Abstract
The hypothesis that the antibacterial effect of copper depends on its state was tested. It was studied the antibacterial effect of copper applied to the fabric, copper in chelated and free (ionic) forms on the growth intensity of Staphylococcus aureus 124 and Pseudomonas aeruginosa 18 in the in vitro system after a single or “primary” contact. Classical microbiology methods were used. Copper was applied to the fabric by magnetron and arc planar discharge systems, and the culture of microalgae Dunaliella viridis, resistant to the action of high concentrations of copper, was used to obtain copper in chelated form. It was shown that a thin layer of copper (3 μm) applied to the fabric showed pronounced antibacterial activity against Staphylococcus (85 % compared to the antibiotic meropenem) and less pronounced activity against Pseudomonas, which is resistant to meropenem. Copper in ionic form inhibited the growth of Staphylococcus aureus 124 as well as the antibiotic, and also effectively inhibited the growth of Pseudomonas aeruginosa 18 i.e., copper ions did not have species specificity like the antibiotic. Components of Dunaliella viridis microalgae cells had weakly expressed antibacterial effect to these types of bacteria, and supplementary addition of copper sulfate to the biomass of microalgae led to an increase of their antibacterial activity and this is more pronounced for microalgae culture in which the ratio « chelated/ionic » forms of copper is shifted to the ionic form. It was shown that the antibacterial activity of microalgae biomass after the first introduction into the tested bacterial cultures depends on the amount of free or “weakly bound” with cell components copper ions. It is suggested that the antibacterial effect of fabric with a thin layer of copper may be determined by two mechanisms: the action of copper ions and mechano-bactericidal effects, while chelated forms of copper may have a prolonged effect on bacterial cultures.
The discovery of penicillin by A. Fleming in 1928 and the industrial production of antibiotics, which began in the 40s of the 20th centuries and continues today, had three huge consequences. The introduction of antibiotics has had a profound impact on the development of biotechnology, leading to significant advancements in the field of medicine. And after a period of approximately 60–70 years during which antibiotics were widely employed in the treatment of a diverse range of bacterial infections, a new global issue has emerged. This issue concerns the growing resistance of numerous nosocomial infectious microorganisms to the antibiotics used to treat them. The pervasive use of antibiotics in medical and veterinary practice has resulted in the emergence of antibiotic resistance among microorganisms. This phenomenon has been observed in a wide range of antibiotics [1]. The search and development of new antibacterial agents has become one of the most relevant problems of modern biotechnology and medicine [[2], [3], [4]].
It is known that many metal ions have antibacterial activity [[5], [6], [7]]. At the same time, the development of modern nanotechnology and the production of nanoparticles have renewed interest in the mechanism of their action on bacterial cells [[8], [9], [10]]. Copper ions are of particular interest in this regard.
There are several reasons for this: copper ions have well-defined antibacterial effects. At the same time, they are much cheaper and more affordable than silver ions, which have a similar effect. Also, copper is an essential element and can be quickly eliminated from the organism. In addition, copper can exist in different states - metalized, chelated with proteins and other ligands, form nanoparticles, or can be in the ionic form. It can be assumed that different states of copper may have different biological activities, including antibacterial activity. Studying the influence of metalized copper, copper in chelated and ionic forms on the growth of Staphylococcus aureus 124 and Pseudomonas aeruginosa 18 will develop the range of applications as an antibacterial agent and contribute to the understanding of its mechanism of action on biological systems. It can be assumed that the duration of the biological effect (short-term or prolonged) may also be a function of the state of copper. It can be expected that in the case of prolonged action of copper, it is possible to provide a longer and more selective effect on bacteria and the organism. Therefore, the antibacterial effects of copper applied to tissue, copper ions, and copper in chelate form on the growth intensity of two types of common nosocomial bacterial infections, namely Staphylococcus aureus 124 and Pseudomonas aeruginosa 18, were determined in an in vitro system following a single primary exposure to these widespread hospital infections.
Knowledge of the antibacterial activity of copper, which is found in various forms, can greatly expand the applications of its use as an antibacterial agent and provide the body with the necessary prolonged intake of this essential element.
1. Methodology
Determination of the antibacterial activity of three forms of copper was carried out by the disk-diffusion method and the agar well diffusion method. As test cultures, we used Staphylococcus aureus 124 and Pseudomonas aeruginosa 18, which are contained in the collection of the I. Mechnikov Institute of Microbiology and Immunology National Academy of Medical Sciences of Ukraine.
Preparation of the microbial suspension of strains (microorganisms) was carried out using the Densi-La-Meter device (manufactured by PLIVA-Lachema, Czech Republic; wavelength 540 nm). The microbial suspension of the strains was prepared according to the instructions attached to the device and the information sheet on innovations in the health care system No. 163–2006 ″Standardization of preparation of microbial suspensions", Kyiv. Synchronization of strain cultures was carried out using a low temperature (4oC). The microbial load was 107 microbial cells per 1 ml of medium and was determined according to the McFarland standard. In the experiments, 18–24-h cultures of strains of microorganisms were used. Muller-Hinton agar was used for cultivation (Production - India “HIMedia Laboratorles Pvt. Ltd India")
Determination of the antimicrobial activity of the studied samples using the agar well diffusion method was carried out on two layers of a nutrient medium poured into Petri dishes (diameter 100 mm and height 15 mm). In the lower layer, a “starvation” unseeded medium (agar-agar, water, salts) was used. This layer is a medium substrate with a volume of 10.0 ± 0.3 ml. On this layer, 6 thin-walled stainless-steel cylinders with a diameter of 8 mm and a height of 10 mm were installed horizontally. Around the cylinders, the upper layer is poured, consisting of nutrient agar medium, melted and cooled to a temperature of 40.0 ± 0.5 °C. Then the appropriate standard of the daily test culture of the microorganisms two strains was introduced to the medium. Previously, the upper layer was well mixed until a homogeneous mass was formed. After solidification, the cylinders were pulled out with sterile tweezers and the test substance in a volume of 0.3 ml was placed in the holes formed. The volume of the medium for the upper layer was 15.0 ± 0.5 ml. The cups were dried for 30–40 min at room temperature while maintaining sterile conditions and placed in a thermostat for 18–24 h. After incubation, the dishes were removed, photographed and the diameters of the microbial growth retardation zones were determined and expressed in mm.
