Abstract
The development of effective cancer treatments is a popular in contemporary medical research. Immunotherapy, the fourth most common cancer treatment method, relies on activating autoimmune function to eradicate tumors and exhibits advantages such as a good curative effect and few side effects. In recent years, tumor vaccines that activate the stimulator of interferon genes (STING) pathway are being actively researched in the field of immunotherapy; however, their application is still limited because of the rapid clearance rate of tumor-related lymph nodes and low efficiency of antigen presentation. The rise of nanomedicine has provided new opportunities for solving these problems. By preparing materials with adjuvant effects nanoparticles, the small size of nanoparticles can be exploited to enable the entry of vaccines into tumor-related lymph nodes to accurately deliver STING agonists and activate the immune response. Based on this, this paper reviews various types of nano-adjuvants based on metals, platinum chemotherapy drugs, camptothecin derivatives, deoxyribonucleic acid, etc. and highlights the transformation prospects of these nano-adjuvants in tumor vaccines to provide a reference for promoting the development of nano-medicine and tumor vaccinology.
Keywords: Immunotherapy, Tumor vaccine, Nano adjuvant, cGAS-STING
1. Nano-adjuvants as tumor vaccines
1.1. Epidemiology and treatment of cancer
According to the latest global cancer data published by the International Agency for Research on Cancer in 2020, China has become a veritable “big cancer country.” Data show that, of the 19.29 million new cancer cases registered worldwide in 2020, 4.57 million of these new cancer cases were diagnosed in China, accounting for 23.7 % of the new cancer patients worldwide. In 2020, 9.96 million cancer deaths were recorded worldwide, which included 5.53 million males and 4.43 million females. Of the total number of deaths worldwide because of cancer in 2020, 3 million cancer deaths, which included 1.82 million males and 1.18 million females, were recorded in China. Thus, records indicate that China has the highest number of new cases and deaths related to cancer worldwide (see Fig. 1, Fig. 2, Fig. 3, Fig. 4).
Fig. 1.
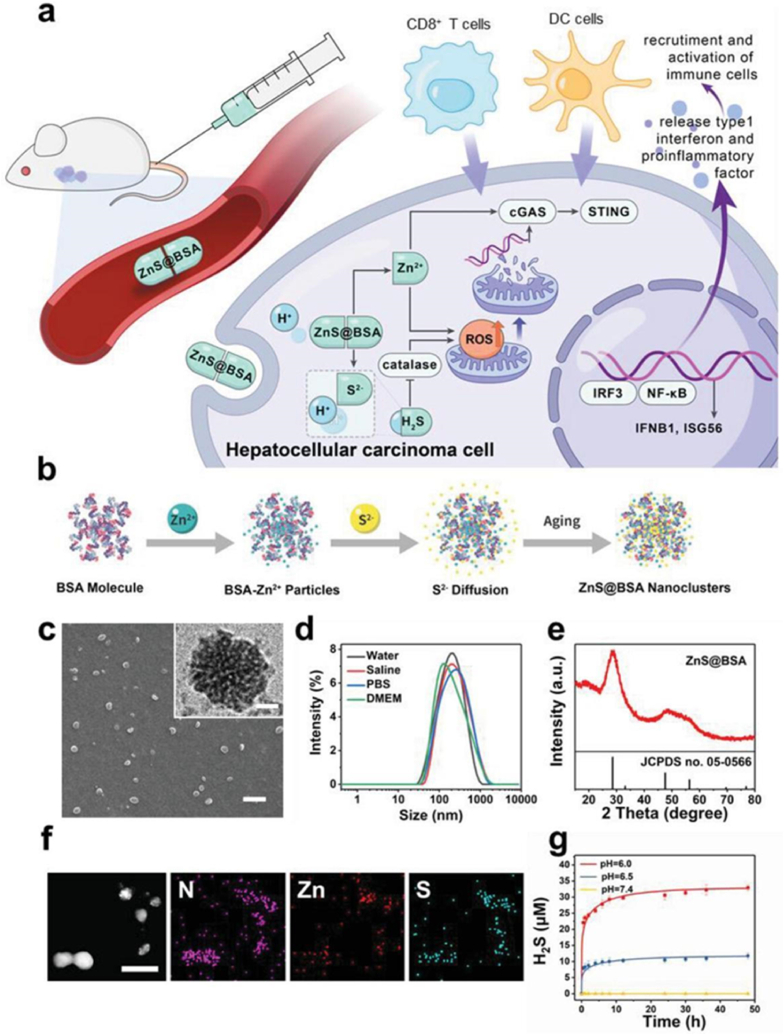
Schematic and characteristics of ZnS@BSA nanoclusters. a) The therap eutic process of ZnS@BSA nanoclusters. b)The synthesis routine of ZnS@BSA nanoclusters. c) Scanning electron microscopy image of ZnS@BSA nanoclusters. Scale bar: 1 μm. Inset: High-resolution transmission electron microscopy imag e. Scale bar: 20 nm. d) Hydrodynamic size of ZnS@BSA nanoclusters in diffe rent solutions. e) X-ray diffraction pattern of ZnS@BSA nanoclusters. f) Eleme nt mapping of ZnS@BSA nanoclusters. Scale bar: 200 nm. g) Release profile of H2S from ZnS@BSA in solutions with different pH of 7.4, 6.5, and 6.0 (n = 3, mean ± standard deviation (SD)). (Reprinted from ref. (79) under the terms and conditions of the Creative Commons Attribution (CCBY) license (http://creativecommons.org/licenses/by/4.0/).).
Fig. 2.
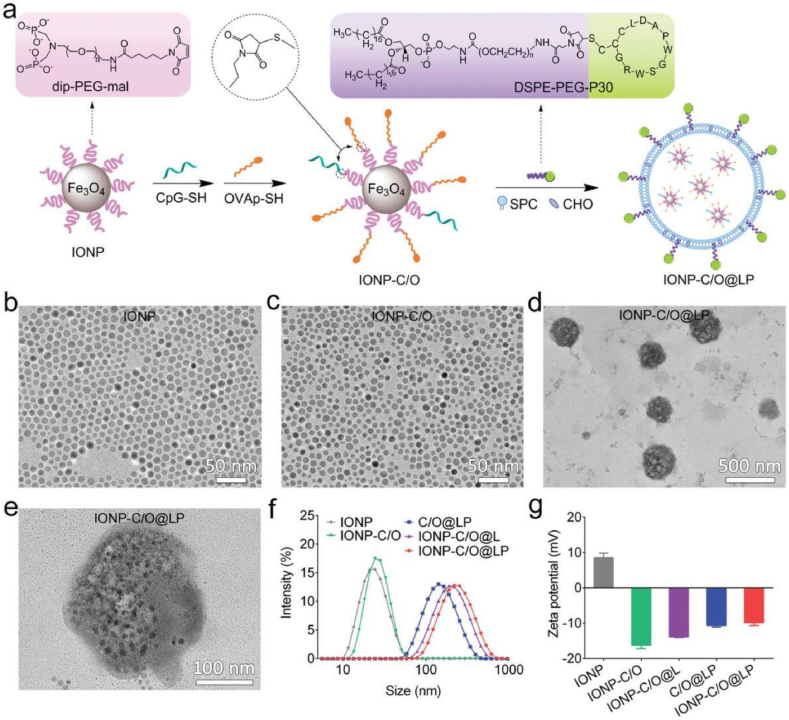
a) Schematic drawing to show the preparative procedures for IONP-C/O@LP. b–e) TEM images of IONP, IONP-C/O conjugates, and IONP-C/O@LP under different magnifications, respectively. f-g) Hydrodynamic size profiles and zeta potentials of IONP,IONP-C/O,IONP-C/O@L,C/O@LP,IONP-C/O@LP nanoformulations, respectively. Data are shown as mean ± SD (n = 3). (Reprinted f rom ref. (82) under the terms and conditions of the Creative Commons Attribution (CCBY) license (http://creativecommons.org/licenses/by/4.0/).).
Fig. 3.
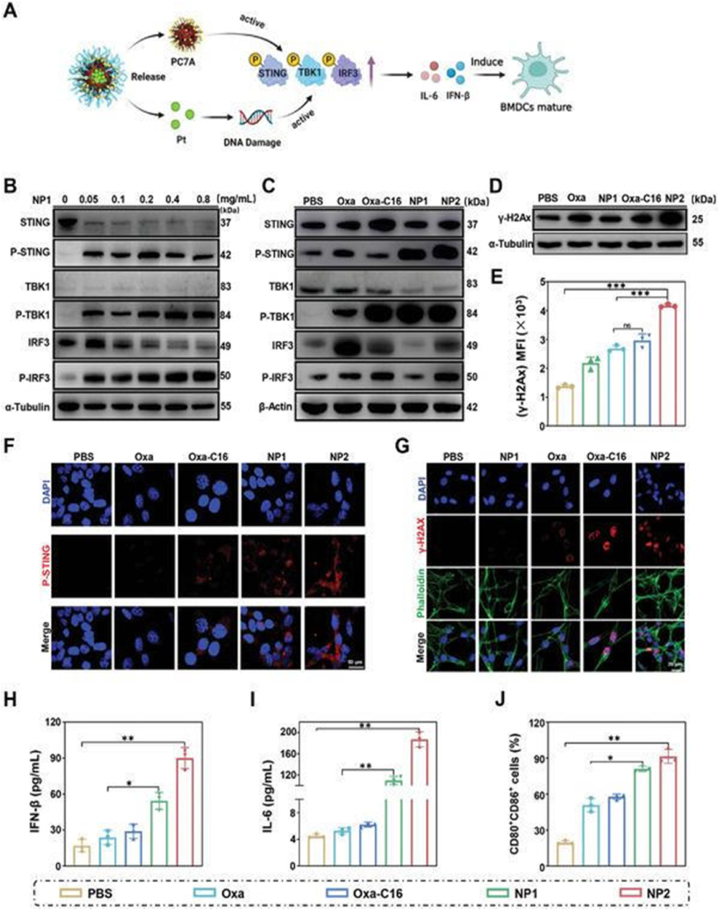
Evaluation of the ability of Oxa, Oxa‐ C16, NP1, or NP2 to intervene in CT26 cancer cells by the STING pathway for therapeutically enhanced com bined chemotherapy and immunotherapy. A) Schematic illustration of the mecha nism of action of NP2 to active the STING pathway. B) Change in the expres sion levels of STING pathway associated proteins upon concentration dependent treatment with NP1 determined by Western Blot analysis. C) Change in the expression levels of STING pathway associated proteins upon treatment determined by Western Blot analysis. D) Change in the expression levels of γ‐ H2A u pon treatment determined by Western Blot analysis. E) Comparison of the expr ession levels of γ‐ H2A determined by flow cytometry. Data are means ± SD. ns = no statistical difference, ∗∗∗p < 0.001 determined by ordinary one‐ way A NOVA and Tukey post‐ hoc tests. F) Immunofluorescence confocal laser scanni ng microscopy images of P‐ STING upon treatment. Scale bar = 50 μm. G) Im munofluorescence confocal laser scanning microscopy images of γ‐ H2AX upon treatment. Scale bar = 20 μm. H) Change of IFN‐ β levels upon treatment deter mined by an ELISA assay (n = 3 independent experiments). I) Change of IL‐ 6 levels upon treatment determined by an ELISA assay (n = 3 independent experim ents). J) Maturation of mouse bone marrow‐ derived dendritic cells determined by flow cytometry. H–J) n = 3 independent experiments. Data are means ± SD. ∗p < 0.05, ∗∗p < 0.01 determined by ordinary one‐ way ANOVA and Tukeypost‐ hoc tests. (Reprinted from ref. (95) under the terms and conditions of th e Creative CommonsAttribution (CCBY)license (http://creativecommons.org/licenses/by/4.0/).).
Fig. 4.
