoid content (TFC)
A volume of 100 μL of the extract (E) at a concentration of 1 mg/mL, or fractions H/W, as well as standard solutions of quercetin ranging from 0 to 200 μg/mL in methanol, were mixed with 100 μL of a 2 % AlCl3 solution. The reaction mixtures were then incubated for 1 h at room temperature. Following incubation, the absorbance of each mixture was measured using a microplate reader (Infinite F200 Pro, Tecan Group Ltd., Männedorf, Switzerland) at a wavelength of 415 nm. All samples were read in triplicate to ensure accuracy. Quercetin was used as the standard to develop a standard curve for the assay [2,19]. The total flavonoid content was expressed as milligrams of Quercetin Equivalent (QE) per gram of dry propolis extract or fractions [2].
2.8. DPPH free radical scavenging assay
The free radical scavenging activity of the H. itama propolis extract (E) and its solvent fractions (H and W) against DPPH radicals was determined following the method outlined in Santhanam et al. [2]. Initially, a 0.1 mM DPPH solution in methanol was prepared, and each test sample was diluted in methanol to various concentrations ranging from 0 to 200 μg/mL. Subsequently, a reaction mixture containing equal volumes of DPPH solution (100 μL) and the test samples (100 μL) was prepared and kept in the dark at room temperature. After a 30-min incubation period, the absorbance of the reaction mixture was measured at 517 nm using a microplate reader (Infinite F200 Pro, Tecan Group Ltd., Männedorf, Switzerland). Ascorbic acid served as the positive control, and all measurements were conducted in triplicate.
The DPPH radical scavenging activity was calculated using formula (3):
| (3) |
Where: Abscontrol represents the absorbance of DPPH radical + methanol, Abssample indicates the absorbance of DPPH radical + sample extract/fractions/standard.
2.9. Anti-collagenase assay
The gelatin digestion assay was conducted following the method outlined by Santhanam et al. [2] with minor adjustments. Agarose (2 %) was prepared in collagenase buffer containing 50 mM TrisHCl, 10 mM CaCl2, and 0.15 M NaCl at pH 7.8, along with 0.15 % porcine gelatin. This mixture was poured into a Petri dish and allowed to solidify for approximately 1 h at room temperature. Subsequently, wells were created using a sterile 200 μL microtip. Samples E, H and W at concentrations of 200 μg/mL, 100 μg/mL, and 50 μg/mL were incubated for 1 h with bacterial collagenase-1 (0.1 mg/mL). Following incubation, the reaction mixture (50 μL) was loaded into the wells and incubated overnight. Epigallocatechin gallate (EG) served as the positive control. The degree of gelatin digestion within the agarose gel was visualized using Coomassie Brilliant Blue staining. Gelatinase inhibition activity was determined by measuring the area of the light translucent zone over a blue background formed after destaining.
2.10. Cytotoxicity
The HaCaT cell line, a human immortalized keratinocyte cell line, was acquired from the American Type Culture Collection (ATCC) in Rockville, USA. These cells were cultured in Dulbecco's modified Eagle's medium with high glucose content, supplemented with 10 % fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin, all sourced from Sigma Aldrich, USA. The cells were kept in a humidified environment at 37 °C with 5 % CO2. For all treatments, propolis extracts were initially dissolved in DMSO and then adjusted to the required concentration using a complete cell culture medium, ensuring a final maximum DMSO concentration of 0.01 % w/v. Sub-confluent cells (80 % confluence) were seeded at a density of 1 x 104 cells and treated with varying concentrations (0–200 μg/mL) of propolis samples (ethanol extract, hexane and water fractions) or with the vehicle alone (DMSO at 0.01 % v/v in media), which served as the control, for 24 h. Following treatment, 20 μL of MTT solution (5 mg/mL) was added to each well and incubated in darkness for 3 h. Formazan crystals formed were dissolved using 100 μL of DMSO, and the absorbance was measured at 570 nm using a microplate reader (Infinite F200 Pro, Tecan Group Ltd., Männedorf, Switzerland) [2].
2.11. LC-MS/MS analysis of the active fraction of H.itama propolis
The LC-MS/MS technique was employed to identify the chemical constituents present in the active fraction (W) of H. itama propolis extracts. An ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) system was utilized for high-throughput mass screening of propolis samples. This system combined a UPLC (Waters Acquity, Milford, MA) with a triple quadrupole-linear ion trap tandem mass spectrometer (Applied Biosystems 4000 Q TRAP; Life Technologies Corporation, Carlsbad, CA). Separation of compounds was achieved using an Inert Sustain C18 column (5 μm, 250 mm × 4.6 mm i.d.), followed by ionization through an electrospray ionization (ESI) source for mass detection. The mobile phase of the UPLC system consisted of solvent A (water with 0.1 % formic acid) and solvent B (methanol with 0.1 % formic acid). Prior to injection, samples were filtered using a 0.2-μm nylon membrane filter. Enhanced mass spectra (EMS) scan mode with two parallel Enhanced Product Ion (EPI) runs was set up using information-dependent acquisition (IDA) to capture mass fragments of compounds. The acquisition was conducted in both positive and negative modes, covering a range from m/z 50 to 1500. The ion source's capillary temperature and voltage were maintained at 400 °C and 5.5 kV (−4.5 kV), respectively. Nitrogen was utilized for nebulization (40 psi), solvent drying (40 psi), and as curtain gas (10 psi). The scan rate was set at 1000 amu/s. Data acquisition and processing were performed using Analyst 1.4.2 software. Compounds were identified by comparing their molecular masses and fragmentation patterns with those in the Compound Discoverer 2.1 software, as well as with published data [19].
2.12. Statistical analysis
The data are presented as means with standard deviations, Mean ± SD. Statistical analysis involved conducting One-way ANOVA followed by the Dunnett test to identify significant differences among the groups.