When evaluating the antibacterial activity of the studied extracts and their modifications, the following criteria were used.
-
-
the absence of growth stunting zones of microorganisms around the hole, as well as a stunting zone of up to 10 mm, indicates that the microorganism is not sensitive to the drug introduced into the hole or the concentration of the antimicrobial substance;
-
-
zones of stunted growth with a diameter of 10–15 mm indicate low sensitivity of the culture to the tested concentration of the antimicrobial substance;
-
-
zones of stunted growth with a diameter of 15–25 mm are considered as an indicator of the sensitivity of the microorganism to the tested substance;
-
-
zones of stunted growth, the diameter of which exceeds 25 mm, indicates the high sensitivity of microorganisms to the antimicrobial substance.
The disk-diffusion method was also used. A 3 mm diameter disc, previously moistened with the solution of the test sample or a small quantity of microalgae biomass, was placed on the agar surface seeded with the corresponding microorganism. When evaluating the antibacterial activity of the studied extracts and their modifications, the following criteria were used.
-
-
the absence of growth stunting zones of microorganisms around the disc, indicates that the microorganism is not sensitive to the drug or the concentration of the antimicrobial substance;
-
-
zones of stunted growth with a diameter of 5–8 mm indicate a low sensitivity of the culture to the tested concentration of the antimicrobial substance;
-
-
zones of stunted growth with a diameter of 9–14 mm are considered as an indicator of the sensitivity of the microorganism to the concentration of the tested substance;
-
-
zones of stunted growth, the diameter of which exceeds 15 mm, indicates the high sensitivity of microorganisms to the tested concentration of the antimicrobial substance.
Methods of obtaining samples of thin-layer metalized copper. In our laboratory, we have developed the technology of vacuum-plasma formation of metallic copper coatings with high adhesion and layer thickness 0.01–3 μm using magnetron and arc planar atomizing systems [11].
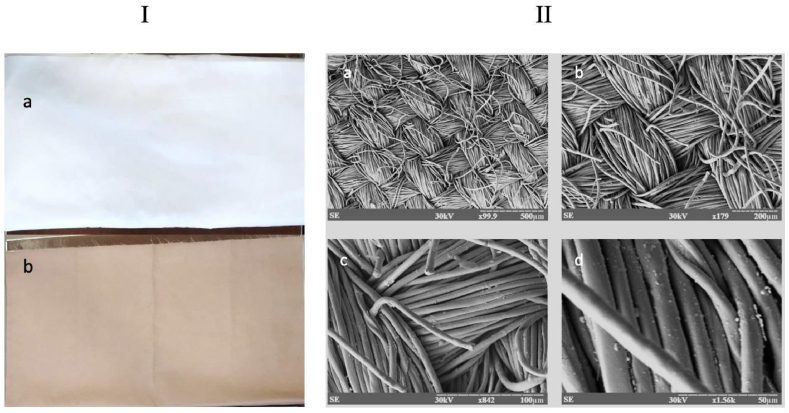
During the application of copper to the fabric, the discharge voltage of the magnetron sputtering system was 600 V, with a current of 10 A and a vacuum in the application chamber of 8x10-2 Pa. A fabric with a copper layer of 3 μm was obtained, sample sizes were 1.0 × 0.6 m2. The thickness of the copper layer was determined using Linnik interferometer on witness samples, placed in the area of application. The appearance of the copper-applied fabric differed from the original fabric (Fig. 1 I).
Fig. 1.
The appearance of the fabric base (a) on which a layer of copper 3 μm thick is applied (b); II - the image of the surface relief of metalized fabric with copper at various magnifications (а – х99.9, b – х179, c – х842, d – х1.56k).
The microstructure of the copper coating was investigated using a scanning transmission electron microscope REM-101(Fig. 1. II).
Determination of the antibacterial activity of copper-metalized tissue was carried out as already described, and the same tissue without copper application was used as a control variant.
1.1. Method of obtaining chelate form of copper in cells of microalgae Dunaliella viridis
Earlier in our laboratory a strain of microalgae D. viridis was obtained, capable of surviving in medium with a high concentration of sulfuric acid copper - 75 mg/l, i.e. resistant to high concentration of copper - D.v.CuR, in contrast to the original strain that is sensitive to copper ions - D. v.CuS [12].
More than 90 % of copper in cells is bound to proteins and most of it is located in the cytosol of cells. To obtain copper ions bound to cell components, Dunaliella viridis culture was precipitated by centrifugation at 3000g for 15 min at room temperature and the cell precipitate was destroyed by osmotic shock followed by homogenization. Unbroken cells and cell fragments were removed after centrifugation at 6000g for 15 min at room temperature. The obtained water phase, which included copper ions in complex with proteins, was used as chelate forms of copper. D. viridis cell culture, which was cultured on a standard medium without copper (D.v.CuS), was used as a control.
To determine the dependence of the antibacterial activity of the ionic form of copper and chelate form of copper, copper sulfate was supplementary added to homogenates of Dunaliella cells to final concentrations of 1, 7 and 15 g/l. The culture was stirred and incubated for 30 min. The cell suspension was then homogenized and the samples were centrifuged at 6000 g for 15 min at room temperature. In the precipitation of cell fragments and the obtained aqueous phase, after precipitation of cell fragments, the copper content was determined on an atomic adsorption spectrophotometer iCE3500, as described in work [13], and in aliquots antibacterial activity against S. aureus 124 and P. aeruginosa 18.