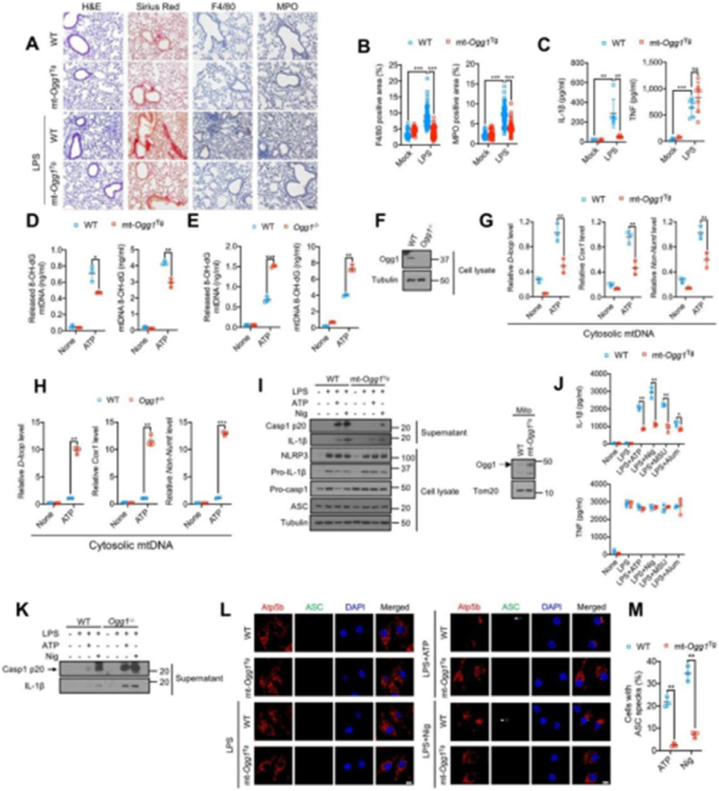
Base excision repair inhibits Ox-mtDNA production and attenuates NL RP3 inflammasome activation.(A) H&E, Sirius red, F4/80 and myeloperoxidase (MPO) antibody staining of lung tissue from mice challenged with 5 mg/kg LP S 24 h prior to tissue collection. n =3 for mock treatment and n = 6–7 mice for LPS treatment. 10–12 images per mouse were analyzed. Scale bar, 100 μm (B) Area (in%) occupied by F4/80 or MPO positive cells in lung sections from(A). Data are means ± s.d.(C) IL-1β and TNF concentrations in BALF from (A) measured by ELISA. Data are means ± s.d. (D and E) 8-OH-dG content of mtDNA from cytosol (left) or mitochondria (right) of LPS (200 ng/mL, 4 h)-primed WT and mt-OggTg(D) or WT and Ogg1−/− (E) BMDM stimulated −/+ ATP (4 mM, 1 h) (F) Immunoblot (IB) analysis of lysates of WT and Ogg1−/− BMDM.(G and H) Relative cytosolic mtDNA amounts in LPS-primed WT and(H) BMDM stimulated −/+ ATP. The relative ratios of D-loop mtDNA, Cox1 mtDNA, or non-NUMT mtDNA are shown. (I) IB analysis of Casp1 p20 and mature IL-1β in supernatants and NLRP3, Pr o-IL-1β, Pro-Casp1, and ASC in lysates of LPS-primed WT or mt-Ogg1Tg BM DM stimulated −/+ ATP (4 mM, 1 h) or nigericin (Nig) (10 μM, 1 h) (left). M itochondrial OGG1 IB in WT and mt-Ogg1Tg BMDM (right) is shown.(J) IL-1 β and TNF secretion by LPS-primed WT or mt-Ogg1Tg BMDM challenged wi th different NLRP3 activators (ATP, 4 mM for 1 h, nigericin, 10 μM for 1 h, MSU, 600 μg/mL for 6 h and alum, 500 μg/mL for 6 h) (K) IB of Casp1 p20 and mature IL-1β in supernatants of LPS-primed WT and Ogg1−/− BMDM sti mulated −/+ ATP (4 mM) or nigericin (10 μM) for 1 h.(L) Representative fluor escent microscopy images of WT or mt-Ogg1Tg BMDM co-stained for Atp5b and ASC before or after LPS priming followed by ATP (4 mM) or nigericin (10 μM) stimulation for 1 h. DAPI stains nuclei. Arrows indicate ASC specks. Scale bar, 5 μm (M) Percentages of cells shown in (L) with ASC specks. n = 15 0 cells per group from 3 independent experiments.IBs are one representative ou t of 3 independent experiments. Results in (D, E, G, H, J and M) are mean ± s.d. (n = 3). ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001. ns, not significant. Two-sided unpaired t-test.(Reprinted from ref. (109) under the terms and conditions of the Cr eativeCommonsAttribution(CCBY)license(http://creativecommons.org/licenses/by/4.0/).
Currently, the clinical treatment methods for tumors primarily include surgical treatment (surgical resection of primary tumors or metastases), radiotherapy (treatment of malignant tumors using α, β, rays and various X-rays generated by radioisotopes), and chemotherapy (treatment of advanced tumors using chemical drugs such as DNA alkylating agents, anti-metabolic drugs, and molecular targeted drugs). These treatment methodologies can effectively treat tumors to a certain extent, but exhibit several drawbacks. For example, tumor resection is only effective only in cases of early detection and in certain tumors without distant metastasis. Moreover, radiotherapy and chemotherapy kill the surrounding healthy tissues along with the malignant tumors, which may lead to tissue necrosis, reduce the activity of natural killer cells, and cause side effects such as hair loss and sweat gland dysfunction. Therefore, new treatment methods must be identified to achieve accurate, safe, and efficient treatment of tumors.
1.2. Immunotherapy
As a new cancer treatment method, cancer immunotherapy is recognized as the most active and promising method for in the comprehensive treatment of cancer in the 21st century. it is also the only method that is expected to completely kill tumor cells at present. It primarily targets the human immune system, activates human immune function in various ways, and kills tumor cells and tumor tissues in the blood by relying on autoimmune function. Currently, the commonly used immunotherapy methods include adoptive lymphocyte therapy [[1], [2], [3]], cytokine therapy [[4], [5], [6]], gene therapy [[7], [8], [9]], anti-tumor antibody therapy [[10], [11], [12]], immune checkpoint (programmed death ligand 1 (PD-L1)/programmed death ligand 2, cytotoxic T-lymphocyte–associated antigen 4) blocking therapy [[13], [14], [15]], and tumor vaccines [[16], [17], [18]].
Immunotherapy has the following advantages: 1) Good curative effect: For patients who are unable to undergo surgery or have been diagnosed with cancer cell recurrence and metastasis, immunocyte therapy can rapidly relieve their clinical symptoms, and most patients experience the therapeutic effect of tumor shrinkage or even disappearance, translating to long-term survival even with tumor. 2) Tail effect: Immunotherapy enhances the recognition and anti-tumor ability of immune cells by activating the human immune system; immune cells have a memory function, which can be maintained for a long time once immunotherapy becomes effective. 3) Broad-spectrum anti-cancer activity: It is effective for a variety of tumors (including tumors that are not suitable for surgery, insensitive to radiotherapy, and resistant to chemotherapy) and can effectively eliminate residual cancer cells and small lesions after surgery to prevent tumor recurrence and metastasis. 4) Small-scale side effects: Studies have shown that immune-related adverse reactions are primarily mild or moderate.
1.3. Adjuvant for tumor vaccine and stimulator of interferon genes (STING) pathway
Tumor vaccines, which are composed of tumor antigens and adjuvants, have emerged as a popular topic in recent research [19]. Their principle of action involves the introductionof tumor antigens into patient bodies in various forms, such as tumor cells, tumor-related proteins or peptides, and genes expressing tumor antigens. This process aims to enhance the ability of the immune systemo eliminate tumors by inducing specific anti-tumor immune responses or reversing the immunosuppressive microenvironment of the tumor. However, the development of tumor vaccines is challenging because of the esfollowing reasons: 1) Limited antigen expression: Tumor vaccines may not fully express all tumor antigens, limiting their ability to effectively prevent or treat tumor diseases. The diversity and complexity of tumor antigens make it difficult to completely optimize the functionality of all possible antigens. 2) Uncertainty in the immune system response: The immune response activated by tumor vaccines may be insufficient to completely eliminate tumors and may simultaneously attack normal cells, leading to adverse reactions. 3) Antigen-intrinsic limitations: Tumor-related proteins, peptides, or genes expressing tumor antigens are prone to degradation in the body, potentially affecting the ability of nano-vaccines to induce immune responses.
The mechanism of using tumor vaccine adjuvants to enhance the specific anti-tumor immune response of the body is primarily based on stimulating innate immune signaling pathways, such as Toll-like receptors (TLRs) [20.21.22] and STING, to activate antigen-presenting cells and stimulate the co-stimulation signals needed to initiate the T-cell response, thus achieving the effect of immunotherapy [20].
The activation of the STING pathway has become a popular research topic in recent years. Professor Joan Massagu é of the Sloan Kettering Cancer Center reported in the academic journal Nature that the inhibition of STING signal can be observed in many cancer cell lines, and that the expression of STING protein decreases with the progression of certain types of cancer (such as melanoma). These findings indicate that the STING signaling pathway is a key inhibitor of cancer metastasis progression, and STING agonists or drugs that activate the STING pathway in the tumor environment may have anti-tumor effects, which can provide checkpoints for preventing the metastasis progression of dormant cancer cells, thus providing a treatable strategy for preventing tumor recurrence [21].
The cyclic guanosine monophosphate (GMP)–adenosine monophosphate (AMP) synthase (cGAS)-STING pathway is a crucial immune signaling pathway in cells. It detects and responds to exogenous and endogenous DNA via the activities of cGAS and STING. In this pathway, cGAS serves as a sensor protein capable of recognizing and binding to cytoplasmic DNA. When cGAS binds to DNA, it catalyzes the synthesis of a secondary signaling molecule called cyclic GMP-AMP (cGAMP). This cGAMP interacts with STING protein, activating it, which then stimulates the production of type I interferon (IFN-I), nuclear factor kappa-B (NF-κB), and other immune-related cytokines. The release and production of these signaling molecules trigger the activation of immune cells, converting many “immunologically cold tumors” into “immunologically hot tumors,” leading to a robust immune response and enhanced cancer immunotherapy that efficiently eliminates tumor cells [22].
Research has shown that in mouse macrophages, the activation of the STING pathway induces the expression of IFN-I-dependent genes, thereby executing macrophage-mediated anti-tumor functions. Conversely, the STING pathway primarily mediates the expression of IFN-I-independent genes in T cells, ultimately promoting T-cell apoptosis. Notably, studies in melanoma and colon cancer mouse models have revealed extensive cluster of differentiation 8 positive (CD8+) T-cell death and rapid tumor growth, which are partially attributable to the independent IFN-I that is mediated by the STING pathway [[23], [24], [25], [26]].
Moreover, in chromosomally unstable tumor cells, after micronuclear rupture induced by chromosomal instability, the release of DNA activates the cGAS-STING pathway, resulting in the production of interleukin-6, which significantly enhances micronuclear rupture and tumor survival [27]. Despite these challenges, activation of the STING immune pathway can induce immune cells to release IFN-I [28,29], mobilizing the activation of both innate and adaptive immune cells to eliminate pathogens or tumor cells, thus exhibiting therapeutic efficacy against most tumors [30].
The activation of the STING pathway typically occurs via two mechanisms. First, the cGAS senses the presence of heterogeneous double-stranded DNA (dsDNA) in the cytoplasm, inducing the production of cGAMP, the natural activating ligand of STING [31,32]. Second, small-molecule STING agonists are delivered directly from the external environment and bind directly to the STING protein to activate downstream pathways [33].
STING agonists can be classified into three categories: 1) drugs that induce DNA damage in cancer cells, such as cisplatin and camptothecin (CPT) [34,35], which leak DNA into the cytoplasm to activate the cGAS-STING signaling pathway; 2) nucleotide-based STING agonists, including interferon-stimulatory DNA, cyclic dinucleotides, and synthetic cyclic dinucleotide analogs [36]; 3) non-nucleotide STING agonists, such as 2′-3′-cGAMP [37]. However, because of their instability and low bioavailability [38], these STING agonists often fail to effectively activate the cGAS-STING pathway, ultimately leading to off-target inflammation and autoimmunity, thus limiting their clinical applications.
1.4. Nano-adjuvant as tumor vaccine
The rise of nanomedicine [39] brings new opportunities for delivering STING agonists and activating immune responses. By preparing materials with an adjuvant effect as nanoparticles, drugs can be loaded and the cross-presentation ability of antigens by antigen-presenting cells can be improved; moreover, nano-materials can be used to promote the activation of immune cells and induce efficient and specific immune responses. Furthermore, nanoparticles can smoothly enter tumor-related lymph nodes and remain in them because of their nano-size, thus improving the accumulation of nano-adjuvants in lymph nodes. Further modification of surface ligands can also enable the directional delivery of specific immune cells, which demonstrates the potential for new applications based on the accurate delivery of STING agonists and activation of the immune response, achieving synergistic activation.