3. Results and discussion
3.1. SPF value determination and UV absorption spectra
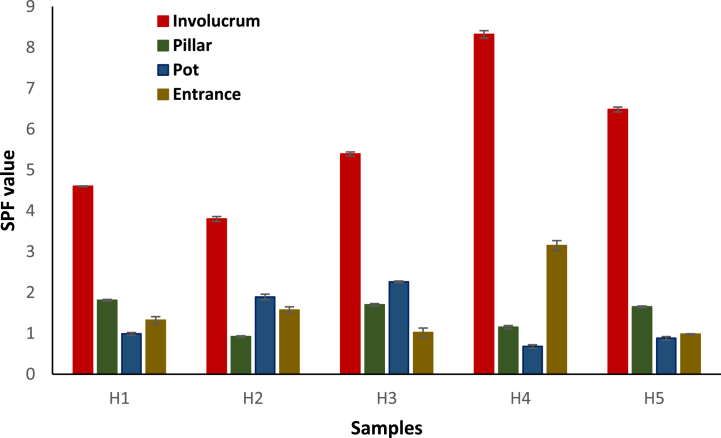
Among the tested 20 samples, the propolis from the involucrum layer of H4 exhibited the highest SPF value (8.23 ± 0.09 at 100 μg/mL), suggesting a potential correlation between chemical composition and photoprotective efficacy (Fig. 2). Notably, this SPF value is comparable to previous studies on ethanolic red propolis extracts, which reported SPF values ranging from 4 to 10 at concentrations of 200–400 μg/mL [16]. This highlights the impressive photoprotective capability of the involucrum extract from H. itama propolis, as it achieved a similar SPF at a significantly lower concentration of 100 μg/mL. Moreover, our results align with other previous studies indicating that propolis composition can vary significantly based on factors such as botanical origin, environmental conditions, and collection methods [20,21]. The involucrum, being the outermost layer of the nest structure, likely accumulates a unique blend of botanical resins, waxes, and other compounds [22]. The higher SPF value of the involucrum extract may be attributed to its rich content of polyphenols, flavonoids, and other bioactive constituents, as mentioned in propolis extracts known for their UV-absorbing capabilities [23,24]. Polyphenols, for instance, are renowned for their ability to scavenge free radicals generated by UV radiation, thereby mitigating oxidative stress and DNA damage in exposed organisms. Similarly, flavonoids possess anti-inflammatory and photoprotective properties, contributing to enhanced skin protection against UV-induced damage [25].
Fig. 2.
SPF value of H. itama propolis obtained from various nest structures such as involucrum, pillar, pot and entrance within H. itama beehives. H1-H5 indicates beehive numbers 1 to 5.
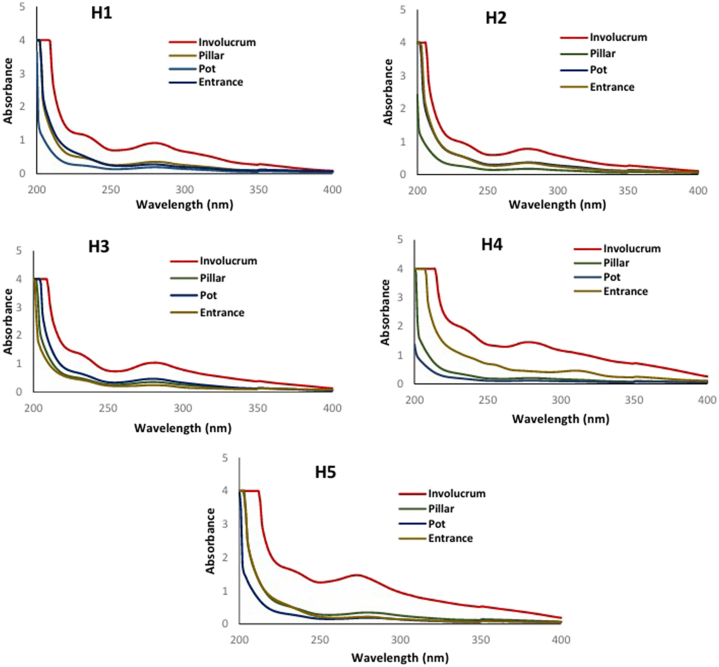
Referring to Fig. 3, the broad UV absorption spectra observed in the involucrum extracts compared to other structures in the beehive further substantiate their enhanced photoprotective capacity. This spectrum indicates that the involucrum extracts can effectively absorb a wide range of UV wavelengths, including both UVA and UVB, which are known to contribute to skin damage and ageing [26]. This broader UV absorption profile can be attributed to the complex mixture of compounds present in the involucrum, including polyphenols, flavonoids, terpenes, and other UV-absorbing molecules [27]. The broader UV absorption spectra of the involucrum extract not only indicate its ability to absorb a wider range of harmful UV radiation but also suggest a more comprehensive protection mechanism against UV-induced skin damage. This aligns with the observed higher SPF value of the involucrum extracts, highlighting their suitability for formulating photoprotective products with broad-spectrum UV protection.
Fig. 3.
UV absorption spectra of H. itama propolis obtained from various nest structures such as Involucrum, Pillar, Pot, Entrance within H. itama bee hives. H1-H5 represent beehive numbers 1 to 5.
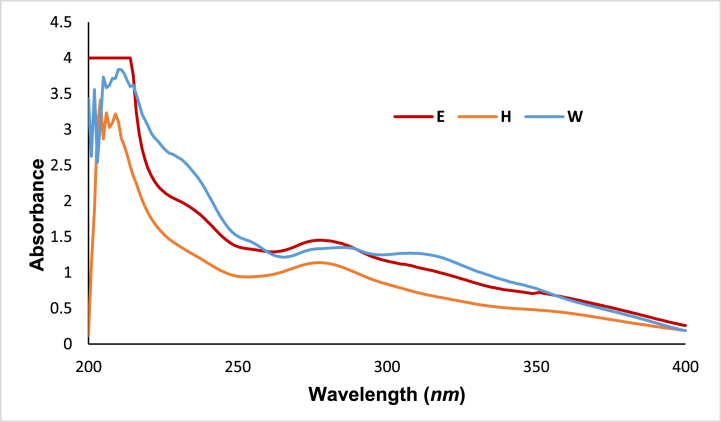
Table 1 and Fig. 4 illustrate the intriguing insights into the photoprotective properties of the hexane and water fractions obtained from the fractionation of the involucrum extract from H4. The fraction (W), displaying a high SPF value (16.55 ± 0.24) and pronounced broad absorption spectra than H fraction (5.70 ± 0.45), highlights the importance of solvent selection in extracting bioactive compounds with photoprotective potential. The higher SPF value of fraction W can be attributed to the presence of hydrophilic compounds such as polyphenols, flavonoids, and other water-soluble compounds. Additionally, the UV absorption spectra of the fraction (W) further support its photoprotective efficacy; its broad and intense absorption spectrum indicates efficient absorption of UV radiation across a wide range of wavelengths.