The absorption spectra of water-soluble components were determined on a Shimadzu UV-2600 spectrophotometer (Japan) at wavelengths of 200–400 nm in order to determine the change in the degree of extractability of microalgae components after copper sulfate addition.
When evaluating the antibacterial action of copper ions, an aqueous solution of copper sulfate was used at concentrations of 0.25, 0.5, 1.0, 7.0 15.0 20.0 and 25.0 g/L, which was tested for antibacterial activity against two species of bacteria as already described.
The electrical conductivity of the studied samples was measured on a Rohde & Schwarz ZNB40 vector network analyzer (Germany).
All experiments were repeated at least three times, with several analytical repetitions. The obtained results were subjected to statistical processing of the results using the Statistica 5.0 software package using the nonparametric Mann-Whitney U test. The tables and graphs show the mean values with their standard errors. Differences between the data of the control and experimental variants were considered significant at p < 0.05.
2. Results
2.1. Resistance of Staphylococcus aureus 124 and Pseudomonas aeruginosa 18 to fabric coated with a thin layer of copper (3 μm)
Determining antibacterial activity, we used the antibiotic meropenem, which is an antimicrobial agent of systemic action, as a control. Meropenem is known to inhibit the synthesis of cell wall components of gram-positive and gram-negative bacteria by binding to PBP proteins (the same proteins that bind penicillin). It is known that various strains of Enterobacteriaceae, Pseudomonas aeruginosa and Acinetobacter can exhibit resistance to meropenem, and therefore it is recommended to test its effect on local strains.
It was found that meropenem provides growth inhibition for S. aureus 124: stunting zone was 24.9 + 0.5 mm using the disk diffusion test (Fig. 2 A). At the same time, this antibiotic did not inhibit the growth of P. aeruginosa 18, which was manifested in the absence of growth inhibition of this bacterial culture (Fig. 2 A).
Fig. 2.
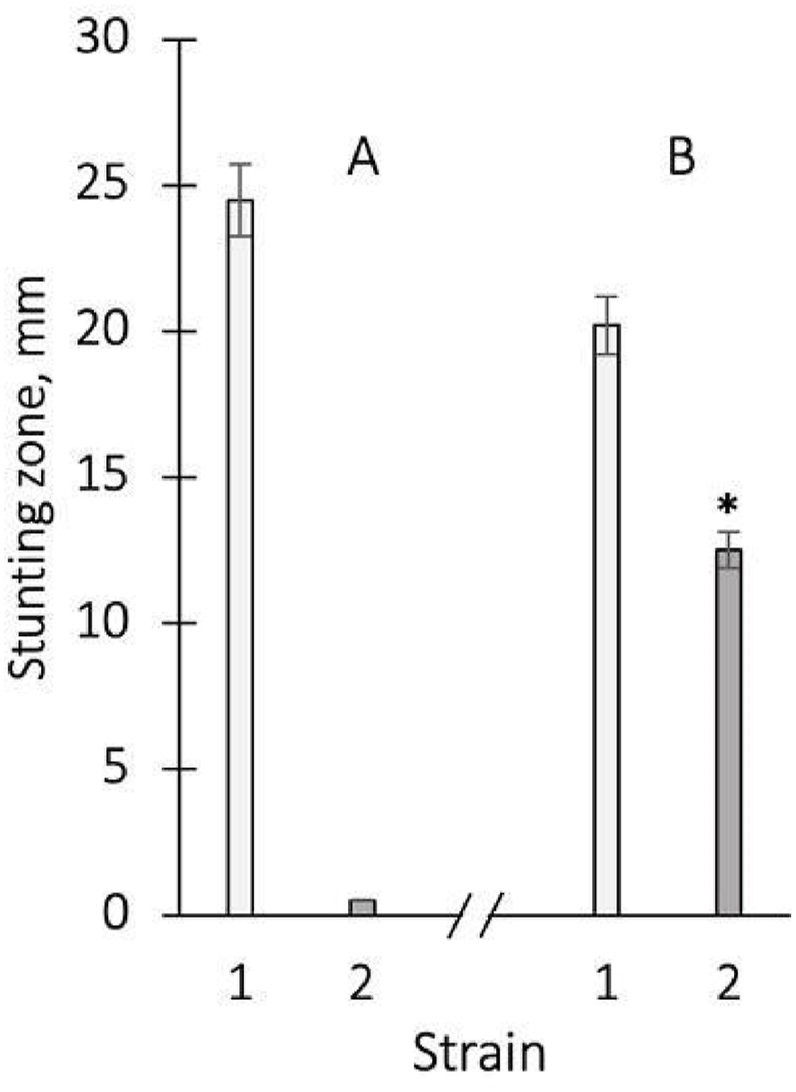
Zones of stunted growth of S. aureus 124 (1) and P. aeruginosa 18 (2) using the disk diffusion test with the antibiotic meropenem (A), as well as fabric with a 3 μm thick copper layer applied (B). The average values from 3 to 5 experiments and their standard errors are presented. ∗ - differences between antibiotic and fabric for variants are significant with P < 0.05.
Consequently, meropenem exhibited significant antibacterial activity against S. aureus 124 and had no effect on P. aeruginosa 18. The results confirm the presence of significant bacterial species sensitivity to the action of this antibiotic.
The fabric with a layer of copper of 3 μm provided a stunted growth of S. aureus 124 by 21.2 mm, which was 85 % compared to meropenem. It can be an indicator of the high sensitivity of this culture to the action of copper applied to the fabric (Fig. 2 B). It is important to note that fabric with a thin layer of copper inhibited the growth of not only Staphylococcus, but also Pseudomonas, in contrast to the antibiotic. But this effect was less pronounced, only by 50,2 % compared to the antibiotic for S. aureus 124 (Fig. 2 B). Consequently, a thin layer of copper applied to the fabric exhibited significant antibacterial activity against Staphylococcus and weakly expressed activity against Pseudomonas.