Based on this, this paper reviews certain nano-adjuvants that have been used to activate the STING pathway in tumor vaccine research in recent years (Table 1), and examines the application prospects of these nano-adjuvants in tumor vaccines to provide a reference for promoting the development of nano-medicine and tumor vaccinology.
Table 1.
Different nano-adjuvants activate STING pathway.
| Drug | Materials | Effect and mechanism | Reference position |
|---|---|---|---|
| Metal-based nanoparticle adjuvants | |||
| CMPCDA | MnCl2,1 mg/mL CDNs solution,DOPE-H11 | Adding Mn2+to different concentrations of cGAMP can significantly enhance the type I IFN response of THP1 cells expressing hSTINGR232, hSTINGH232 and hSTINGHAQ. CMP can significantly enhance the activation of ST agonist,ST activation and IFN-β response of THP1 cells in vitro. | [40] |
| HBMn-FA NPs | 0.625 mol/L (NH₄)₂CO₃ solution (containing 8mg/mLHemin),13.3 mg/mL PLGA/DCM solution),1 mol/L MnCl₂ solution,10 mg/mL BSO solution,BSA solution,DSPE-PEG-FA,Milli-Q water | Specifically activating and amplifying cGAS-STING pathway in both tumor cells and antigen presenting cells; skillfully avoiding the delivery difficulty of STING agonists via the activation of STING by endogenous signaling; and.significantly enhancing the therapeutic efficacy of immune check point blockade (a PD-1)to activate systematic immunotherapy. | [41] |
| TriNV | MON (Mesoporous Organosilica Nanoparticle),10 mg/mL Polyethyleneimine (PEI) Solution, KMnO4, H2O, Oleic Acid (OA), 5 mg/mLPoly (allylamine hydrochloride) Solution (PAH), 5 mg/mL,Poly (acrylic acid) Solution (PAA):,Amino-terminated PEG (mPEG-5K-NH2), EDC (Likely 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide, a crosslinking agent), Indocyanine Green (ICG) | ISTVs improve the uptake of TAA by DC cells in the tumor microenvironment, enhance their acquired immunity, and finally, due to the release of Mn2+ in the tumor microenvironment from ISTVs and the synergistic effect with dsDNA released from the rupture of cancer cells caused by photothermal therapy, the activation of the cGAS-STING pathway is promoted, which further promotes the maturation of DC and the polarization of macrophages to M1, thus amplifying the T cell immune response. | [42] |
| CM@Mn | KMnO4,OA,4T1The tumor cell membrane (CM) | A large amount of reactive oxygen species (ROS) is produced in cells to promote cell death, which leads to a large amount of DNA release to activate STING pathway and stimulate the activation of immune cells | [43] |
| G5-pBA/OVA@Mn | G5-PAMAM dendrimer, OVA,3-(bromomethyl) benzoic acid (mBA) and 4-(bromomethyl) benzoic acid (pBA),MnCl2 solution | Because tumor antigen OVA is attached to G5-pBA, DC cells uptake tumor antigen and accelerate the release of Mn2 +, thus activating STING pathway to accelerate the maturation of immune cells such as CD+8 and release immune factors such as IFN-γ. | [44] |
| ZnS@BSA | Bovine serum albumin (BSA),Zn(CH3COO) 2,Na2S | Zn ∼ (2 +) and S ∼ (2-) are released in the acidic environment of tumor cells. Sulfur ion and H ∼ (2 +) in tumor cells lead to the accumulation of ROS and the release of mitochondrial DNA, which activates the STING pathway. Zn ∼ (2 +) enhances the activity of cGAS catalytic enzyme and enhances the STING signal | [45] |
| CFCP | methanol,ethanol, Acetone,1,3,5-Tris (4-aminophenyl)benzene (TAPB),Benzene-1,3,5-tricarbaldehyde (BTC),glacial acetic acid, FeCl3.6H2O, curcumin,H2PtCl6,NaBH4 | Under the mediation of CFCP and its standardization, the cell membrane of tumor cells is destroyed, accelerating cancer cell death in synergy with iron ions. This process releases dsDNA and activates the STING pathway, promoting dendritic cell (DC) maturation. Additionally, it amplifies immune stimulation through interferon induction, thereby inducing systemic immunity | [46] |
| IONP-C/O@LP | denoted as dip-PEG-mal, Fe3O4,thiol-modified CpG,thiol-modified OVAp | Through targeted delivery of OVAp and CpG to the immature DC cytoplasm and lysosomes, the activation rate of DC cells is enhanced and the accumulation of reactive oxygen species is induced, further activating the STING pathway. | [47] |
| Advantages and Disadvantages | Advantages: 1.Metal-based nano-adjuvants for tumor vaccines leverage the size advantage of nanomaterials, enabling them to concentrate more easily in lymph nodes, spleens, and other lymphatic organs. This improves their effectiveness in stimulating the immune response.2.Metal nano-adjuvants, while delivering antigens or drugs, can also activate immune pathways on their own. Metal ions such as Mn2+, Zn2+, and Ca2+ have the ability to trigger immune pathways, further enhancing the immunogenicity of the vaccine Disadvantages:1.During the delivery process, metal nano-adjuvants may be influenced by the internal environment, resulting in unstable drug release or inaccurate targeting to the desired site. This can compromise their therapeutic effectiveness.2.The immunological mechanisms underlying the actions of metal nano-adjuvants in tumor vaccines are not yet fully understood. This lack of clarity may hinder the prediction and optimization of their therapeutic outcomes. |
||
| Nano adjuvant based on platinum/camptothecin -based chemotherapy drugs | |||
| PC7APolymeric Nanoparticles | ester coupling of 2,2,5-trimethyl-1,3-dioxane-5-carboxylic acid,2- (azepan-1-yl)ethanol,1-ethyl-3-(3-dimethylaminopropyl) carbodiimide, 1,3-dioxane heterocycle trifluoroacetic acid,ethyl-2,6-diisocyanatohexanoate,polyethylene glycol,1H and 13C NMR,Oxa-C16,1,2-distearoyl-sn-glycero-3 phosphoethanolamine-N-[polyethyleneglycol2000]ammonium (DSPE-PEG 2000) | PC7A is capable of binding to STING protein and activating STING pathway effectively. Moreover, cisplatin within it can induce DNA damage, releasing DNA to further activate STING pathway | [48] |
| CPT-SS-OA/CM | Oleic acid (OA),2,2-dithiodiethanol or hexanediol, dichloromethane (DCM),CPT,DMAP, Triphosgene,ethanol, Cremophor EL | CPT-SS-OA/CM can be easily internalized into cells and converted into active ester CPT, which activates the cGAS-STING pathway, promoting the maturation of DC cells and the tumor infiltration of CD8+ T cells. Additionally, it reduces the inherent toxicity of camptothecin. | [49] |
| CPT-S-S-LA | OH-S-S-OH(DTDE),triethylamine, tetrahydrofuran (THF),dichloromethane (DCM),NaHCO3,NaN3,N-Dimethylformamide (DMF),lactobionic acid,trifluoroacetic acid | This medication can target the accumulation of CPT at the site of liver cancer tumors, release CPT in tumor cells, induce apoptosis in tumor cells, release tumor DNA, activate a strong STING immune response, and have fewer toxic side effects from CPT. | [50] |
| CPT-Pt (Ⅳ) | Mercaptoacetic acid, trifluoroacetic acid, acetone, lithium aluminium hydride, triphosgene, succinic anhydride, 1,2,4,5-cyclohexa netetracarboxylic dianhydride, mPEG2k-DSPE, and 3-(4,5-dimethylth iazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) Hexadecyl iso cyanate, Cisplatin,Irinotecan hydrochloride trihydrate (CPT-11) | CPT-Pt (Ⅳ) can facilitate the maturation of BMDCs, leading to the accumulation of reactive oxygen species (ROS) in cancer cells, the disruption of mitochondria and the release of DNA, activation of the cGAS-STING pathway, and the subsequent release of type I interferon, promoting DC cell maturation. | [51] |
| SSC-1,SSC-2,and SSC-3 | K2CO3,allylbromide,N,N-Dimethylformamide (DMF)4-Dimethylaminopyridine,acetic anhydride,dry pyridine,dry CH2Cl2,C8H12N4(AIBN),2-mercaptoethoxy ethanol, compound 7,DMTrCl, Camptothecin | Promoting the accumulation of reactive oxygen species (ROS) triggers the disruption of mitochondrial membrane in cancer cells, leading to the release and assembly of mitochondrial DNA into double-stranded DNA (dsDNA). The binding of y with the CGAS protein inside cancer cells activates the STING pathway. | [52] |
| Advantages and disadvantages | Advantages: 1.Due to their nanoscale size, nano-adjuvants are capable of penetrating deep into tumor cells and precisely releasing chemotherapy drugs within the tumor microenvironment, thus effectively killing a large number of cancer cells.2.The extensive death of cancer cells caused by chemotherapy drugs releases a significant amount of dsDNA, which in turn strongly activates the cGAS-STING pathway, leading to a robust immune response. Disadvantages: 1.Chemotherapy drugs can cause damage to healthy cells as well, making the timing and location of their release a major concern. Ensuring the drugs are delivered specifically to tumor cells and released at optimal intervals is crucial.2.The loading capacity of nano-adjuvants for chemotherapy drugs determines their ability to trigger an immune response. Low drug loading represents a significant obstacle in the development of nano-adjuvants carrying chemotherapy drugs. |
||
| DNA nano-adjuvant | |||
| DNA-based nanotubes | oligonucleotides, Atto488-dUTP,Cy3-dUTP,CoCl2,terminal transferase enzyme | CpG oligonucleotides with the DNA tubes promote the production of TNF-α, thereby activating the STING pathway. | [53] |
| Advantages and disadvantages | Advantages: 1. DNA nano-adjuvant tumor vaccines leverage the unique properties of nanomaterials to achieve efficient targeted delivery of drugs or antigens, ensuring their precise localization to tumor tissues while minimizing off-target distribution.2.By optimizing the antigen delivery system, DNA nano-adjuvants can enhance the immune response to vaccines, thereby improving their immunogenicity. Disadvantages: 1.The preparation process of DNA nano-adjuvants is relatively complex, requiring precise control over parameters such as nanoparticle size, shape, and surface properties to ensure efficient delivery and stable release in vivo.2.Due to the complexity of the preparation process and the specificity of the required materials, the cost of DNA nano-adjuvants tends to be relatively high. |
||
| Other | |||
| PC7A NP | antigen, polymeric nanoparticle | It induces a strong cytotoxic T cell response and reduces the expression of systemic cytokines. Mechanistically, PC7A NP achieves effective cytoplasmic delivery of tumor antigens to APCs in draining lymph nodes, leading to increased surface presentation while activating the STING pathway, subsequently activating type I interferon-stimulated genes | [54] |
| STING-NPs | Butyl methacrylate (BMA), poly (ethylene glycol)4-cyano-4-(phenylcarbonothioylthio)pentanoate, Mn = 2000 Da and Mn = 10,000 Da (PEG-CPADB),4cyano-4-(phenylcarbonothioylthio)pentanoate (CPADB), N,N′-Dicyclohexylcarbodiimide (DCC),4-(Dimethylamino)pyridine (DMAP), DL-Dithiothreitol (DTT), 4,4′-azobis (4 Cyanovaleric acid) (V501), dichloromethane,1,4-dioxane,and poly (ethylene glycol) methyl ether (Mn = 5,000Da)2-(Diethylamino)ethyl methacrlate (DEAEMA) 2,2′-Azobis (4 methoxy-2,4-dimethylvaleronitrile) | Increased bioactivity of cGAMP enhances the STING signaling in the tumor microenvironment and sentinel lymph nodes, transforming the immunosuppressive tumor into an immunogenic tumor-killing microenvironment. This leads to enhanced therapeutic efficacy of cGAMP, suppressing tumor growth, improving long-term survival rates, amplifying response to immune checkpoint blockade, and inducing immune memory to protect against tumor re-challenge. | [55] |
| DMXAA | flavone-acetic,5,6-dimethylxanthenone-4-acetic,Flavone-8-Acetic Acid | Activate the immune system to increase the quantities of white blood cells, interleukin-6, tumor necrosis factor, and chemokines, inducing cellular apoptosis and activating the STING pathway. | [56] |
| MOF-Cp G-DMXAA | Fumaric aicd,CpG ODNs,DMXAA,MOF-801,Zr6 clusters,DNA (50 μm,uniform size and zeta potential at around +45 mV) | Can polarize TAMs in the cell to promote the maturation of DC cells, including DMXAA, which can activate the STING pathway. Metal-Organic Framework (MOF-801) can be recognized by TLR4 to activate the STING-NF-κB pathway, thereby promoting the maturation of dendritic cells. | [57] |
| MONCPT | 2O(S)-camptothecin,10-hydroxy-20(S)-camptothecin,10-methoxy-20-(S)-camptothecin,DMSO solution | Regulate the expression of matrix metalloproteinase-9, control the cell cycle, induce extensive cell death, release DNA to activate the cGAS-STING pathway, and promote DC cell maturation. | [58] |
CMPCDA:CMP,CDN-Mn2+particle,CDN,cyclicdinucleotide; CDA,c-di- AMP,AMP,a denosine monophosphate; CM@Mn:CM,tumor cell membrane; G5-PAMAM,Poly(a midoamine) dendrimer; ZnS@BSA: BSA,bovine serum albumin; CFCP: iron-base d covalent organic framed nanoadjuvant doped with curcumin and platinum; IO NP-C/O@LP: IONP, iron oxide nanoparticles; L,encapsulated by lipid film; P,bearin g a DC-targeting cyclic peptide P30; PC7A,encapsulation of chemotherapeutic pl atinum complexes with a polymer with a cyclic seven-membered ring; CPT-SS- OA: CPT,camptothecin; SS,disulfide bond; OA,oleic acid; CPT-S-S-LA: LA,lactose; SSC,Aptamer−drug conjugates; PC7A NP, a minimalist nanovaccine by a simplephysical mixture of an antigen with a synthetic polymeric nanoparticle; STING- NPs: STING, agonists o stimulator of interferon genes; DMXAA, Vadimezan; MOF-CpG-DMXAA:MOF,metal-organic framework; CpG,cytosine-phosphate-guanine; MONCPT,a topoisomerase I inhibitor.