Table 1.
SPF value of the crude ethanol extract (E) of H. itama propolis from the involucrum layer of H4 and its solvent fractions (H) and (W).
| Wavelength | Ethanol crude Extract (E) (100 μg/mL) | Water Fraction (W) (100 μg/mL) | Hexane Fraction (H) (100 μg/mL) |
|---|---|---|---|
| SPF | 8.23 ± 0.09 | 16.55 ± 0.24 | 5.70 ± 0.45 |
Fig. 4.
UV absorption spectra of the crude ethanol extract (E) of H. itama propolis from the involucrum layer of H4 and its solvent fractions (H) and (W).
In contrast, the differences in SPF values and UV absorption spectra between the H and W fractions underscore the differential extraction of bioactive compounds based on their solubility properties. While the fraction (H) may contain lipophilic compounds like terpenes and fatty acids, the fraction (W) likely concentrates hydrophilic and UV-absorbing molecules, contributing to its higher SPF value and UV absorption efficacy. These findings emphasize the significance of solvent-solvent fractionation in enriching specific bioactive compounds with photoprotective properties. Notably, the UV absorption spectra of the water fraction are higher compared to Brazilian ethanolic propolis extracts reported in previous studies [16]. This suggests that the water (W) fraction from the involucrum of H4 has superior UV protection potential, making it a promising candidate for developing natural sunscreens or skincare products with enhanced UV protection.
3.2. Total phenolic and flavonoid content of the active propolis extract and its fractions
From Table 2, it is evident that the fraction (H) exhibits the highest total phenolic content (TPC) value of 388.95 ± 4.54 mgGAE/g DW, followed by the fraction (W) with 286.76 ± 6.48 mgGAE/g DW, and the crude extract (E) with 91.83 ± 4.12 mgGAE/g DW (Table 2). This indicates that the fractions (H and W) are richer in phenolic compounds compared to the extract, with the H fraction showing the highest phenolic content. In contrast, the W fraction exhibits the highest total flavonoid content (TFC) value of 79.82 ± 6.21 mgQE/g DW, followed by the H fraction with 45.56 ± 0.05 mgQE/g DW, and the extract (E) with 34.57 ± 1.11 mgQE/g DW.
Table 2.
Total phenol and flavonoid content of the crude ethanol extract (E) of H. itama propolis from the involucrum layer of H4 and its solvent fractions (H) and (W).
| Involucrum (H4) - (200 μg/mL) | TPC- mgGAE/g DW | TFC- mgQE/g DW |
|---|---|---|
| Ethanol crude Extract (E) | 91.83 ± 4.12 | 34.57 ± 1.11 |
| Water Fraction (W) | 286.76 ± 6.48 | 79.82 ± 6.21 |
| Hexane Fraction (H) | 388.95 ± 4.54 | 45.56 ± 0.05 |
These results clearly illustrate the impact of solvent-solvent fractionation on the composition and photoprotective properties of propolis extracts from the involucrum of H4. The contrasting compositions suggest distinct mechanisms contributing to the photoprotective efficacy of each fraction. The high phenolic content in the H fraction indicates a concentration of lipophilic phenolic compounds, which may contribute to its SPF value However, the lower flavonoid content in this fraction suggests a potential limitation in terms of UV absorption capabilities, as flavonoids are well-known for their UV-absorbing properties [27].
In contrast, the water fraction (W), with its high flavonoid content and lower phenolic content, shows strong potential for achieving a high SPF value and effective UV absorption. The lower phenolic and flavonoid contents in the whole extract (E) suggest that fractionation effectively concentrates bioactive compounds by separating them based on their polarity. The less polar hexane fraction may contain compounds that are less effective at absorbing UV light, while the more polar water fraction may include compounds with stronger photoprotective properties. Thus, the SPF value of each fraction reflects the specific compounds present, and fractionation allows for the isolation of those with the highest photoprotective potential [2].
A recent study comparing stingless bee propolis from H. itama and G. thoracica in East Kalimantan found that the TPC was higher in H. itama propolis, reaching approximately 880 mgGAE/100g compared to G. thoracica's 875 mgGAE/100g. Moreover, H. itama propolis exhibited enhanced antioxidant activity at 100 ppm, demonstrating about 84 % inhibition, whereas G. thoracica propolis showed 76.5 %. This suggests that H. itama propolis has greater antioxidant potential compared to G. thoracica propolis [28].
Similarly, investigations into Malaysian stingless bee propolis, specifically H. itama, revealed a significant correlation (r = 0.282) among TPC, TFC, and antioxidant activity. This correlation implies that higher levels of TPC and TFC correspond to increased antioxidant activity, underscoring the medicinal potential of stingless bee propolis as a natural product [22,[28], [29]].
3.3. Antioxidant activity of the propolis extracts against DPPH free radicals
From Table 3, it is evident that the extract (E) derived from the involucrum layer of H4 exhibited a modest antioxidant capacity, indicated by an EC50 value of 120 ± 5.65 μg/mL. This observation suggests a limited presence of highly active compounds within the extract capable of efficiently neutralizing DPPH radicals. Alternatively, the observed antioxidant activity may be influenced by synergistic interactions among the bioactive components, potentially impacting the overall bioactivity.
Table 3.
DPPH free radical scavenging of the crude ethanol extract (E) of H. itama propolis from the involucrum of H4 and its solvent fractions (H) and (W).
| Samples (μg/mL) | Ascorbic Acid (AA) | Ethanolic Extract (E) | Hexane Fraction (H) | Water Fraction (W) |
|---|---|---|---|---|
| EC50 | 5.2 ± 0.19 | 120 ± 5.65∗∗∗ | 34.39 ± 4.66∗∗∗ | 39.51 ± 3.54∗∗∗ |
Dunnett's Multiple Comparison Test was performed using GraphPad Prism software to compare the antioxidant activity of ascorbic acid (AA) with the ethanol extract (E), hexane fraction (H), and water fraction (W). The test assessed the significance of differences in EC50 values, with significance levels indicated as follows: ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Studies have shown that H. itama propolis extract exhibits significant antioxidant efficacy, with a DPPH assay result of 84 %, surpassing G. thoracica propolis at 76.5 % [30]. The IC50 value of H. itama propolis extract was determined to be 50.61 ppm, indicating a moderate level of antioxidant activity [30].