The development of methods for producing and using copper applied on fabric and other materials is of great practical interest as potential antibacterial agents. However, this requires knowledge of the mechanisms of the antibacterial action of such materials. To test this hypothesis, we conducted an experiment in which we applied copper to tissue and observed the antibacterial effect. Our hypothesis was that the antibacterial effect of copper is due to the ionization of copper in contact with biological substrates, which has an inhibitory effect on bacterial cells.
2.2. Resistance of Staphylococcus aureus 124 and Pseudomonas aeruginosa 18 to the action of copper ions
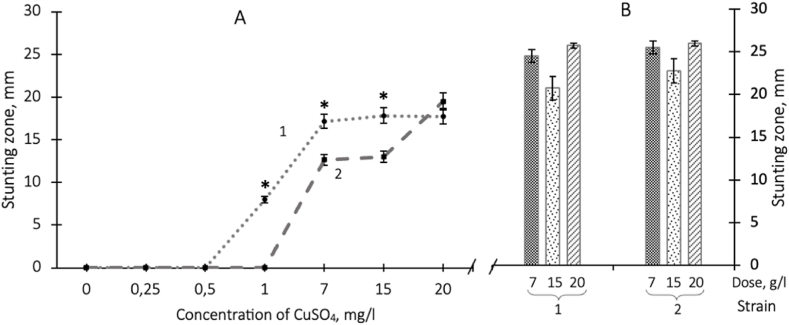
Testing the resistance of S. aureus 124 to the action of copper ions (water solution of copper sulfate) using the disk diffusion test, it was found that concentrations of 0.25 and 0.5 g/l did not affect the growth of this strain. When the concentration of copper sulfate was increased to 1 g/l there was a slight inhibition of growth. However, the antibacterial activity of copper ions was inferior in inhibiting the bacterial growth of copper that was applied to the tissue (Fig. 3, Fig. 1). A further increase of the concentration of copper sulfate did not affect the inhibition of the growth rate of S. aureus 124, i.e., the antibacterial activity curve reached a plateau (Fig. 3, Fig. 1).
Fig. 3.
Zones of stunted growth of S. aureus 124 (1) and P. aeruginosa 18 (2) with applying of copper sulfate to paper disks in different doses after cultivation for 24 h at a temperature of 37 °C (A) and with applying of copper sulfate to agar wells in doses: 7, 15 and 20 g/l– for S. aureus 124 (1) and P. aeruginosa 18 (2) (B). The means and their standard errors from three independent experiments are presented. ∗ - the difference between variants S. aureus 124 and P. aeruginosa 18 is significant at P < 0.05.
At the same time, during the disk diffusion test P. aeruginosa 18 had less sensitivity to copper ions compared to S. aureus 124 (Fig. 3 A). It should be noted that at high concentrations of copper sulfate of 7–20 g/l, the growth retardation for P. aeruginosa 18 is similar to that for S. aureus 124 (Fig. 3 A).
As is known, the zone of stunted growth of bacterial cultures depends not only on the concentration of the studied compounds, but also on the rate of diffusion of antibacterial compounds into the gel. In this regard, zones of delayed growth of crops were determined with the application of copper sulfate using the agar well diffusion method. It was found that in this case, the effectiveness of growth inhibition at high concentrations of copper sulfate was the same as the effect of copper applied to the fabric and was not inferior to meropenem. However, no species differences in bacterial resistance to copper ions were detected when antibacterial activity was assessed by the well method (Fig. 3 B).
Consequently, copper ions have the ability to inhibit the growth of S. aureus 124 and P. aeruginosa 18. The effectiveness of growth inhibition depends on the concentration, and this dependence has a saturating character. P. aeruginosa 18 had less sensitivity compared to S. aureus 124 at relatively low concentrations, and when high concentrations were reached (7–20 g/l), the efficiency of growth inhibition by copper ions was the same for both strains.
At the next stage of the work, we tested the assumption that the application of copper to the fabric was accompanied by the formation of complexes of copper ions with the cellulose structures of the tissue i.e., the antibacterial effect of copper, which was applied to the fabric, could be related to the antibacterial effect of copper chelate complexes. As an alternative model i.e., the study of chelate forms of copper on antibacterial action, we used cells of Dunaliella microalgae, which contain copper in chelate form.
2.3. Resistance of Staphylococcus aureus 124 and Pseudomonas aeruginosa 18 to the action of copper in chelate form
The ability to survive in a medium with a high concentration of copper sulfate (75 mg/l) of these microalgae was ensured by several mechanisms: the deposition of copper ions in cells in chelate form; restructuring of metabolism and synthesis of specific stress proteins.
At the next stage of the work, homogenates of two strains of D. viridis were obtained: with a low, i.e. physiological, content of copper ions, a natural strain of microalgae D. viridis - (D.v-Cu.S) and a strain with a high content of copper ions - (D.v-Cu.R). Then the ability of such homogenates (biomass) of these microalgae to exhibit antibacterial activity was determined.
It was found that the biomass of microalgae D. viridis, with low, i.e. physiological, the content of copper ions (D.v-Cu.S) provided a delay in bacterial growth by 11–12 mm with using disk diffusion test and by 13–14.7 mm with applying suspensions to wells, and this was manifested to the same extent for S. aureus 124 and P. aeruginosa 18 (see Table 1). The same degree of stunted growth of these bacterial cultures was exhibited by the biomass of microalgae - (D.v-Cu.R) (see Table 1), which exceeded D.v-Cu.S in the amount of copper by 215 times (Fig. 6, stage 1).
Table 1.