2. Nanoparticle adjuvants activate the STING pathway to enhance tumor immunotherapy
2.1. Metal-based nanoparticle adjuvants
Numerous studies have indicated a significant role of metal ions in immune regulation [33,[59], [60], [61]]. For instance, manganese, zinc, and iron ions play pivotal roles in regulating innate immunity. Manganese and zinc ions enhance the sensitivity of cGAS toward dsDNA, thereby strengthening the innate immune response against tumors [62,63]. Iron ions can promote the formation of reactive oxygen species (ROS) via the Fenton reaction, thus activating the NF-κB inflammatory signaling pathway and inducing substantial pro-inflammatory immune responses [64]. Calcium ions act as second messengers in signaling pathways, regulating immune cell activation, differentiation, and proliferation, and are crucial for the activation of immune cells, such as T cells, B cells, and macrophages, as well as the release of cytokines and chemokines. They activate dendritic cells (DCs), promote their maturation, and initiate adaptive immune responses [65]. Magnesium ions promote the activation of CD8+ T cells by binding to molecules associated with lymphocyte function [66]. Although metal ions exhibit clear advantages in immune regulation, delivering them efficiently and effectively to target tissues for safe and potent anti-tumor and immune-activating effects remains a critical challenge.
2.1.1. Manganese nano-adjuvant
cGAS, as a cytoplasmic DNA receptor, can be activated by DNA and/or manganese ions, and uses adenosine triphosphate and guanosine triphosphate to synthesize the second messenger 2′3′-cGAMP. This further activates STING, induces phosphorylation of interferon regulatory factor 3, and stimulates the production of IFN-Is and other cytokines, thereby mediating tumor immune responses [67]. Therefore, manganese ions are important metal elements for activating the STING pathway [68,69]. Moreover, manganese ions can induce ROS generation [70,71] and stimulate the maturation and polarization of immune cells [72] (such as DCs and M2 macrophages), leading to anti-tumor immune responses.
Sun et al. showed that metal ions can enhance the activity of STING agonists, with manganese ions exhibiting the best amplification effect [40]. In their experiments, the manganese-based nano-vaccine CMPDDA was observed to significantly improve the efficiency of DC uptake of tumor antigens and have a remarkable enhancing effect on STING activation and the response of interferon-β, demonstrating significant potential in tumor immunity [73].
Qiang et al. [41] pioneered an in situ tumor vaccine (ISTVs) with triple-enhanced anti-tumor immunity. They assessed the photothermal properties of specific materials to induce in situ photothermal ablation of tumor-associated antigens (TAAs). Meanwhile, the ISTVs facilitated the uptake of TAAs via DCs in the tumor microenvironment, thereby enhancing adaptive immunity. Moreover, the release of Mn2+ from the ISTVs, in tandem with the dsDNA released from the disrupted tumor cells during photothermal ablation, synergistically activated the cGAS-STING pathway, promoting DC maturation and macrophage M1 polarization, further amplifying the T-cell immune response.
Liang et al. [42] introduced a ferroptosis-induced mitochondrial DNA- (mtDNA-)-guided tumor immunotherapy nanoplatform (HBMn-FA). The primary advantage of this platform lies in its ability to mediate ferroptosis, resulting in high levels of ROS within the tumor, triggering mitochondrial stress and the release of mtDNA. In collaboration with manganese, this mtDNA specifically activates the cGAS-STING pathway. Furthermore, dsDNA released by HBMn-FA after inducing tumor cell death further activates the cGAS-STING pathway, rapidly initiating systemic anti-tumor immunity and enhancing the therapeutic effect of checkpoint blockade, ultimately suppressing tumor growth in both local and metastatic tumor models.
However, because of the poor stability of manganese, MnO2, which is a more stable form of manganese, has become a populartopic in nanocatalyst research [74]. MnO2 exhibits superior characteristics, such as a high surface area, strong oxidizing ability, high catalytic activity, and a broad range of spectral absorption [75,76]. In biological organisms, MnO2 can undergo redox reactions with glutathione (GSH) [77]. Under weakly acidic conditions, MnO2 catalyzes the decomposition of hydrogen peroxide into oxygen [78], demonstrating its applicability in the field of biomedicine. Various methods are available for preparing MnO2, with the commonly used ones including hydrothermal redox, and biomineralization methods, which are relatively convenient. When considering the mechanism of action of MnO2 [62], in a slightly acidic environment, excess hydrogen peroxide and GSH in the surroundings are reduced to manganese ions [79]. In the reaction with hydrogen peroxide, MnO2 acts as a catalyst to decompose hydrogen peroxide into oxygen [80]. The reduction of MnO2 provides a sufficient reducing agent for GSH, which is oxidized to form disulfide bonds in GSH, whereas MnO2 is reduced to manganese ions, thereby activating the cGAS-STING pathway.
2.1.2. Zinc nano-adjuvant
Recent studies have shown that zinc ions play a significant role in STING activation. They promote the phase separation of cGAS proteins and enhance their enzymatic activities [81,82]. Similar to other metal nano-adjuvants, zinc ions can induce the accumulation of ROS in tumor cells, disrupt mitochondria, and release mitochondrial DNA, thus activating the cGAS-STING signaling pathway [[83], [84], [85], [86], [87]] and leading to interferon production.
Cen et al. [45] developed ZnS@bovine serum albumin nano-clusters by encapsulating zinc and sulfur ions with bovine serum albumin as the carrier. Under acidic conditions, these nano-clusters release zinc and sulfur ions, which act on tumor cells. Zinc ions induce ROS generation in cells, thereby increasing the catalytic ability of cGAS and enhancing the efficiency of cGAS-STING pathway activation, promoting the release of IFN-Is and cytokines, and accelerating the infiltration and maturation of DCs. Moreover, sulfur ions combine with free hydrogen ions in the body to produce hydrogen sulfide gas, which can inhibit the activity of hydrogen peroxide enzymes, leading to the accumulation of ROS and the promotion of tumor cell apoptosis.
2.1.3. Iron nanoparticle adjuvant
Iron plays an important role in the growth and metastasis of tumors. Iron tumor therapy using iron nanoparticles as carriers has become a new approach in tumor treatment. Choi [88] conducted a study to prepare iron oxide nanoparticles with different loading contents and compared the effects of different loading amounts on targeting efficiency. The results showed that the iron oxide nanoparticles were effectively wrapped in nanocarriers, with a loading efficiency of over 95 %. This experiment also indirectly indicated that the mechanical properties of the nanocarriers for loaded iron oxide nanoparticles can be conveniently changed by adjusting the loading amount, thus providing iron nano-adjuvants with significant advantages in tumor immunotherapy.
Xu et al. [46] designed an iron-based covalent organic framed nano-adjuvant doped with curcumin and platinum. This iron ion-– bound nano-adjuvant increased the release of dsDNA, activated the STING pathway to promote DC maturation, and induced a systemic immune response via interferon amplification, resulting in efficient tumor treatment. Moreover, iron nano-adjuvants can synergistically enhance tumor immune combination therapy with antigens.
Meng et al. [47] proposed a lipid-encapsulated iron oxide nanoparticle that was delivered as a nanovaccine. Utilizing endocytosis, peptide antigens and cytosine-guanosine phosphate (CpG) DNA were delivered to DCs. The results showed that iron oxide promoted ROS in cells, facilitated DC maturation, activated the STING pathway, inhibited tumor growth, and improved animal survival. As nano-adjuvants, iron oxide nano-adjuvants possess sufficient targeting efficiency and the ability to prolong half-life in vivo.
2.2. Nano-adjuvant based on platinum-based chemotherapy drugs
Cisplatin is a nano-adjuvant based on platinum chemotherapy drugs. It releases its DNA into cytoplasm to activate STING pathway by causing DNA damage in tumor cells and inhibiting DNA repair, thus transforming “cold tumor” into “hot tumor,” providing better anti-tumor effect for clinical chemotherapy and immunotherapy [[89], [90], [91], [92]]. However, cisplatin exhibits poor water and fat solubility, insufficient specificity for tumor cells, low bioavailability, and high toxicity and side effects, which limit its clinical application [93]. Liposomes, one of the most mature nano-drug delivery systems, exhibit considerable potential for drug delivery. Encapsulating cisplatin in liposomes can improve its bioavailability in the human body and enhance immune responses [94].
Phenanthriplatin is a monovalent platinum (II)-based complex that can rapidly bind to DNA and cause serious DNA damage in tumor cells; however, it is easily removed from the human body. Monroe et al. [95] showed that phenanthrene platinum differs from cisplatin in damaging the anti-tumor mechanism of DNA. Phenanthrene platinum primarily binds to guanosine residues in a monodentate manner without distorting DNA and inhibits transcription and evades the DNA repair mechanism. Studies have suggested that phenanthrene platinum may not have similar toxicity and side effects as cisplatin because of its different mechanism of activating apoptosis from cisplatin. However, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide–based detection indicated that phenanthrene platinum inhibited survival ability to a similar extent as cisplatin, which could cause toxicity via multiple cell death pathways [96,97]. This nanoparticle can rapidly enrich in tumor sites by size effect, induce DNA damage of tumor cells, activate the cGAS-STING pathway, promote the transformation of “cold tumor” into “hot tumor,” and achieve tumor immune activation [98,99].
Oxaliplatin is a third-generation platinum chemotherapy drug that can induce DC maturation, promote CD8+ T-cell infiltration into tumor tissues, block the tumor-related macrophage cycle in the M1 phase, efficiently activate the STING pathway, improve the immunosuppressive tumor microenvironment, and enhance anti-tumor effects [100]. However, considering the limited effect of a single STING agonist on anti-tumor immunity, the immune activation of oxaliplatin must be combined with the immune enhancement of nano-materials. PC7A is a synthetic biodegradable polymer material, whose seven-membered tertiary amine can directly bind to the STING protein, thus activating the immune response. Based on this, Gao et al. [48] designed a multi-mode nano-preparation that modified the cyclic seven-membered ring (PC7A) as an effective STING agonist on the polymer skeleton, coated the platinum complex with it, and self-assembled to form nanoparticles. This nano-adjuvant is responsive to the pH of the tumor microenvironment. After entering the tumor microenvironment, oxaliplatin induces DNA damage in cancer cells and activates the classical cGAS- STING pathway. PC7A can directly bind to STING to form STING-PC7A aggregates, which activate the PC7A-STING pathway. Drugs and nano-materials synergistically activate the STING pathway, improve the tumor microenvironment of immunosuppression, increase the inherent anti-tumor immune response, enhance the effect of immune checkpoint blocking therapy mediated by the PD-L1 monoclonal antibody, and finally achieve the best anti-tumor effect, providing a new strategy for clinical tumor treatment.