Furthermore, chemical analysis of H. itama propolis extract identified flavonoids, phenolic acids, terpenes, and aromatic acids, known for their antioxidant properties attributed to hydroxyl, amine, carbonyl, and ester functional groups [[31], [32]]. Notably, H. itama propolis extract showed a higher TPC of 50.61 μg/mL compared to G. thoracica propolis at 42.55 μg/mL Despite the abundance of flavonoids and phenolic compounds, the antioxidant activity of H. itama propolis extract does not solely correlate with these constituents, suggesting that other components may also contribute to its antioxidant characteristics [[30], [33]].
In this study, the fraction (H) exhibited a significantly lower EC50 value of 34.39 ± 4.66 μg/mL compared to extract (E), indicating a significant antioxidant activity. This suggests that non-polar compounds in the fraction (H) contribute substantially to the antioxidant potential of H. itama propolis [17]. These compounds may include lipophilic antioxidant compounds, which are known to exhibit substantial radical scavenging activity [[32], [34]].
On the other hand, the water fraction showed moderate potent antioxidant activity among the tested samples, with an EC50 value of 39.51 ± 3.54 μg/mL. This result suggests that polar compounds extracted into the fraction (W) may have lower antioxidant capacity compared to those extracted into the fraction (H). This comprehensive analysis of total phenol and flavonoid content, coupled with the DPPH free radical scavenging assay, provides valuable insights into the composition and antioxidant potential of propolis extracts. The contrasting compositions of the fractions suggest distinct mechanisms contributing to their photoprotective efficacy, underscoring the importance of solvent selection and fractionation in maximizing the bioactivity of propolis extracts [[32], [34]].
3.4. Anti-collagenase properties of the propolis extracts
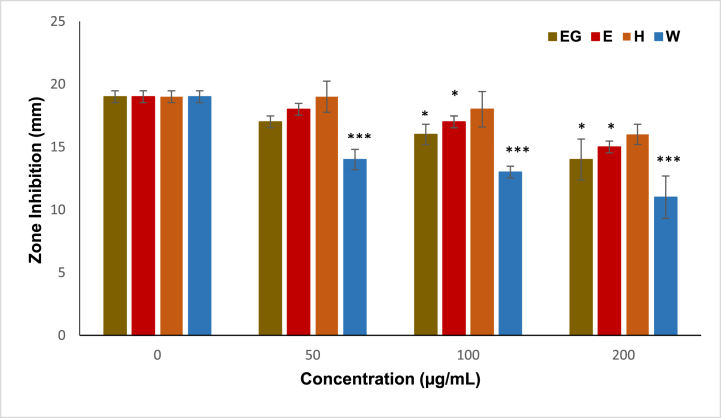
The propolis extract and its fractions inhibited collagenase activity dose-dependently across a range of concentrations (0–200 μg/mL) in Fig. 5. Notably, water fraction exhibited particularly remarkable collagenase inhibition properties, with its effectiveness increasing with higher concentrations. Strikingly, the inhibition observed with the fraction (W) surpassed that of the positive control, EG, at certain concentrations.
Fig. 5.
Collagenase inhibition properties of the crude ethanol extract (E) of H. itama propolis from the involucrum of H4 and its solvent fractions (H) and (W), epigallocatechin gallate (EG). (EG) at various concentrations (0–200 μg/mL), compared with the negative control, Statistical analysis was performed using One-way ANOVA followed by Dunnett's test, with significance levels denoted as ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 for comparisons against the positive control (EG).
This finding underscores the potency of the fraction (W) in inhibiting collagenase activity, suggesting its potential as a highly effective natural collagenase inhibitor. The increased inhibition observed with fraction (W) could be linked to the presence of distinct bioactive compounds extracted into this fraction, including hydrophilic phenolic compounds and flavonoids. These compounds are known for their antioxidant and anti-inflammatory properties, which are conducive to mitigating collagen degradation and preserving skin elasticity. Propolis obtained from various locations, such as Greek propolis and Brazilian propolis, is reported to possess significant anti-collagenase properties, which support their usage in anti-ageing formulations [13,35].
3.5. Cytotoxic properties of the crude ethanol extract (E) of H. itama propolis from the involucrum (H4) and its solvent fractions (H) and (W)
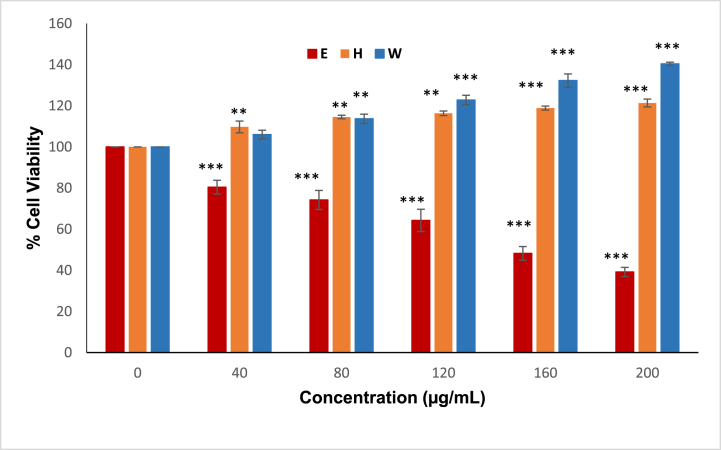
The cytotoxic characteristics of the crude ethanol extract (E) and its solvent fractions (H and W) from the involucrum layer of H4 produced by H. itama propolis reveal novel insights into their impact on cell viability. The extract (E) was cytotoxic to HaCaT cells, indicating possible negative effects on cellular health. Contrastingly, the fractions (H and W) demonstrated distinct behaviour, as shown in Fig. 6. These fractions exhibited non-toxic effects on the cells and, intriguingly, even stimulated cell proliferation. Such findings suggest that the bioactive compounds responsible for cytotoxicity in the extract (E) may have been selectively concentrated or removed during the fractionation process.
Fig. 6.
Cytotoxicity of the crude ethanol extract (E) of H. itama propolis from the involucrum layer of H4 and its solvent fractions (H) and (W) at various concentrations (0–200 μg/mL) against HaCaT cells (n = 3). Statistical analysis involved conducting One-way ANOVA followed by the Dunnett test to identify significant differences among the groups (∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001).