Zones of stunted growth (mm) for S. aureus 124 and P. aeruginosa 18 after application of a cell homogenate of two strains of microalgae: sensitive (D.v-Cu.S) and resistant (D.v-Cu.R) to copper ions, to paper disks and wells. The means of 3 experiments and their standard errors are presented.
| Test Method | Test sample | Bacterial strain |
|
|---|---|---|---|
| S. aureus 124 | P. aeruginosa 18 | ||
| Disk diffusion test | D.v.-CuS | 11,7 ± 0,42 | 11,5 ± 0,50 |
| D.v.-CuR | 12,2 ± 0,60 | 12,0 ± 0,63 | |
| Agar well diffusion method | D.v.-CuS | 14,0 ± 0,41 | 14,5 ± 1,19 |
| D.v.-CuR | 13,0 ± 0,41 | 14,8 ± 0,75 | |
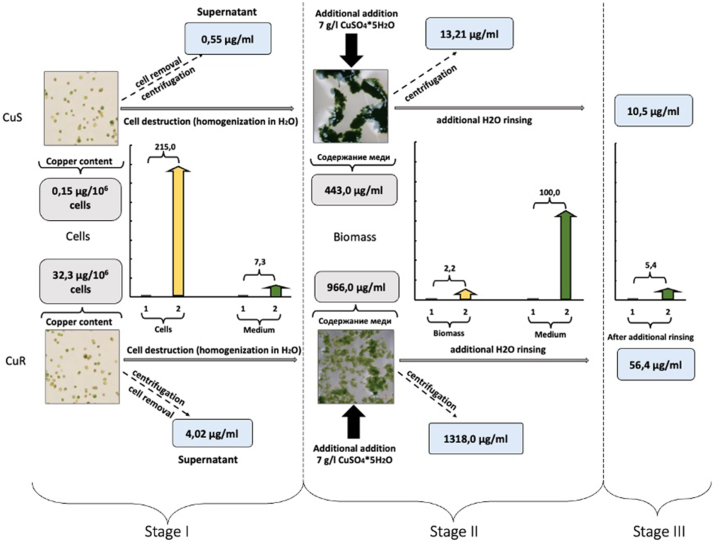
Fig. 6.
The stages of determining the content of copper ions in the studied samples. At Stage I, the number of copper ions in native cells D.v-Cu.S and D.v-Cu.R was determined (the number of cells of the two strains in the suspension was the same - 3.9 × 109 and the volume of cell suspensions was equalized) and the number of copper ions that were present in their culture medium. At Stage II, the cells were destroyed, and 7 g/l of copper sulfate was added to the obtained biomass (its amount was the same for the two strains), followed by determination of the amount of copper bound to the D.v-Cu.S and D.v-Cu.R biomass and the amount of remaining – unbound copper in the medium. At Stage III, the amount of easily removed (“weakly bound”) copper with biomass D.v-Cu.S and D.v-Cu.R was determined by water extraction. The squares represent the average values of the amount of copper from three determinations, and the histograms represent the comparative differences in the amount of copper ions in the studied samples between D.v-Cu.S and D.v-Cu.R.
Consequently, cell biomasses of D. viridis of two different strains, which included copper ions in chelate form in different amounts, exhibited poorly expressed antibacterial activity, which was manifested to the same extent for Staphylococcus aureus 124 and Pseudomonas aeruginosa 18.
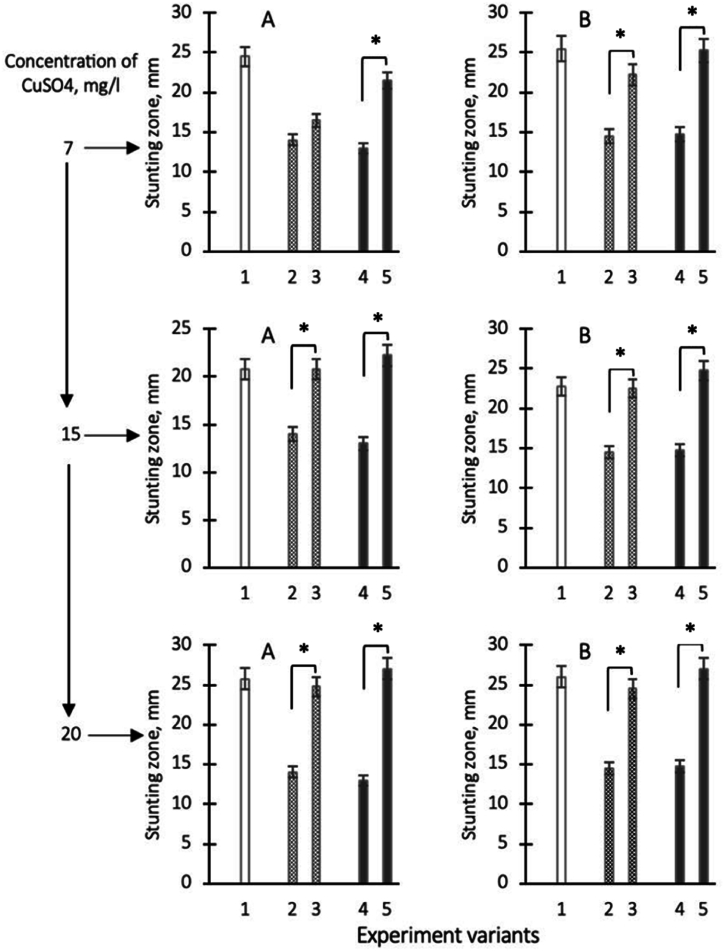
To investigate the concentration dependence of the antibacterial action of copper chelate forms, in the following series of experiments, copper sulfate was additionally added to the biomass of microalgae D.v-Cu.S and D.v-Cu.R at higher concentrations: 7; 15 and 20 g/L per equal number of cells (3.9 × 109), from which cell biomasses with increased amounts of copper chelate forms were obtained.