2.3. Nano-adjuvant based on camptothecin derivatives
CPT, a nano-adjuvant based on CPT derivatives [101,102], is a natural product and belongs to topoisomerase inhibitor. It blocks DNA replication and repair in cancer cells by inhibiting topoisomerase I activity, thus playing an anti-tumor role, particularly in solid tumors such as colorectal cancer and ovarian cancer [103]. However, CPT exhibits certain limitations, such as low bioavailability, hightoxicity and side effects, and weak activation ability of the STING pathway when used alone.
Therefore, the preparation of CPT as a nano-adjuvant to improve its immunotherapeutic effect has become an important research topic. CPT based prodrug CPT-SS-OA was prepared by linking CPT with oleic acid via a disulfide bond. Because of the presence of the oleic acid group, CPT lactone was stably used to improve its anti-tumor effect. Li et al. [49] constructed CPT-SS-OA/CM by embedding CPT-SS-OA into bone ash micelles (CM). In vitro studies have shown that CPT-SS-OA/CM is easily internalized into cells, transforms into active lactone CPT, activates the cGAS-STING pathway, promotes the development and maturation of DCs and tumor infiltration of CD8+ T cells, and reduces the original toxicity of CPT, thus providing a new idea for tumor immunotherapy.
Lu et al. [50] prepared the CPT prodrug CPT-S-S-LA by connecting CPT to stearic acid via a disulfide bond. The prodrug can self-assemble into nanoparticles in water owing to the presence of stearic acid moieties. In response to GSH, CPT-S-S-LA can release CPT drug, activate the cGAS-STING pathway, promote the development and maturation of DCs and promote tumor infiltration of CD8+ T cells. Moreover, it significantly reduces the inherent toxicity of CPT, providing a new approach for tumor immunotherapy.
To further enhance the activation of the STING pathway, Lei et al. [51] prepared CPT-Pt (IV) by linking CPT with cisplatin, which was then self-assembled with the ROS- sensitive polymer and lipid polymer 1,2-distearoyl-snglycero-3-phosphoethanolamine-N-[methoxy (polyethylene glycol)-2000] to form a nano-adjuvant. The prepared nanoparticles aggregated at tumor sites and improved the utilization rate of drugs. Moreover, the nanoparticles induced double DNA damage by releasing cisplatin and CPT, activating the cGAS-STING pathway, inducing DC maturation, and enhancing the tumor infiltration of CD8+ T cells in a mouse colorectal cancer model. Thus, “immune cold tumors” can be transformed into “immune hot tumor,” and tumor inhibition can be enhanced by combined chemical immunotherapy. Moreover, the nano-preparation overcomes the limitation of poor water solubility of CPT, enabling CPT to exert maximum efficacy. The combined action of the two causes rapid DNA release, which can stimulate a strong immune response, thereby killing tumor cells with maximum efficiency.
7-Ethyl-10-hydroxy-camptothecin (SN38) is an active metabolite of CPT and exhibits higher anti-tumor activity than CPT. Animal experiments have shown that SN38 plays a significant role in inducing DCs o mature and release interferon-β. Further study identified that after the SN38 damaged DNA, DNA fragments were released in exosomes, which were absorbed by immune cells such as DCs to activate the STING pathway. Because SN38 exhibits poor water solubility, easy in vivo cleaning, and systemic toxicity, Wang et al. [52] chemically modified SN38, such that SN38 can could self-assemble into stable micelles, which significantly improved its anti-tumor activity. Gao et al. [104] prepared SN38 conjugates with tumor-targeting abilities by automatically coupling compounds containing phosphoramide with aptamers, which can induce apoptosis by releasing the parent SN38 and inhibiting tumor growth.
2.4. DNA nano-adjuvant
DNA nano-adjuvants exhibit precise chemical structures and significant assembly ability; consequently, they have attracted considerable attention in the field of nanotechnology. Currently, common DNA nanotechnology includes DNA brick self-assembly, DNA origami, and other methods, that can perform a specific function under the stimulation of specific conditions and chemically modify it, which can significantly expand its functional range [[105], [106], [107]]. Rehberg et al. [53] assembled a DNA nanotube as a delivery vector for the CpG sequence, and evaluated the ability of the DNA nanotubes to induce immune stimulation. The results showed that DNA nanotubes were absorbed by macrophages via internalization, which activated the inflammatory pathway of the surrounding cells, recruited immune cells, promoted the maturation of DCs, and effectively activated the cGAS-STING pathway, thus achieving tumor immunity [108].
Interferon-stimulated DNA can activate the cGAS-STING pathway and induce a strong anti-tumor immune response. However, owing to its low bioavailability, easy decomposition, poor stability, and poor specific selection for tumors, its clinical use is challenging. Mitochondrial DNA (mtDNA), which escapes to intracellular or extracellular compartments under different conditions, causes inflammation by activating the cGAS-STING pathway. Xian et al. [109] identified that oxidized mtDNA (Ox-mtDNA) can bind to cytoplasmic nucleotide-binding domain, leucine-rich–containing family, pyrin domain–containing-3 (NLRP3) to trigger the activation of inflammatory corpuscles. Simultaneously, Ox-mtDNA activates cGAS-STING signal transduction and produces pro-inflammatory extracellular DNA. NLRP3 inflammatory corpuscles are initiated by TLRs, which sense pathogens or damage-associated molecular patterns. They activate caspase-1-mediated proteolysis of interleukin-1 β and interleukin-18 processing secretion. Another target of cytoplasmic mtDNA is the DNA-sensing enzyme loopcGAS, which leads to the activation of STING) and production of IFN-I, thus further amplifying inflammation.
2.5. Others
Luo et al. [54] constructed ovalbumin-PC7A nanoparticle (OVA-PC7A NP) with OVA as an antigen and identified that the number of mature DCs increased significantly under OVA-PC7A NP treatment. Further studies showed that PC7A biotin in mice increased significantly after OVA-PC7A NP treatment. PC7A biotin could retain STING to the greatest extent, STING could directly bind to PC7A, and there was a special interaction between them, which further promoted the activation of the cGAS-STING pathway.
Cyclic dinucleotide (CDN) agonists are promising immunotherapy drugs that can activate innate immunity and increase the immunogenicity of tumors [[110], [111], [112]]. However, the effectiveness of CDNs is affected by disorders of drug delivery, poor cell targeting, rapid clearance, and low efficiency of transport to the STING-localized cytoplasm. Shae et al. [55] introduced STING-activated nanoparticles (STING-NPs) for the cytoplasmic delivery of endogenous CDN ligands (2, 3- cGAMP). STING-NPs can improve the biological efficacy of cGAMP and enhance the STING signal of the tumor microenvironment and sentinel lymph nodes to transform immunosuppressive tumors into an immunogenic microenvironment, which can enhance the therapeutic effect of cGAMP, effectively inhibit tumor growth, improve tumor response to immune checkpoint blockade, and induce immune memory to prevent tumor recurrence.
5,6-Dimethylxanthenone-4-acetic acid (DMXAA) is a synthetic mouse agonist of STING, which exhibits potential anti-tumor activity in clinic [56]. The encapsulation of DMXAA in nanoparticles can enhance its enrichment in tumor tissues, reduce its influence on normal tissues, and improve its anti-tumor effect. Although DMXAA can not bind to human STING, it remains a promising tumor vascular disrupting agent and shows high efficiency in combination with various anti-tumor drugs, including cisplatin [113].
Chen et al. [57] identified that CpG oligodeoxynucleotides and DMXAA were introduced into metal-organic frames-801 (MOF-801) via coordination bonds to self-assemble MOF-CpG-DMXAA, which was recognized by TLR-4and could activate cGAS-STING-NF-κ B signaling pathway at a significantly low dose (70 ng mL-1), thus accelerating DC maturation. The data showed that MOF-CpG-DMXAA can enhance the activation of STING; therefore, it can still efficiently activate the CGAS-STING pathway at lower concentrations and produce efficient tumor-killing results.
Moreover, DMXAA can specifically induce the closure of blood flow in tumors without destroying normal tissues; therefore, DMXAA has been widely studied in cancer treatment research, and remarkable results have been achieved not only in laboratories, but also in phase I/II clinical trials [56]. Unfortunately, DMXAA failed in Phase III clinical trials, because it only binds to mouse STING and not to human STING [114]. DMXAA can cause irreversible destruction of tumor blood vessels and completely block tumor blood flow [115]; thus, it is a promising vascular blocker with great research value.
Studies have shown that 10-methoxy-9-nitrocamptothecin (MONCPT) has strong antiproliferative and antiangiogenic activities in vitro and in vivo. According to Yang et al. [58], MONCPT can effectively inhibit the invasion and migration of melanoma cells in mouse melanoma models without affecting cell survival. As a CPT analog, MONCPT exhibits good anti-tumor activity and is a potential and efficient anti-tumor drug. Compared to CPT MONCPT has higher DNA damage ability, activates cGAS-STING pathway, induces DC cell maturation, and has significant inhibitory effect on the growth of transplanted human non-small cell lung cancer [116] However, the solubility and stability of MONCPT must be improved.
3. Discussion
The current format of utilizing nanoparticles coupled with antigens has emerged as a novel approach for cancer immunotherapy. The delivery of antigens via nano-sized particles, which is possible owing to their small particle size and large specific surface area, significantly enhances the targeted delivery efficiency of antigens. Via rational surface modification and targeted design, nano-adjuvants can specifically recognize and bind to antigen-presenting cells (such as DCs and macrophages), thereby efficiently delivering antigens to these cells and improving their antigen presentation efficiency. Ultimately, this facilitates the differentiation of DCs into specific mature states [117].
Furthermore, nano-adjuvants can alter the physicochemical properties of antigens by increasing their solubility and stability, thereby enhancing their immunogenicity. Particularly, nano-adjuvants derived from certain metal nanoparticles (e.g., manganese and) can modulate the activity and function of immune cells via their interactions, thereby influencing antigen presentation and immune response processes.
Moreover, nano-adjuvants can mimic pathogens and activate the innate immune response of T cells, thereby inducing adaptive immune responses. They can also activate TLRs or the cGAS-STING pathway, leading to an increase in the number and activity of CD8+ T cells and a reduction in the number of regulatory T cells. This approach aids in improving the immunosuppressive state within the tumor microenvironment and enhances the antitumor capabilities of the immune system.
4. Outlook
Research on nano-adjuvants has achieved certain milestones such as, enabling the precise modulation of the immune system via accurate control of their size, shape, surface charge, and functional modifications. Nano-adjuvants also serve as effective carriers for vaccines, efficiently delivering antigens to target cells and enhancing their immunogenicity and stability. However, most of these studies are still in the laboratory incubation and research phases, requiring considerable research and development efforts before practical clinical applications. A series of challenges related to nano-adjuvants remain to be addressed.
First, as novel drug delivery systems, nano-adjuvants must undergo stringent regulatory approval for ensuring their safety and efficacy. This includes a comprehensive evaluation of their physicochemical properties, biological activities, immunogenicities, and safety profiles. Second, there are potential risks of toxicity or side effects associated with nano-adjuvants in vivo, such as excessive immune responses or organ damage.
Nevertheless, advancements in nanotechnology have propelled the development of nano-adjuvants, offering the potential to synthesize more efficient and stable formulations. Thus, with the continuous accumulation of research on nano-adjuvants, the development of a new generation of n ano-adjuvants can be expected in the foreseeable future.
Funding
This study was supported by the National Natural Science Foundation of China (82372109).
CRediT authorship contribution statement
Zicong Xu: Writing – original draft. Yihong Wu: Writing – original draft. Junjie Hu: Writing – original draft. Zhaozhao Mei: Writing – original draft. Yutong Zhao: Writing – review & editing. Keda Yang: Writing – review & editing. Yi Shi: Writing – review & editing. Xiaoling Xu: Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Keda Yang, Email: kdyang@scicalq.com.