The result is consistent with previous research indicating the existence of bioactive chemicals in ethanol extracts of propolis with cytotoxic characteristics against certain cell types such as HaCaT, HeLa, and MCF-7 [36,[11], [37]]. In a study by Bae et al. [36], the cytotoxic effects of Korean propolis were investigated using HaCaT cells. Interestingly, the crude ethanol extract of propolis exhibited cytotoxicity, highlighting potential concerns regarding its direct application. However, upon fractionation with water and methylene chloride, distinct patterns emerged. The lipophilic fraction displayed slightly higher cytotoxicity compared to the crude extract, suggesting the presence of bioactive compounds that may contribute to this effect. In contrast, the hydrophilic fraction demonstrated significantly reduced cytotoxicity, indicating a more favourable profile in terms of cellular safety.
The differences in the cytotoxicity between the crude ethanol extract (E) and fractions (H and W) underscore the importance of fractionation in modulating the biological effects of propolis extracts. Fractionation serves as a crucial tool for isolating and concentrating specific bioactive compounds, thereby altering the overall biological activity of the extract [38]. In the context of this study, fractionation not only mitigated the cytotoxic effects of the crude ethanol extract but also promoted cell proliferation, suggesting the presence of potentially beneficial compounds that warrant further exploration.
This highlight the complex chemical composition of propolis and the nuanced effects that different extraction methods and solvent fractions can impart on cellular physiology [39]. Moreover, they emphasize the significance of fractionation techniques in harnessing the therapeutic potential of natural products like propolis while mitigating potential adverse effects.
3.6. Chemical constituents identification in the active water fraction of H. itama propolis using LC-MS/MS analysis
From Table 4, it is clear that the active fraction (W) of propolis from the involucrum layer of H4 exhibited diverse chemical structures and classes, including flavonoids, terpenoids, polyphenols, and lignans, all known for their potential photoprotective properties. Flavonoids such as dihydrokaempferol, pinobanksin-5-methyl-ether, pinocembrin, catechin, and myricetin are recognized for their UV-absorbing capabilities, which contribute to photoprotection by reducing UV-induced damage to the skin [40]. This effectiveness can be attributed to their structure, specifically the presence of aromatic rings and hydroxyl groups, which enhance UV absorption and antioxidant activities.
Table 4.
Chemical constituents identified in the active water fraction (W) of H. itama propolis from the involucrum layer of H4 using LC-MS/MS analysis.
| No | RT | Compound Name | Mode | Molecular Weight | m/z ratio | Class | References |
|---|---|---|---|---|---|---|---|
| 1 | 6.68 | Matairesinol | – | 358 | 357, 221, 137, 122, 83 | Lignan | [15] |
| 2 | 7.03 | 5-[2-(4-Allyl-2 methoxy phenoxy)propyl]-1,2,3 trimethoxy benzene | + | 390 | 373, 151 | Ether | Database |
| 3 | 7.27 | Pratensein | + | 300 | 301, 286, 269 | Flavonoid | Database |
| 4 | 7.76 | 1,5-Bis(2,5-dimethoxyphenyl) pentane 1,5-dione | + | 372 | 373, 177, 151,137 | Diketones | Database |
| 5 | 8.07 | Dihydrokaempferol | – | 288 | 287,259, 177 | Flavonoid | [16] |
| 6 | 8.57 | Methoxyflavonone | + | 440 | 441, 317, 183, 165, 139 | Flavonoid | [16] |
| 7 | 8.67 | Pinobanksin-5-methyl-ether | + | 286 | 285, 165, 137 | Flavonoid | [16] |
| 8 | 8.79 | Ethyl 3-({[(3R,4S)-4-{2-[(3,4 dimethoxybenzyl)amino]-2-oxoethyl}-3 ethyl-1 piperidinyl]carbonyl}amino)benzoate | + | 386 | 387, 151 | Benzenoids | Database |
| 9 | 8.88 | Pinocembrin | – | 256 | 255, 213, 171, 151 | Flavonoid | [16] |
| 10 | 9.28 | Gallyol catechin | – | 442 | 441, 289, 151 | Flavonoid | [19] |
| 11 | 9.61 | Quercetin | – | 302 | 283, 255 | Flavonoid | [16] |
| 12 | 9.84 | Gallyol afzelechin | + | 426 | 425,273, 151 | Polyphenols | [41] |
| 13 | 12.73 | Ursolic acid derivative | – | 486 | 485, 455, 391 | Terpenoid | Database |
| 14 | 13.02 | Puerarol | + | 422 | 423, 367,311, | Flavonoid | Database |
| 15 | 16.14 | Oleanolic acid | + | 456 | 457, 439, 95 | Terpene | [16] |
| 16 | 17.23 | 3-Hydroxyurs-12-en-23-oic acid | – | 456 | 455, 437 | Terpene | Database |
| 17 | 18.06 | (3E)-3-(Hydroxymethyl)-2 oxo-5-[(1S,8aS)-5,5,8a-trimethyl-2 methylenedecahydro-1-naphthalenyl] 3-pentenoic acid | + | 398 | 399, 137, 95 | Terpene | [16] |
| 18 | 19.45 | 18-β-Glycyrrhetinic acid | – | 470 | 469, 407 | Terpene | Database |
| 19 | 19.49 | Myricetin | – | 602 | 601, 313,109 | Flavonoid | [16] |
| 20 | 26.03 | Oleanolic acid | + | 438 | 203, 191, 109, 95 | Terpene | [16] |
Quercetin and methoxyflavonone are notable among the flavonoids due to their documented photoprotective properties. Quercetin's structure, characterized by multiple hydroxyl groups and an aromatic ring, enhances its ability to scavenge free radicals and counter UV-induced oxidative stress, making it a promising candidate for photoprotection [42]. Similarly, methoxyflavonone, with its higher molecular weight and additional methoxy groups, exhibits increased UV absorption and photoprotective efficacy. Research indicates that polymethoxyflavone and hydroxy-polymethoxyflavone, which contain multiple methoxy groups, show promising anti-photoaging effects, suggesting that methoxy substitutions in flavonoids enhance their photoprotective properties [43].
Terpenoids such as oleanolic acid derivatives and 18-β-glycyrrhetinic acid also demonstrate photoprotective potential due to the presence of specific functional groups. These compounds possess anti-inflammatory properties that can mitigate UV-induced inflammation, a key aspect of photodamage [44]. Additionally, their antioxidant activity aids in neutralizing reactive oxygen species generated upon UV exposure, contributing to skin protection. Ursolic Acid Derivative and 3-Hydroxyurs-12-en-23-oic Acid might contribute to photoprotection through similar mechanisms, with specific functional groups that support their anti-inflammatory and antioxidant actions.