It was found that the supplementary addition of copper sulfate to final concentrations of 7; 15 and 20 g/l to microalgae cell homogenates was accompanied by an increase in antibacterial activity for both D.v-Cu.S and D.v-Cu.R biomass against S. aureus 124, compared to the biomasses of these cells without additional copper (Fig. 4 A). During testing these samples against P. aeruginosa 18, the effect of increasing antibacterial activity after supplementary addition of copper sulfate was manifested only for samples D.v-Cu.R and after the addition of copper sulfate of large concentrations - 15 and 20 g/l (Fig. 4 B).
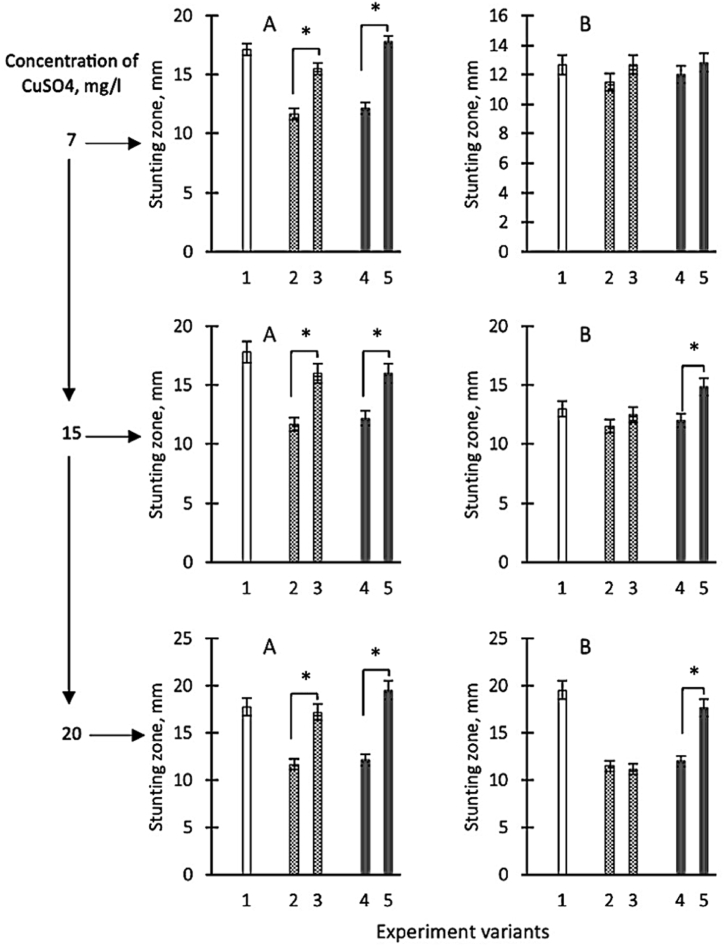
Fig. 4.
Growth inhibition zones of Staphylococcus aureus 124 (A) and Pseudomonas aeruginosa 18 (B) when tested on paper discs after applying copper sulfate solution (ionic form) at different concentrations to the disc: 7; 15 and 20 g/l (shown on an additional scale) respectively they are shown on the ordinate axis as variants 1, variants 2 - zones of growth retardation in case of application to discs of homogenates of D.v-Cu.S cells characterised by low copper content in chelate form, variants 3 - zones of growth retardation after application of homogenates of D.v-Cu. cells. S with the additional, preliminary introduction of sulfuric acid copper in cell homogenates respectively 7; 15 and 20 g/l of sulfuric acid copper, variant 4 - zones of growth retardation after application on discs of homogenates of cells D.v-Cu. R cells (which initially contained a relatively large amount of copper in the chelate form) and variant 5 - growth retardation zones after application of D.v-Cu.R cells homogenates on discs with additional preliminary introduction of 7; 15, and 20 g/l of copper sulfate into homogenates, respectively. The average values from three experiments and their standard errors are presented. ∗ - differences between options 2–3 and 4–5 are significant with P < 0.05.
During testing antibacterial activity using the agar well diffusion method, these effects were even more significant (Fig. 5). Thus, the effect of enhancing antibacterial activity after adding supplementary concentrations of copper sulfate was manifested not only against S. aureus 124, but also against the more resistant P. aeruginosa 18 (Fig. 5).
Fig. 5.
Growth inhibition zones of Staphylococcus aureus 124 (A) and Pseudomonas aeruginosa 18 (B) when tested by diffusion (well method) after application of copper sulfate solution (ionic form) at different concentrations: 7; 15 and 20 g/l (shown on an additional scale) respectively they are shown on the ordinate axis as variants 1, variants 2 - zones of growth retardation in case of application of homogenates of D.v-Cu.S cells on discs, which are characterised by the low copper content in chelate form, variants 3 - zones of growth retardation after application of homogenates of D.v-Cu. S with the additional, preliminary introduction of copper sulphoxide into cell homogenates respectively 7; 15, and 20 g/l of copper sulphoxide, variant 4 - zones of growth retardation after application on discs of cell homogenates D.v-Cu. R cells (which initially contained a relatively large amount of copper in the chelate form) and variant 5 - zones of growth retardation after applying homogenates of D.v-Cu.R cells to discs with additional preliminary introduction of 7; 15 and 20 g/l of copper sulfate into homogenates, respectively. The average values from three experiments and their standard errors are presented. ∗ - differences between options 2–3 and 4–5 are significant with P < 0.05.
The results allow us to conclude: 1- supplementary addition of copper sulfate to the biomasses of D.v-Cu.S and D.v-Cu.R cells increased their antibacterial activity towards the studied bacterial strains from 20 to 110 %. It was more significant for D.v -Cu.R; 2 - there is no direct correlation between increasing the dose of copper sulfate introduced into the cell biomass and antibacterial activity; 3 - the rate of growth inhibition of S. aureus 124 and P. aeruginosa 18 cell biomass to which copper sulfate was supplementary added did not exceed the antibacterial activity of a pure solution of copper sulfate (Fig. 4, Fig. 5); 4 – copper applied to the fabric exhibited the same antibacterial activity as a solution of copper sulfate at the highest concentration and greater than the antibacterial activity of the original cellular biomass of microalgae; 5 - the greatest differences in the increase in antibacterial activity for the cell biomass of D.v-Cu.R compared to D.v-Cu.S occurred after supplementary addition 7 g/l of copper sulfate to the cell biomass.