Yi Shi, Email: lh2918@shutcm.edu.cn.
Xiaoling Xu, Email: ziyao1988@zju.edu.cn.
References
- 1.Rath J.A., Arber C. Engineering strategies to enhance TCR-based adoptive T cell therapy. Cells. 2020 18;9(6):1485. doi: 10.3390/cells9061485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Restifo N.P., Dudley M.E., Rosenberg S.A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 2012 22;12(4):269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunert A., Debets R. Engineering T cells for adoptive therapy: outsmarting the tumor. Curr. Opin. Immunol. 2018;51:133–139. doi: 10.1016/j.coi.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 4.Silk A.W., Margolin K. Cytokine therapy. Hematol. Oncol. Clin. N. Am. 2019;33(2):261–274. doi: 10.1016/j.hoc.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y., Adu-Berchie K., Brockman J.M., Pezone M., Zhang D.K.Y., Zhou J., Pyrdol J.W., Wang H., Wucherpfennig K.W., Mooney D.J. Cytokine conjugation to enhance T cell therapy. Proc. Natl. Acad. Sci. U. S. A. 2023 3;120(1) doi: 10.1073/pnas.2213222120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohno M., Natsume A., Wakabayashi T. Cytokine therapy. Adv. Exp. Med. Biol. 2012;746:86–94. doi: 10.1007/978-1-4614-3146-6_7. [DOI] [PubMed] [Google Scholar]
- 7.Culver K.W., Blaese R.M. Gene therapy for cancer. Trends Genet. 1994;10(5):174–178. doi: 10.1016/0168-9525(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 8.Albelda S.M., Wiewrodt R., Sterman D.H. Gene therapy for lung neoplasms. Clin. Chest Med. 2002;23(1):265–277. doi: 10.1016/s0272-5231(03)00072-8. [DOI] [PubMed] [Google Scholar]
- 9.Palmer D.H., Young L.S., Mautner V. Cancer gene-therapy: clinical trials. Trends Biotechnol. 2006;24(2):76–82. doi: 10.1016/j.tibtech.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Abedi Kiasari B., Abbasi A., Ghasemi Darestani N., Adabi N., Moradian A., Yazdani Y., Sadat Hosseini G., Gholami N., Janati S. Combination therapy with nivolumab (anti-PD-1 monoclonal antibody): a new era in tumor immunotherapy. Int. Immunopharm. 2022;113(Pt A) doi: 10.1016/j.intimp.2022.109365. [DOI] [PubMed] [Google Scholar]
- 11.Jeong S., Park E., Kim H.D., Sung E., Kim H., Jeon J., Kim Y., Jung U.J., Son Y.G., Hong Y., Lee H., Lee S., Lim Y., Won J., Jeon M., Hwang S., Fang L., Jiang W., Wang Z., Shin E.C., Park S.H., Jung J. Novel anti-4-1BB×PD-L1 bispecific antibody augments anti-tumor immunity through tumor-directed T-cell activation and checkpoint blockade. J Immunother Cancer. 2021;9(7) doi: 10.1136/jitc-2021-002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams G.P., Weiner L.M. Monoclonal antibody therapy of cancer. Nat. Biotechnol. 2005;23(9):1147–1157. doi: 10.1038/nbt1137. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y., Zheng J. Functions of immune checkpoint molecules beyond immune evasion. Adv. Exp. Med. Biol. 2020;1248:201–226. doi: 10.1007/978-981-15-3266-5_9. [DOI] [PubMed] [Google Scholar]
- 14.Li B., Chan H.L., Chen P. Immune checkpoint inhibitors: basics and challenges. Curr. Med. Chem. 2019;26(17):3009–3025. doi: 10.2174/0929867324666170804143706. [DOI] [PubMed] [Google Scholar]
- 15.Haanen J.B., Robert C. Immune checkpoint inhibitors. Prog Tumor Res. 2015;42:55–66. doi: 10.1159/000437178. [DOI] [PubMed] [Google Scholar]
- 16.Peng M., Mo Y., Wang Y., Wu P., Zhang Y., Xiong F., Guo C., Wu X., Li Y., Li X., Li G., Xiong W., Zeng Z. Neoantigen vaccine: an emerging tumor immunotherapy. Mol. Cancer. 2019 23;18(1):128. doi: 10.1186/s12943-019-1055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang J., Mei J., Yi S., Feng C., Ma Y., Liu Y., Liu Y., Chen C. Tumor associated macrophage and microbe: the potential targets of tumor vaccine delivery. Adv. Drug Deliv. Rev. 2022;180 doi: 10.1016/j.addr.2021.114046. [DOI] [PubMed] [Google Scholar]
- 18.Wu H., Fu X., Zhai Y., Gao S., Yang X., Zhai G. Development of effective tumor vaccine strategies based on immune response cascade reactions. Adv. Healthcare Mater. 2021;10(13) doi: 10.1002/adhm.202100299. [DOI] [PubMed] [Google Scholar]
- 19.Morse M.A., Gwin WR 3rd, Mitchell D.A. Vaccine therapies for cancer: then and now. Targeted Oncol. 2021;16(2):121–152. doi: 10.1007/s11523-020-00788-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ou L., Zhang A., Cheng Y., Chen Y. The cGAS-STING pathway: a promising immunotherapy target. Front. Immunol. 2021;9(12) doi: 10.3389/fimmu.2021.795048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Jing, Sánchez-Rivera Francisco J., Wang Zhenghan, Johnson Gabriela N., Ho Yu-Jui, Ganesh Karuna, Umeda Shigeaki, Gan Siting, Mujal Adriana M., Delconte Rebecca B., Hampton Jessica P., Zhao Huiyong, Kottapalli Sanjay, de Stanchina Elisa, Iacobuzio-Donahue Christine A., Pe'er Dana, Lowe Scott W., Sun Joseph C. Joan Massagué,STING inhibits the reactivation of dormant metastasis in lung adenocarcinoma. Nature. 2023;616(7958):806–813. doi: 10.1038/s41586-023-05880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu Ying, Ye Runxin, Su Jiaming, Rui Yajuan, Yu Xiao-Fang. cGAS-STING-mediated novel nonclassic antiviral activities. J. Med. Virol. 2024;96(2) doi: 10.1002/jmv.29403. [DOI] [PubMed] [Google Scholar]
- 23.Tan Jia, et al. Advanced nanomaterials targeting activation of STING for enhanced cancer immunotherapy. Coord. Chem. Rev. 2023;493 [Google Scholar]
- 24.Xue C., Dong N., Shan A. Trends Immunol. 2022;43:513–522. doi: 10.1016/j.it.2022.04.011. [DOI] [PubMed] [Google Scholar]
- 25.Liu S., Guan W. Mediat. Inflamm. 2018;2018 doi: 10.1155/2018/1202797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sivick K.E., Desbien A.L., Glickman L.H., Reiner G.L., Corrales L., Surh N.H., Hudson T.E., Vu U.T., Francica B.J., Banda T., Katibah G.E., Kanne D.B., Leong J.J., Metchette K., Bruml J.R., Ndubaku C.O., McKenna J.M., Feng Y., Zheng L., Bender S.L., Cho C.Y., Leong M.L., van Elsas A., Dubensky T.W., Jr., McWhirter S.M. Cell Rep. 2018;25:3074–3085.e3075. doi: 10.1016/j.celrep.2018.11.047. [DOI] [PubMed] [Google Scholar]
- 27.Hong C., Schubert M., Tijhuis A.E., Requesens M., Roorda M., van den Brink A., Ruiz L.A., Bakker P.L., van der Sluis T., Pieters W., Chen M., Wardenaar R., van der Vegt B., Spierings D.C.J., de Bruyn M., van Vugt M.A.T.M., Foijer F. Nature. 2022;607:366–373. doi: 10.1038/s41586-022-04847-2. [DOI] [PubMed] [Google Scholar]
- 28.Snell L.M., McGaha T.L., Brooks D.G. Type I interferon in chronic virus infection and cancer. Trends Immunol. 2017;38(8):542–557. doi: 10.1016/j.it.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boukhaled G.M., Harding S., Brooks D.G. Opposing roles of type I interferons in cancer immunity. Annu. Rev. Pathol. 2021 24;16:167–198. doi: 10.1146/annurev-pathol-031920-093932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan Jia, et al. Understanding structure–function relationships of nanoadjuvants for enhanced cancer vaccine efficacy. Adv. Funct. Mater. 2022;32(16) [Google Scholar]
- 31.Samson N, Ablasser A. The cGAS-STING pathway and cancer. Nat. Can. (Ott.). 202266;3(12):1452-1463. [DOI] [PubMed]
- 32.Gan Y., Li X., Han S., Liang Q., Ma X., Rong P., Wang W., Li W. The cGAS/STING pathway: a novel target for cancer therapy. Front. Immunol. 2022 3;12 doi: 10.3389/fimmu.2021.795401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J.X., Ren H., Zhang Y.M. Metal-based nano-vaccines for cancer immunotherapy. Coord. Chem. Rev. 2022;454 [Google Scholar]
- 34.Sakasai R., Wakasugi M., Matsui T., Sunatani Y., Saijo M., Matsunaga T., Iwabuchi K. Camptothecin compromises transcription recovery and cell survival against cisplatin and ultraviolet irradiation regardless of transcription-coupled nucleotide excision repair. DNA Repair. 2022;113 doi: 10.1016/j.dnarep.2022.103318. [DOI] [PubMed] [Google Scholar]
- 35.Havelka A.M., Berndtsson M., Olofsson M.H., Shoshan M.C., Linder S. Mechanisms of action of DNA-damaging anticancer drugs in treatment of carcinomas: is acute apoptosis an "off-target" effect? Mini Rev. Med. Chem. 2007;7(10):1035–1039. doi: 10.2174/138955707782110196. [DOI] [PubMed] [Google Scholar]
- 36.Gogoi H., Mansouri S., Jin L. The age of cyclic dinucleotide vaccine adjuvants. Vaccines (Basel) 2020 13;8(3):453. doi: 10.3390/vaccines8030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y., Luo J., Alu A., Han X., Wei Y., Wei X. cGAS-STING pathway in cancer biotherapy. Mol. Cancer. 2020 4;19(1):136. doi: 10.1186/s12943-020-01247-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shae D., Becker K.W., Christov P., Yun D.S., Lytton-Jean A.K.R., Sevimli S., Ascano M., Kelley M., Johnson D.B., Balko J.M., Wilson J.T. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 2019;14(3):269–278. doi: 10.1038/s41565-018-0342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H., Luo Q., Zhang H., Ma X., Gu Z., Gong Q., Luo K. Nanomedicine embraces cancer radio-immunotherapy: mechanism, design, recent advances, and clinical translation. Chem. Soc. Rev. 2023 3;52(1):47–96. doi: 10.1039/d2cs00437b. [DOI] [PubMed] [Google Scholar]
- 40.Sun X., Zhang Y., Li J., et al. Amplifying STING activation by cyclic dinucleotide-manganese particles for local and systemic cancer metalloimmunotherapy. Nat. Nanotechnol. 2021;16:1260–1270. doi: 10.1038/s41565-021-00962-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Qiang, et al. Boosting antitumor immunity via a tumor microenvironment‐responsive transformable trifecta nanovaccine. Adv. Funct. Mater. 2024 [Google Scholar]
- 42.Liang J.L., Jin X.K., Zhang S.M., Huang Q.X., Ji P., Deng X.C., Cheng S.X., Chen W.H., Zhang X.Z. Specific activation of cGAS-STING pathway by nanotherapeutics-mediated ferroptosis evoked endogenous signaling for boosting systemic tumor immunotherapy. Sci. Bull. 2023 Mar 30;68(6):622–636. doi: 10.1016/j.scib.2023.02.027. [DOI] [PubMed] [Google Scholar]
- 43.Gao Z.L., Xu W., Zheng S.J., et al. Orchestrated cytosolic delivery of antigen and adjuvant by manganese ion-coordinated nanovac‐cine for enhanced cancer immunotherapy. Nano Lett. 2023;23:1904–1913. doi: 10.1021/acs.nanolett.2c04970. [DOI] [PubMed] [Google Scholar]
- 44.Zhao Z., Dong S., Liu Y., et al. Tumor microenvironment-activable manganese-boosted catalytic immunotherapy combined with PD-1 checkpoint blockade. ACS Nano. 2022;16:20400–20418. doi: 10.1021/acsnano.2c06646. Zhao Z, Dong S, Liu Y, et al. Tumor microenvironment-activable. [DOI] [PubMed] [Google Scholar]