Polyphenols such as ellagic acid glucoside, characterized by its lactone ring and multiple hydroxyl groups, offer photoprotective benefits by counteracting UV-induced oxidative stress and inflammation [45]. 5-[2-(4-allyl-2-methoxyphenoxy) propyl]-1,2,3-trimethoxybenzene and 1,5-bis(2,5-dimethoxyphenyl) pentane-1,5-dione exhibit potential antioxidant properties due to their methoxy groups and aromatic structures. Lignans such as matairesinol, with its dibenzylbutane skeleton, may also contribute to photoprotection through their antioxidant actions, although further research is needed to explore their specific photoprotective mechanisms [46].
The diverse array of phytochemical compounds listed presents a rich source of natural photoprotective agents. Their mechanisms, ranging from UV absorption to antioxidant and anti-collagenase activities, collectively contribute to shielding against UV-induced skin damage, highlighting their potential in skincare formulations for photoprotection.
One of the main limitations of this study is the potential variability in the chemical composition of propolis between different hives and geographic regions [10,12]. Propolis is known to be highly dependent on local environmental factors, including plant sources and climate, which can lead to differences in its bioactive components [21]. While we focused on propolis from Heterotrigona itama colonies in a specific region, it is important to acknowledge that the composition of propolis could vary significantly in other areas. Future studies could explore the geographic variability in greater detail to strengthen the generalizability of these findings. Further investigation with a larger sample size would allow for a more comprehensive understanding of the bioactivity of propolis across different colonies.
Finally, while we optimized our experimental conditions to closely mimic real-world application scenarios, there are inherent limitations in in-vitro models and lab-based sunscreen formulations compared to clinical applications.
4. Conclusion
In conclusion, this research highlights the promising photoprotective potential of the water fraction derived from H. itama propolis, specifically from the involucrum structure of the beehive. Comprehensive analysis, including SPF assessment, UV absorption, antioxidant activity, and chemical profiling, revealed significant findings. The water fraction demonstrated superior SPF efficacy compared to the hexane fraction and crude ethanol extract, alongside notable antioxidant and anti-collagenase activities. Importantly, it exhibited non-toxicity towards HaCaT cells, unlike the ethanol extract. Furthermore, LC-MS/MS analysis identified bioactive flavonoids within the water fraction, including pratensein, quercetin, and myricetin. These findings underscore the potential of the water fraction from H. itama propolis as a natural and effective ingredient for sunscreen and skincare formulations. Its strong photoprotective properties, combined with antioxidant and anti-aging effects, make it an attractive candidate for the development of innovative skincare products. The natural origin and non-toxic nature of the water fraction offer additional consumer appeal, aligning with the increasing demand for sustainable and safe alternatives in the cosmetics industry. Further research on the specific bioactive compounds in propolis and formulation studies, including testing in human in vitro models, could leverage these insights to develop novel photoprotective products with enhanced efficacy and safety profiles, potentially paving the way for new advancements in natural UV protection solutions.
CRediT authorship contribution statement
Sivakumar Mohan: Writing – original draft, Methodology, Formal analysis, Data curation, Conceptualization. Wahizatul Afzan Azmi: Writing – review & editing, Supervision, Formal analysis, Data curation. Rameshkumar Santhanam: Writing – review & editing, Writing – original draft, Validation, Methodology, Formal analysis, Data curation, Conceptualization. Nor Ehsan Abd Rahman: Visualization, Resources, Investigation. Wan Iryani Wan Ismail: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Data and code availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Rameshkumar Santhanam, Email: ramesh@umt.edu.my.
Wan Iryani Wan Ismail, Email: waniryani@umt.edu.my.
References
- 1.Raymond-Lezman J.R., Riskin S.I. Benefits and risks of sun exposure to maintain adequate vitamin D levels. Curēus. 2023 doi: 10.7759/cureus.38578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santhanam R., Karunakaran T., Sowndhararajan K., Zulkifli M.F., Kothandaraman M.G., Aravindhan V., Ismail W.I.W. Photoprotective potential, cytotoxicity, and UPLC-QTOF/MS analysis on bioactive solvent fractions of moringa concanensis nimmo bark. Evid. base Compl. Alternative Med. 2022:1–10. doi: 10.1155/2022/3781189. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tong S., Bambrick H., Beggs P.J., Chen L., Hu Y., Ma W., Steffen W., Tan J. Current and future threats to human health in the Anthropocene. Environ. Int. 2022;158 doi: 10.1016/j.envint.2021.106892. [DOI] [PubMed] [Google Scholar]
- 4.Bernhard G., Neale R.E., Barnes P.W., Neale P.J., Zepp R.G., Wilson S.R., Andrady A.L., Bais A.F., McKenzie R.L., Aucamp P.J., Young P., Liley B., Lucas R., Yazar S., Rhodes L.E., Byrne S.N., Hollestein L., Olsen C.M., Young A.R.…White C.C. Environmental effects of ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2017. Photochem. Photobiol. Sci. 2018;17(2):127–179. doi: 10.1039/c7pp90043k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawda D., Shinde P. Effects of solar radiation on the eyes. Curēus. 2022 doi: 10.7759/cureus.30857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vechtomova Y.L., Телегина Т.А., Buglak A.A., Kritsky M.S. UV radiation in DNA damage and repair involving DNA-Photolyases and cryptochromes. Biomedicines. 2021;9(11):1564. doi: 10.3390/biomedicines9111564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moeller M., Pawlowski S., Petersen‐Thiery M., Miller I., Nietzer S., Heisel-Sure Y., Kellermann M.Y., Schupp P.J. Challenges in current coral reef protection – possible impacts of UV filters used in sunscreens, a critical review. Front. Mar. Sci. 2021;8 doi: 10.3389/fmars.2021.665548. [DOI] [Google Scholar]