The obtained data can indicate that the addition of copper ions to microalgae biomass is accompanied by the formation of different structural-functional complexes which may have different antibacterial activity, and the presence of antibacterial effects is not only due to the action of copper ions This may be supported by the data on the presence, although not strongly expressed, of the antibacterial action of homogetans of microalgae cells. It cannot be excluded that in the presence of high concentrations of copper in the medium, the ‘extractivity’ of the cellular components of microalgae, which have different antibacterial activity, may change. The study of this issue is important in understanding the possible mechanisms of antibacterial effects.
To test this hypothesis, in the next series of experiments we determined the copper content in microalgae cells before the addition of additional amount of sulfuric acid copper to them, after the addition of sulfuric acid copper to them, as well as the features of extraction (transfer into aqueous solution) of copper ions and cell components, which may affect the manifestation of antibacterial activity.
2.4. Study of the “ability” of the biomass of microalgae strains D.v-Cu.S and D.v-Cu.R to bind copper ions
It was found that D.v-Cu.S cells, which were cultured under standard conditions in Artari medium, contained 0.15 μg of copper ions per 106 cells, which is a physiological level for these microalgae. D.v-Cu.R cells, which were adapted to growth on a medium containing copper ions and had been cultivated for more than 25 years on Artari medium with a supplementary addition of 75 mg/l copper sulfate, contained 32.3 μg of copper in 106 cells, which is 215 times more then D.v-Cu.S (Fig. 6 stage I).
It is important to note that in the culture medium where D.v-Cu.S culture was growing (where copper sulphoxide was not added), 0.55 μg/ml of copper was present, which may be a background level for this culture medium (Fig. 6 stage I). The culture medium of D.v-Cu.R, which was cultured on a medium containing 75 mg/l of copper sulphoxide, contained only 7 times more copper compared to the culture medium of D.v-Cu.S, not 215 times as in the cells of these strains (Fig. 6 stage I). This indicates that copper ions, after being added to the medium where D.v-Cu.R was cultivated, quickly penetrated and accumulated in the cells, binding to proteins and other cell components. So, most copper is found in cells in chelated form.
In order to determine the ability of cell biomass to additionally “bind” copper ions, microalgae cells were destroyed by homogenization and cell biomass D.v-Cu.S and D.v-Cu.R were obtained, respectively, and additional copper sulfate was added to these biomass up to 7 g/l. Then after incubation for 15 min, cell fragments (biomass) were separated from the water medium by centrifugation at 15000g for 15 min. It was found that the biomass of D.v-Cu.S cells contained 443.0 μg/ml of copper ion biomass, i.e. the amount of copper in the biomass increased 2950 times compared to the amount of copper in the cells (Fig. 6, stage II). Under the same conditions and with the same amount of D.v-Cu.R biomass, 966.0 μg/ml of copper was bound, i.e. the amount of copper in the D.v-Cu.R biomass increased after the supplementary addition of 7 g/l copper sulfate by only 29 times compared to its copper content in the cells (Fig. 6 stage II).
Consequently, D.v-Cu.R biomass which initially already contained copper bound 100 times less copper compared to D.v-Cu.S biomass under the same conditions.
In favour of the fact that D.v-Cu.R cell biomass binds relatively fewer copper ions compared to D.v-Cu.S after the addition of 7 g/l of copper sulphide, the results on the content of copper ions that remained in the aqueous medium after precipitation of cell biomass also testify. Thus, in the variant with D.v-Cu.S biomass, a small amount of copper ions (13.2 μg/ml) remained in the water medium i.e. most of the added copper was associated with the cell biomass. At the same time for the D.v-Cu.R variant, the water medium under the same conditions contained 100 times more copper compared to the water medium for D.v-Cu.S (Fig. 6, stage II). These results may indicate that the biomass of D.v-Cu.R. cells almost reached its ‘limit’ of copper ion saturation, in contrast to the biomass of D.v-Cu.S. microalgae cells.
Moreover, the “degree” of copper ion binding to the D.v-Cu.S cell biomass was much “stronger” compared to the D.v-Cu.R cell biomass. Thus, if the biomass “loaded” with copper was subjected to water extraction 10.5 μg/ml of copper ions from the D.v-Cu.S biomass passed into the water phase, while from the D.v-Cu.R biomass passed 5 times more of copper ions (56.4 μg/ml) (Fig. 6 Stage III).
Consequently, in the biomass of D.v-Cu.S and D.v-Cu.R cells, the ratios between the ionic form and the chelate form of copper are different, moreover, the degree of copper binding to the components of D.v-Cu.R cells is less strong compared to D.v-Cu.S. It can be assumed that the D.v-Cu.S and D.v-Cu.R biomasses will also differ in the ability of their components to “transition” into the water phase, i.e. extractivity, which may also influence differences in antibacterial activity.