- 45.Cen D., Ge Q., Xie C., et al. ZnS@BSA nanoclusters potentiate efficacy of cancer immunotherapy. Adv. Mater. 2021;33 doi: 10.1002/adma.202104037. [DOI] [PubMed] [Google Scholar]
- 46.Xu D., Hu J., Mei J., Zhou J., Wang Z., Zhang X., Liu Q., Su Z., Zhu W., Liu H., Zhu C. Nanoadjuvant-triggered STING activation evokes systemic immunotherapy for repetitive implant-related infections. Bioact. Mater. 2024 24;35:82–98. doi: 10.1016/j.bioactmat.2024.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meng J., Zhang P., Chen Q., Wang Z., Gu Y., Ma J., Li W., Yang C., Qiao Y., Hou Y., Jing L., Wang Y., Gu Z., Zhu L., Xu H., Lu X., Gao M. Two-pronged intracellular Co-delivery of antigen and adjuvant for synergistic cancer immunotherapy. Adv. Mater. 2022;34(21) doi: 10.1002/adma.202202168. [DOI] [PubMed] [Google Scholar]
- 48.Gao X., Lei G., Wang B., et al. Encapsulation of platinum prodrugs into PC7A polymeric nanoparticles combined with immune checkpoint inhibitors for therapeutically enhanced multimodal chemotherapy and immunotherapy by activation of the STING pathway. Adv. Sci. 2023;10(4) doi: 10.1002/advs.202205241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li F., Huang Z., Chen H., Yan L., Li J., Su Y., Zhang Q., Huang Z., Zheng Y. Redox-sensitive lipophilic prodrugs: delivering unstable chemotherapeutant for improved cancer therapy. Drug Deliv. 2019 Dec;26(1):1068–1079. doi: 10.1080/10717544.2019.1678696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu Lu, Li Bing, Lin Chuanchuan, Li Ke, Liu Genhua, Xia Zengzilu, Zhong Luo, Cai Kaiyong. Redox-responsive amphiphilic camptothecin prodrug nanoparticles for targeted liver tumor therapy. J. Mater. Chem. B. 2020 6;8(17):3918–3928. doi: 10.1039/d0tb00285b. [DOI] [PubMed] [Google Scholar]
- 51.Cao Lei, Tian Huixiang, Fang Man, Xu Zhe, Tang Dongsheng, Chen Juan, Yin Jiye, Xiao Haihua, Shang Kun, Han Hongbin, Li Xiangping. Activating cGAS-STING pathway with ROS-responsive nanoparticles delivering a hybrid prodrug for enhanced chemo-immunotherapy. Biomaterials. 2022;290 doi: 10.1016/j.biomaterials.2022.121856. [DOI] [PubMed] [Google Scholar]
- 52.Wang J., Hu S., Mao W., Xiang J., Zhou Z., Liu X., Tang J., Shen Y. Assemblies of peptide-cytotoxin conjugates for tumor-homing chemotherapy. Adv. Funct. Mater. 2019;29 [Google Scholar]
- 53.Sellner S., Kocabey S., Nekolla K., Krombach F., T Liedl, Rehberg DNA nanotubes as intracellular delivery vehicles in vivo. M.Biomaterials. 2015;53:453–463. doi: 10.1016/j.biomaterials.2015.02.099. [DOI] [PubMed] [Google Scholar]
- 54.Luo M., Wang H., Wang Z., Cai H., Lu Z., Li Y., Du M., Huang G., Wang C., Chen X., Porembka M.R., Lea J., Frankel A.E., Fu Y.X., Chen Z.J., Gao J. A STING-activating nanovaccine for cancer immunotherapy. Nat. Nanotechnol. 2017 Jul;12(7):648–654. doi: 10.1038/nnano.2017.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shae D., Becker K.W., Christov P., Yun D.S., Lytton-Jean A.K.R., Sevimli S., Ascano M., Kelley M., Johnson D.B., Balko J.M., Wilson J.T. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 2019;14(3):269–278. doi: 10.1038/s41565-018-0342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Daei Farshchi Adli A., Jahanban-Esfahlan R., Seidi K., Samandari-Rad S., Zarghami N. An overview on Vadimezan (DMXAA): the vascular disrupting agent. Chem. Biol. Drug Des. 2018;91(5):996–1006. doi: 10.1111/cbdd.13166. [DOI] [PubMed] [Google Scholar]
- 57.Chen X., Tang Q., Wang J., Zhou Y., Li F., Xie Y., Wang X., Du L., Li J., Pu J., Hu Q., Gu Z., Liu P. A DNA/DMXAA/Metal-Organic framework activator of innate immunity for boosting anticancer immunity. Adv. Mater. 2023;35(15) doi: 10.1002/adma.202210440. [DOI] [PubMed] [Google Scholar]
- 58.Yang X.C., Tu C.X., Luo P.H., Zhu H., Zhu D.F., Wu H.H., Zhou X.L., Lu W., He Q.J., Yang B. Antimetastatic activity of MONCPT in preclinical melanoma mice model. Invest. N. Drugs. 2010;28(6):800–811. doi: 10.1007/s10637-009-9323-8. [DOI] [PubMed] [Google Scholar]
- 59.Wang C., Zhang R., Wei X., et al. Metalloimmunology: the metal ion-controlled immunity. Adv. Immunol. 2020;145:187–241. doi: 10.1016/bs.ai.2019.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Feng W., Liu Z., Xia L., Chen M., Dai X., Huang H., Dong C., He Y., Chen Y. A sonication-activated valence-variable sono-sensitizer/catalyst for autography inhibition/ferroptosis-induced tumor nanotherapy. Angew Chem. Int. Ed. Engl. 2022 25;61(48) doi: 10.1002/anie.202212021. [DOI] [PubMed] [Google Scholar]
- 61.Chen L., Mignani S., Caminade A.M., Majoral J.P. Metal-based phosphorus dendrimers as novel nanotherapeutic strategies to tackle cancers: a concise overview. Wiley Interdiscip Rev Nanomed Nanobiotechnolw. 2019;11(6) doi: 10.1002/wnan.1577. [DOI] [PubMed] [Google Scholar]
- 62.Lv M., Chen M., Zhang R., et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020;30:966–979. doi: 10.1038/s41422-020-00395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Q., Gao Y., Li Q., He A., Xu Q., Mou Y. Enhancing dendritic cell activation through manganese-coated nanovaccine targeting the cGAS-STING pathway. Int. J. Nanomed. 2024 11;(19):263–280. doi: 10.2147/IJN.S438359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zanganeh S., Hutter G., Spitler R., et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat. Nanotechnol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dong C., Dai X., Wang X., Lu Q., Chen L., Song X., Ding L., Huang H., Feng W., Chen Y., Chang M. A calcium fluoride nanozyme for ultrasound-amplified and Ca2+ -Overload-Enhanced catalytic tumor nanotherapy. AdvMater. 2022;34(43) doi: 10.1002/adma.202205680. [DOI] [PubMed] [Google Scholar]
- 66.Lotscher J., Marti I.L.A.A., Kirchhammer N., et al. Magnesium sensing via LFA-1 regulates CD8+ T cell effector function. Cell. 2022;185:585–602.e29. doi: 10.1016/j.cell.2021.12.039. [DOI] [PubMed] [Google Scholar]
- 67.Zhou M., Liang S., Liu D., Ma K., Yun K., Yao J., Peng Y., Hai L., Zhang Q., Wang Z. Manganese-enriched zinc peroxide functional nanoparticles for potentiating cancer immunotherapy. Nano Lett. 2023;23(22):10350–10359. doi: 10.1021/acs.nanolett.3c02941. vb22. [DOI] [PubMed] [Google Scholar]
- 68.Lei H., Li Q., Li G., Wang T., Lv X., Pei Z., Gao X., Yang N., Gong F., Yang Y., Hou G., Chen M., Ji J., Liu Z., Cheng L. Manganese molybdate nanodots with dual amplification of STING activation for "cycle" treatment of metalloimmunotherapy. Bioact. Mater. 2023 8;31:53–62. doi: 10.1016/j.bioactmat.2023.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guan X., Zeng N., Zhao Y., Huang X., Lai S., Shen G., Zhang W., Wang N., Yao W., Guo Y., Yang R., Wang Z., Jiang X. Dual-modality imaging-guided manganese-based nanotransformer for enhanced gas-photothermal therapy combined immunotherapeutic strategy against triple-negative breast cancer. Small. 2023;21 doi: 10.1002/smll.202307961. [DOI] [PubMed] [Google Scholar]
- 70.Chen X., Cheng D., Yu N., Feng J., Li J., Lin L., 15 Tumor-targeting polymer nanohybrids with amplified ROS generation for combined photodynamic and chemodynamic therapy. J. Mater. Chem. B. 2024;9 doi: 10.1039/d3tb02341a. [DOI] [PubMed] [Google Scholar]
- 71.Huang Z., Wang Y., Su C., Li W., Wu M., Li W., Wu J., Xia Q., He H. Mn-Anti-CTLA4-CREKA-Sericin nanotheragnostics for enhanced magnetic resonance imaging and tumor immunotherapy. Small. 2023;27 doi: 10.1002/smll.202306912. [DOI] [PubMed] [Google Scholar]
- 72.Zhao X., Zhang J., Chen B., Ding X., Zhao N., Xu F.J. Rough nanovaccines boost antitumor immunity through the enhancement of vaccination cascade and immunogenic cell death induction. Small Methods. 2023;7(5) doi: 10.1002/smtd.202201595. [DOI] [PubMed] [Google Scholar]
- 73.Qian Z.H.A.O., Fang-min C.H.E.N., Yi L.A.I., Wang Wei, Hai-jun Y.U. Advances of metal nanoadjuvant for cancer immunotherapy. Acta Pharm. Sin. 2023;58(8):2311–2319. doi: 10.16438/j.0513-4870.2023-0554. [DOI] [Google Scholar]
- 74.Gu Yuan, Lin Subin, Wu Yanxian, Pei Xu, Wen Zhu, Wang Yangyun, Cheng Xiaju, Zhang Leshuai W., Stauber Roland H., Wang Yong, Gao Mingyuan. Targeting STING activation by antigen-inspired MnO2Nanovaccines optimizes tumor radiotherapy. Adv. Healthcare Mater. 2023;12(12) doi: 10.1002/adhm.202300028. [DOI] [PubMed] [Google Scholar]
- 75.Ju Q., Huang R., Hu R., Fan J., Zhang D., Ding J., Li R. 19. Phyticacid-modified manganese dioxide nanoparticles oligomer for magnetic resonance imaging and targeting therapy of osteosarcoma. Drug Deliv. 2023;30(1) doi: 10.1080/10717544.2023.2181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang Z., Wang Y., Su C., Li W., Wu M., Li W., Wu J., Xia Q., He H. Mn-Anti-CTLA4-CREKA-Sericin nanotheragnostics for enhanced magnetic resonance imaging and tumor immunotherapy. Small. 2023;27 doi: 10.1002/smll.202306912. [DOI] [PubMed] [Google Scholar]
- 77.Wang X., Huang R., Wu W., Xiong J., Wen Q., Zeng Y., Chen T., Li J., Zhang C., Zhong J.F., Yang S., Zhang X. Amplifying STING activation by bioinspired nanomedicine for targeted chemo- and immunotherapy of acute myeloid leukemia. Acta Biomater. 2023;157:381–394. doi: 10.1016/j.actbio.2022.11.007. [DOI] [PubMed] [Google Scholar]
- 78.Ding B., Yue J., Zheng P., Ma P., Lin J. Manganese oxide nanomaterials boost cancer immunotherapy. J. Mater. Chem. B. 2021;9(35):7117–7131. doi: 10.1039/d1tb01001h. 15. [DOI] [PubMed] [Google Scholar]
- 79.Ma G., Zhang X., Zhao K., Zhang S., Ren K., Mu M., Wang C., Wang X., Liu H., Dong J., Sun X. Polydopamine nanostructure-enhanced water interaction with pH-responsive manganese sulfide nanoclusters for tumor magnetic resonance contrast enhancement and synergistic ferroptosis-photothermal therapy. ACS Nano. 2024;22 doi: 10.1021/acsnano.3c10249. [DOI] [PubMed] [Google Scholar]
- 80.Peng J., Yang Q., Li W., Tan L., Xiao Y., Chen L., Hao Y., Qian Z. Erythrocyte-membrane-coated prussian blue/manganese dioxide nanoparticles as H2O2-responsive oxygen generators to enhance cancer chemotherapy/photothermal therapy. ACS Appl. Mater. Interfaces. 2017;9(51):44410–44422. doi: 10.1021/acsami.7b17022. 27. [DOI] [PubMed] [Google Scholar]