- 8.Gholap A.D., Sayyad S.F., Hatvate N.T., Dhumal V.V., Pardeshi S.R., Chavda V.P., Vora L.K. Drug delivery strategies for Avobenzone: a case study of photostabilization. Pharmaceutics. 2023;15(3):1008. doi: 10.3390/pharmaceutics15031008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ballestín S.S., Bartolomé M.J.L. Toxicity of different chemical components in sun cream filters and their impact on human health: a review. Appl. Sci. 2023;13(2):712. doi: 10.3390/app13020712. [DOI] [Google Scholar]
- 10.Chuttong B., Lim K., Praphawilai P., Danmek K., Maitip J., Vit P., Wu M., Ghosh S., Jung C., Burgett M., Hongsibsong S. Exploring the functional properties of propolis, geopropolis, and cerumen, with a special emphasis on their antimicrobial effects. Foods. 2023;12(21):3909. doi: 10.3390/foods12213909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ismail Wan Iryani Wan, Hussin Nurul Noratikah, Mazlan Siti Nur Farhanim, Hussin Nur Hazlin, Radzi Mohd Naim Fadhli Mohd. Physicochemical Analysis, Antioxidant and Anti Proliferation Activities of Honey, Propolis and Beebread Harvested from Stingless Bee. IOP. Conf. Ser. Mater. Sci. Eng. 2018;440 doi: 10.1088/1757-899X/440/1/012048. [DOI] [Google Scholar]
- 12.Stančiauskaitė M., Marksa M., Rimkienė L., Ramanauskienė K. Evaluation of chemical composition, sun protection factor and antioxidant activity of Lithuanian propolis and its plant precursors. Plants. 2022;11(24):3558. doi: 10.3390/plants11243558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivera-Yañez C.R., Ruiz-Hurtado P.A., Mendoza-Ramos M.I., Reyes-Reali J., García-Romo G.S., Pozo-Molina G., Reséndiz-Albor A.A., Nieto-Yañez O., Méndez-Cruz A.R., Méndez-Catalá C.F., Rivera-Yañez N. Flavonoids present in propolis in the battle against photoaging and psoriasis. Antioxidants. 2021;10(12):2014. doi: 10.3390/antiox10122014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karapetsas A., Voulgaridou G., Konialis M., Tsochantaridis I., Kynigopoulos S., Lambropoulou M., Stavropoulou M., Σταθοπούλου Κ., Aligiannis N., Bozidis P., Goussia A., Gardikis Κ., Panayiotidis M.Ι., Pappa A. Propolis extracts inhibit UV-Induced photodamage in human experimental in vitro skin models. Antioxidants. 2019;8(5):125. doi: 10.3390/antiox8050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stavropoulou M., Σταθοπούλου Κ., Cheilari A., Benaki D., Gardikis Κ., Chinou İ., Aligiannis N. NMR metabolic profiling of Greek propolis samples: comparative evaluation of their phytochemical compositions and investigation of their anti-ageing and antioxidant properties. J. Pharmaceut. Biomed. Anal. 2021;194 doi: 10.1016/j.jpba.2020.113814. [DOI] [PubMed] [Google Scholar]
- 16.Valverde T.M., De Souza Soares B.N.G., Nascimento A.M., Andrade Â.L., Sousa L.R.D., De Abreu Vieira P.M., Santos V.R., Seibert J.B., De Almeida T.C.S., Rodrigues C.F., Oliveira S.R.M., Martins F.P., Júnior J.G.F., Santos V.M.R.D. Anti-Inflammatory, Antimicrobial, antioxidant and photoprotective investigation of Red Propolis extract as sunscreen formulation in Polawax Cream. Int. J. Mol. Sci. 2023;24(6):5112. doi: 10.3390/ijms24065112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fu Y., Wan R., Yang L., Xiong L., Hu J., Tang J., He H., Gu Z., Li L., Li Y. Propolis inspired sunscreens for efficient UV-protection and skin barrier maintenance. Nano Res. 2022;15(9):8237–8246. doi: 10.1007/s12274-022-4434-z. [DOI] [Google Scholar]
- 18.Pangestika N.W., Atmowidi T., Kahono S. Additional nest structures and natural enemies of stingless bees (Hymenoptera: apidae: Meliponinae) JurnalSumberdayaHayati. 2020;4(2):42–47. doi: 10.29244/jsdh.4.2.42-47. [DOI] [Google Scholar]
- 19.Karunakaran T., Goh Y.S., Santhanam R., Murugaiyah V., Bakar M.H.A., Ramanathan S. RP-HPLC-DAD analysis of mitragynine content in mitragyna speciosa korth. (Ketum) leaf extracts prepared using ultrasound assisted extraction technique and their cytotoxicity. Separations. 2022;9(11):345. doi: 10.3390/separations9110345. [DOI] [Google Scholar]
- 20.Lim J.R., Chua L.S., Dawood D.a.S. Evaluating biological properties of stingless bee propolis. Foods. 2023;12(12):2290. doi: 10.3390/foods12122290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woźniak M., Sip A., Mrówczyńska L., Broniarczyk J., Waśkiewicz A., Ratajczak I. Biological activity and chemical composition of propolis from various regions of Poland. Molecules/Molecules Online/Molecules Annual. 2022;28(1):141. doi: 10.3390/molecules28010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menyiy N.E., Bakour M., Ghouizi A.E., Guendouz S.E., Lyoussi B. Influence of geographic origin and plant source on physicochemical properties, mineral content, and antioxidant and antibacterial activities of Moroccan propolis. International Journal of Food Science. 2021:1–12. doi: 10.1155/2021/5570224. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powell N. Stingless bee basics- hive make-up. Australian Native Bee. 2017, February 4 https://www.australiannativebee.com/2016/10/14/hive-make-up/ [Google Scholar]