- 81.Du M.J., Chen Z.J.J. DNA-induced liquid phase condensation of cGAS activates innate immune signaling. Science. 2018;361:704–709. doi: 10.1126/science.aat1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J., Qin X., Wang B., et al. Zinc oxide nanoparticles harness autophagy to induce cell death in lung epithelial cells. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ranoa D.R.E., Widau R.C., Mallon S., et al. STING promotes homeostasis via regulation of cell proliferation and chromosomal stability. Cancer Res. 2019;79:1465–1479. doi: 10.1158/0008-5472.CAN-18-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao M., Wang Y.Z., Li L., et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA mainte‐ nance. Theranostics. 2021;11:1845–1863. doi: 10.7150/thno.50905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nabil A., Elshemy M.M., Asem M., Abdel-Motaal M., Gomaa H.F., Zahran F., Uto K., Ebara M. Zinc oxide nanoparticle synergizes sorafenib anticancer efficacy with minimizing its cytotoxicity. Oxid. Med. Cell. Longev. 2020;28(2020) doi: 10.1155/2020/1362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Motafeghi F., Mortazavi P., Shokrzadeh M. Anticancer activity of zinc oxide nanoparticles on prostate and colon cancer cell line. Toxicol. Res. 2024 16;13(1) doi: 10.1093/toxres/tfad127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tian Y., Tian H., Li B., Feng C., Dai Y. An ultrasound-triggered STING pathway nanoagonist for enhanced chemotherapy-induced immunogenic cell death. Small. 2024;15 doi: 10.1002/smll.202309850. [DOI] [PubMed] [Google Scholar]
- 88.Choi W.I., Kim J.Y., Heo S.U., Jeong Y.Y., Kim Y.H., Tae G., J Control Release The effect of mechanical properties of iron oxide nanoparticle-loaded functional nano-carrier on tumor targeting and imaging. J. Contr. Release. 2012 10;162(2):267–275. doi: 10.1016/j.jconrel.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 89.Xu D., Lu X., Yang F., Jiang Z., Yang S., Bi L., Liu J., Shan H., Li D. STING-targeted PET tracer for early assessment of tumor immunogenicity in colorectal cancer after chemotherapy. Eur. J. Nucl. Med. Mol. Imag. 2024;51(3):641–655. doi: 10.1007/s00259-023-06485-w. [DOI] [PubMed] [Google Scholar]
- 90.Liu X., Cen X., Wu R., Chen Z., Xie Y., Wang F., Shan B., Zeng L., Zhou J., Xie B., Cai Y., Huang J., Liang Y., Wu Y., Zhang C., Wang D., Xia H. ARIH1 activates STING-mediated T-cell activation and sensitizes tumors to immune checkpoint blockade. Nat. Commun. 2023 10;14(1):4066. doi: 10.1038/s41467-023-39920-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee S.E., Jang G.Y., Lee J.W., Park S.H., Han H.D., Park Y.M., Kang T.H. Improvement of STING-mediated cancer immunotherapy using immune checkpoint inhibitors as a game-changer. Cancer Immunol. Immunother. 2022;71(12):3029–3042. doi: 10.1007/s00262-022-03220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fu G., Wu Y., Zhao G., Chen X., Xu Z., Sun J., Tian J., Cheng Z., Shi Y., Jin B. Activation of cGAS-STING signal to inhibit the proliferation of bladder cancer: the immune effect of cisplatin. Cells. 2022 27;11(19):3011. doi: 10.3390/cells11193011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu S., Qin Z., Mao Y., Wang N., Zhang W., Wang Y., Chen Y., Jia L., Peng X. Pharmacological inhibition of MYC to mitigate chemoresistance in preclinical models of squamous cell carcinoma. Theranostics. 2024 1;14(2):622–639. doi: 10.7150/thno.88759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gao Menghan, Deng Hong, Zhang Yiyi, Wang Huimin, Liu Runmeng, Hou Wei, Zhang Weiqi. Hyaluronan nanogel co-loaded with chloroquine to enhance intracellular cisplatin delivery through lysosomal permeabilization and lysophagy inhibition. Carbohydr. Polym. 2024;1 doi: 10.1016/j.carbpol.2023.121415. 323. [DOI] [PubMed] [Google Scholar]
- 95.Monroe J.D., Johnston A.M., Smith M.E. The monofunctional platinum(II) compounds, phenanthriplatin and pyriplatin, modulate apoptosis signaling pathways in HEI-OC1 auditory hybridoma cells. Neurotoxicology. 2020;79:104–109. doi: 10.1016/j.neuro.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Park G.Y., Wilson J.J., Song Y., Lippard S.J. Phenanthriplatin, a monofunctional DNA-binding platinum anticancer drug candidate with unusual potency and cellular activity profile. Proc. Natl. Acad. Sci. U. S. A. 2012 Jul 24;109(30):11987–11992. doi: 10.1073/pnas.1207670109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Riddell I.A., Johnstone T.C., Park G.Y., Lippard S.J. Nucleotide binding preference of the monofunctional platinum anticancer-agent phenanthriplatin. Chemistry. 2016 May 23;22(22):7574–7581. doi: 10.1002/chem.201600236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bonaventura P., Shekarian T., Alcazer V., et al. Cold tumors:a therapeutic challenge for immunotherapy. Front. Immunol. 2019;10:168. doi: 10.3389/fimmu.2019.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu Y.-T., Sun Z.-J.J.T. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021;11(11):5365. doi: 10.7150/thno.58390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Margiotta N., Savino S., Gandin V., Marzano C., Natile G. Monofunctional platinum(II) complexes with potent tumor cell growth inhibitory activity: the effect of a hydrogen-bond donor/acceptor N-heterocyclic ligand. ChemMedChem. 2014;9(6):1161–1168. doi: 10.1002/cmdc.201402028. [DOI] [PubMed] [Google Scholar]
- 101.Wang Hua-Fen, Jia Hui-Zhen, Chu Yan-Feng, Feng Jun, Zhang Xian-Zheng, Zhuo Ren-Xi. Acidity-promoted cellular uptake and drug release mediated by amine-functionalized block polycarbonates prepared via one-shot ring-opening copolymerization. Macromol. Biosci. 2014;14(4):526–536. doi: 10.1002/mabi.201300414. [DOI] [PubMed] [Google Scholar]
- 102.He Wenxiu, Jiang Yue, Qian Li, Zhang Di, Li Zhonghao, Luan Yuxia. A versatile strategy to create an active tumor-targeted chemo-photothermal therapy nanoplatform: a case of an IR-780 derivative co-assembled with camptothecin prodrug. Acta Biomater. 2019;15(84):356–366. doi: 10.1016/j.actbio.2018.11.049. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Qixiong, Zhang Fuzhong, Li Shanshan, Liu Renfeng, Jin Taotao, Yin Dou, Zhou Zhenhua, Zhang Jianxiang. A multifunctional nanotherapy for targeted treatment of colon cancer by simultaneously regulating tumor microenvironment. Theranostics. 2019;31(13):3732–3753. doi: 10.7150/thno.34377. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gao F., Zhou J., Sun Y., Yang C., Zhang S., Wang R., Tan W. Programmable repurposing of existing drugs as pharmaceutical elements for the construction of aptamer-drug conjugates. ACS Appl. Mater. Interfaces. 2021;13:9457–9463. doi: 10.1021/acsami.0c18846. [DOI] [PubMed] [Google Scholar]
- 105.Liu Shaoli, Qiao Jian, Xiao Zhao, Zhao Ruifang, Wang Yuanning, Wang Yiming, Liu Jianbing, Shang Yingxu, Zhao Shuai, Wu Tiantian, Zhang Yinlong, Nie Guangjun, Ding Baoquan. A DNA nanodevice-based vaccine for cancer immunotherapy. Nat. Mater. 2021;20(3):421–430. doi: 10.1038/s41563-020-0793-6. [DOI] [PubMed] [Google Scholar]
- 106.Zhu Jiaojiao, Peng Lanyuan, Jehan Shah, Wang Haiyang, Chen Xiang, Zhao Shuang, Zhou Wenhu. vol. 24. Research (Wash D C); 2024. p. 7. (Activable Photodynamic DNA Probe with an "AND" Logic Gate for Precision Skin Cancer Therapy). 0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Weijun, Lin Mengling, Chen Yan-Ru, Wang Wenqing, Lv Jinrui, Chen Yaxin, Yin Hongwei, Shen Zhifa, Wu Zai-Sheng. Y-shaped backbone-rigidified DNA tiles for the construction of supersized nondeformable tetrahedrons for precise cancer therapies. Anal. Chem. 2024;17 doi: 10.1021/acs.analchem.3c03923. [DOI] [PubMed] [Google Scholar]
- 108.Zhang Wen-Bin. DNA nano-robot: what dreams may come. Acta Polym. Sin. 2021;52(4):335–338. [Google Scholar]
- 109.Xian H., Watari K., Sanchez-Lopez E., Offenberger J., Onyuru J., Sampath H., Ying W., Hoffman H.M., Shadel G.S., Karin M. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity. 2022 9;55(8):1370–1385.e8. doi: 10.1016/j.immuni.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou Shurong, Cheng Furong, Zhang Yu, Su Ting, Zhu Guizhi. Engineering and delivery of cGAS-STING immunomodulators for the immunotherapy of cancer and autoimmune diseases. Acc. Chem. Res. 2023 7;56(21):2933–2943. doi: 10.1021/acs.accounts.3c00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kuttruff Christian A., et al. Discovery of BI 7446: a potent cyclic dinucleotide STING agonist with broad-spectrum variant activity for the treatment of cancer. J. Med. Chem. 2023 27;66(14):9376–9400. doi: 10.1021/acs.jmedchem.3c00510. [DOI] [PubMed] [Google Scholar]
- 112.Xu Li, Deng Hongping, Wu Liang, Wang Dali, Shi Leilei, Qian Qiuhui, Huang Xiangang, Zhu Lijuan, Gao Xihui, Yang Jiapei, Su Yue, Feng Jing, Zhu Xinyuan. Supramolecular cyclic dinucleotide nanoparticles for STING-mediated cancer immunotherapy. ACS Nano. 2023 13;17(11):10090–10103. doi: 10.1021/acsnano.2c12685. [DOI] [PubMed] [Google Scholar]
- 113.Lu L., Liu W., Li S., Bai M., Zhou Y., Jiang Z., Jia Z., Huang S., Zhang A., Gong W. Flavonoid derivative DMXAA attenuates cisplatin-induced acute kidney injury independent of STING signaling. Clin Sci (Lond). 2023 31;137(6):435–452. doi: 10.1042/CS20220728. [DOI] [PubMed] [Google Scholar]
- 114.Daei Farshchi Adli A., Jahanban-Esfahlan R., Seidi K., Samandari-Rad S., Zarghami N. An overview on Vadimezan (DMXAA): the vascular disrupting agent. Chem. Biol. Drug Des. 2018;91(5):996–1006. doi: 10.1111/cbdd.13166. [DOI] [PubMed] [Google Scholar]
- 115.Ching L.M., Zwain S., Baguley B.C. Relationship between tumour endothelial cell apoptosis and tumour bloodflow shutdown following treatment with the antivascular agent DMXAA in mice. Br. J. Cancer. 2004;90:906–910. doi: 10.1038/sj.bjc.6601606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Luo P., He Q., He X., Hu Y., Lu W., Cheng Y., Yang B. Potent antitumor activity of 10- methoxy-9-nitrocamptothecin. Mol. Cancer Therapeut. 2006;5(4):962–968. doi: 10.1158/1535-7163.MCT-05-0385. [DOI] [PubMed] [Google Scholar]
- 117.Dong Heng, et al. Biomaterials facilitating dendritic cell‐mediated cancer immunotherapy. Adv. Sci. 2023;10(18) doi: 10.1002/advs.202301339. [DOI] [PMC free article] [PubMed] [Google Scholar]