- 24.Salleh S.N.a.S., Hanapiah N.a.M., Ahmad H., Johari W.L.W., Osman N.H., Mamat M.R. Determination of total phenolics, flavonoids, and antioxidant activity and GC-MS analysis of Malaysian stingless bee propolis water extracts. Scientifica. 2021:1–11. doi: 10.1155/2021/3789351. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Šuran J., Cepanec I., Mašek T., Radić B., Radić S., Gajger I.T., Vlainić J. Propolis Extract and its Bioactive Compounds—from traditional to modern extraction technologies. Molecules/Molecules Online/Molecules Annual. 2021;26(10):2930. doi: 10.3390/molecules26102930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun M., Deng Y., Cao X., Xiao L., Ding Q., Fuqing L., Huang P., Gao Y., Liu M., Zhao H. Effects of natural polyphenols on skin and hair health: a review. Molecules/Molecules Online/Molecules Annual. 2022;27(22):7832. doi: 10.3390/molecules27227832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gabros S., Nessel T.A., Zito P.M. Sunscreens and photoprotection. StatPearls - NCBI Bookshelf. 2023, July 17 https://www.ncbi.nlm.nih.gov/books/NBK537164/ [PubMed] [Google Scholar]
- 28.Ferreira J.M., Negri G., Salatino M.L.F., Message D., Salatino A. Chemical profile and antioxidant activity of geopropolis from Melipona subnitida collected inside and outside the nest. In Figshare. 2023 doi: 10.6084/m9.figshare.21946330. [DOI] [Google Scholar]
- 29.Ghazi S. Do the polyphenolic compounds from natural products can protect the skin from ultraviolet rays? Results in Chemistry. 2022;4 doi: 10.1016/j.rechem.2022.100428. [DOI] [Google Scholar]
- 30.Kustiawan P.M., Zulfa A.F., Batistuta M.A., Hanifa D.N.C., Setiawan I.M. vol. 18. Malaysian Journal of Medicine & Health Sciences; 2022. (Comparative Analysis of Phytochemical, Total Phenolic Content, Antioxidant and Antibacterial Activity of Two Species Stingless Bee Propolis from East Kalimantan). [Google Scholar]
- 31.Al-Hatamleh M.a.I., Boer J.C., Wilson K., Plebanski M., Mohamud R., Mustafa M.Z. Antioxidant-based medicinal properties of stingless Bee products: recent progress and future directions. Biomolecules. 2020;10(6):923. doi: 10.3390/biom10060923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarıkahya N.B., Varol E., Okkalı G.S., Yücel B., Mărgăoan R., Nalbantsoy A. Comparative study of antiviral, cytotoxic, antioxidant activities, total phenolic profile and chemical content of propolis samples in different colors from Turkiye. Antioxidants. 2022;11(10):2075. doi: 10.3390/antiox11102075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Apak R., Özyürek M., Güçlü K., Çapanoğlu E. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms, and electron transfer (ET)-Based assays. J. Agric. Food Chem. 2016;64(5):997–1027. doi: 10.1021/acs.jafc.5b04739. [DOI] [PubMed] [Google Scholar]
- 34.Rajauria G. In-vitro antioxidant properties of lipophilic antioxidant compounds from 3 Brown seaweed. Antioxidants. 2019;8(12):596. doi: 10.3390/antiox8120596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez B.G., Marcucci M.C., Rocco S.A., Sforça M.L., Eberlin M.N., Hewitson P., Ignatova S., Sawaya A.C.H.F. Preparative fractionation of Brazilian red propolis extract using Step-Gradient Counter-Current chromatography. Molecules/Molecules Online/Molecules Annual. 2024;29(12):2757. doi: 10.3390/molecules29122757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bae I.A., Ha J.W., Choi J.Y., Boo Y.C. Antioxidant effects of Korean propolis in HACAT keratinocytes exposed to particulate matter 10. Antioxidants. 2022;11(4):781. doi: 10.3390/antiox11040781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arung E.T., Ramadhan R., Khairunnisa B., Amen Y., Matsumoto M., Nagata M., Kusuma I.W., Paramita S., Sukemi Yadi, Tandirogang N., Takemoto N., Syafrizal S., Kim Y.U., Shimizu K. Cytotoxicity effect of honey, bee pollen, and propolis from seven stingless bees in some cancer cell lines. ~Al-œMi’galaẗ Al-sa’udiyaẗ Lī-ulum Al-ḥayaẗ. 2021;28(12):7182–7189. doi: 10.1016/j.sjbs.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stéphane F.F.Y., Jules B.K.J., Batiha G.E., Ali I., Bruno L.N. IntechOpen eBooks; 2022. Extraction of Bioactive Compounds from Medicinal Plants and Herbs. [DOI] [Google Scholar]
- 39.Woźniak M., Mrówczyńska L., Kwaśniewska-Sip P., Waśkiewicz A., Nowak P., Ratajczak I. Effect of the solvent on propolis phenolic profile and its antifungal, antioxidant, and in vitro cytoprotective activity in human erythrocytes under oxidative stress. Molecules/Molecules Online/Molecules Annual. 2020;25(18):4266. doi: 10.3390/molecules25184266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Forero A.G., Mantilla D.a.V., Núñez L.A., Ocazionez R.E., Stashenko E.E., Fuentes J.L. Photoprotective and antigenotoxic effects of the flavonoids apigenin, naringenin and pinocembrin. Photochem. Photobiol. 2019;95(4):1010–1018. doi: 10.1111/php.13085. [DOI] [PubMed] [Google Scholar]
- 41.Lim J.R., Chua L.S., Soo J. Study of stingless bee (Heterotrigona itama) propolis using LC-MS/MS and TGA-FTIR. Applied Food Research. 2023;3(1) doi: 10.1016/j.afres.2022.100252. [DOI] [Google Scholar]
- 42.Li L., Chong L., Huang T., Ma Y., Li Y., Ding H. Natural products and extracts from plants as natural UV filters for sunscreens: a review. Animal Models and Experimental Medicine. 2022;6(3):183–195. doi: 10.1002/ame2.12295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li G., Tan F., Zhang Q., An-Qun T., Cheng Y., Zhou Q., Liu M., Tan X., Huang L., Rouseff R.L., Wu H., Zhao X., Liang G., Zhao X. Protective effects of polymethoxyflavone-rich cold-pressed orange peel oil against ultraviolet B-induced photoaging on mouse skin. J. Funct.Foods. 2020;67 doi: 10.1016/j.jff.2020.103834. [DOI] [Google Scholar]
- 44.Kong S., Chen H., Yu X., Xie Z., Feng X., Kang X., Li W., Huang N., Luo H., Su Z. The protective effect of 18β-Glycyrrhetinic acid against UV irradiation induced photoaging in mice. Exp. Gerontol. 2015;61:147–155. doi: 10.1016/j.exger.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 45.Michalak M. Plant-Derived Antioxidants: significance in skin health and the ageing process. Int. J. Mol. Sci. 2022;23(2):585. doi: 10.3390/ijms23020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu Q., Wang Y., Li Q. Matairesinol exerts anti-inflammatory and antioxidant effects in sepsis-mediated brain injury by repressing the MAPK and NF-κB pathways through up-regulating AMPK. Aging. 2021:23780–23795. doi: 10.18632/aging.203649. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.