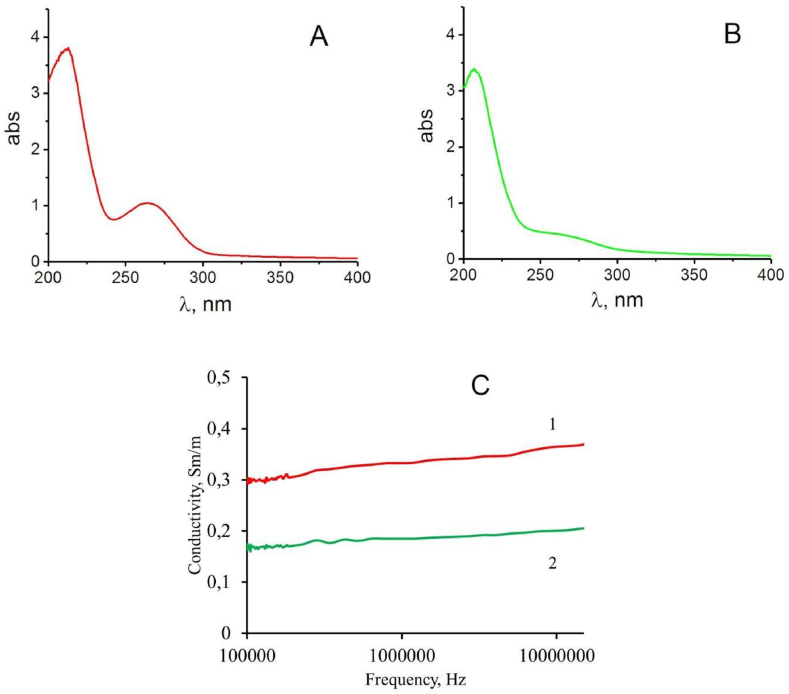
Study of the “extractivity” of biologically active components from D.v-Cu.S and D.v-Cu.R cell biomass after supplementary adding 7 g/l copper sulfate to them.
It was found that after the suspension of native D.v-Cu.S cells, a small amount of amino acids (absorption region 220 nm) and proteins (absorption region 260 nm) were extracted into the water phase (Fig. 7 A). At the same time, from the same number of native D.v-Cu.R cells under the same conditions, significantly fewer amino acids and trace amounts of proteins passed into the water phase compared to D.v-Cu.S (Fig. 7A and B).
Fig. 7.
Absorption spectra in the UV region of the water medium after rinsing the D.v-Cu.S (A) and D.v-Cu.R (B) cells, the electrical conductivity of the water medium after rinsing the D.v-Cu.S (1) and D.v-Cu cells. R (2) (C) and copper content in the water medium after rinsing these strains. To rinse the cells from the salts contained in the culture medium, 10 ml of sterile distilled water were added to cell sediments of equal weight, stirred for 5 min, precipitated again by centrifugation at 3000g, and the studied parameters were determined in the water phase.
These results indicate that the presence of copper ions in D.v-Cu.R cells leads to a decrease in the extractivity of cell components, probably due to the formation of associates not only with phytochelatins and metallothioneins, which specifically bind copper ions [14], but also with other cell components, which can be accompanied by a global cooperative structural and functional restructuring in such cells.
It should be noted that such growth-adapted D.v-Cu.R cells retained the basic characteristics of these microalgae and have been cultivated in the laboratory for more than 25 years.
The low extractivity of cell components and D.v-Cu.R compared to D.v-Cu.S cells is indirectly supported by the results of the difference in electrical conductivity of extractive substances obtained from D.v-Cu.S and D.v-Cu.R (Fig. 7C). Thus, the electrical conductivity of the water medium after extraction of D.v-Cu.S cells was 2 times higher compared to the electrical conductivity of the water medium after extraction of D.v-Cu.R cells (Fig. 7C).
This is explained by the fact that at least 2 times less components passed from D.v-Cu.R cells into the water medium compared to D.v-Cu.S, which ensured an increase in the electrical conductivity of this medium.
If D.v-Cu.R cells differ in their structural and functional organization due to the presence of a large amount of copper in them, compared to D.v-Cu.S cells, then we can expect that they will be more “resistant” to mechanical stress. At the next stage of the work, the cells were homogenized in water medium in order to obtain biomass to evaluate its antibacterial activity.
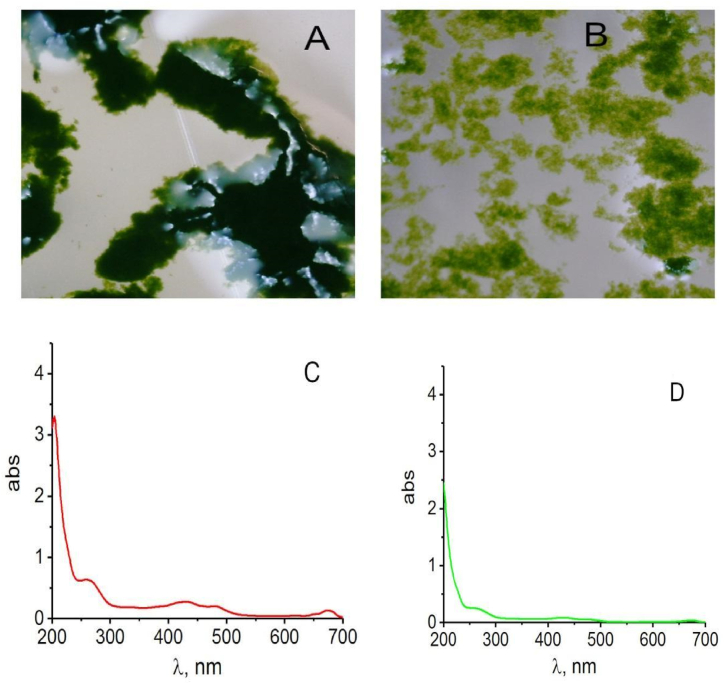
The results showed: 1- biomass obtained from D.v-Cu.S and D.v-Cu.R cells differed in color, which indicates a change in the structure of pigments (chlorophyll, carotenoids) and in the degree of aggregation of cellular components (Fig. 8 A and B); 2 - mechanical destruction of D.v-Cu.S cells was accompanied by the transition into the water medium of a relatively small amount of amino acids, peptides, proteins, which absorbed in the range of 220–280 nm, and pigments, which absorbed in the range of 400–500 and 670 nm (Fig. 8 C).
Fig. 8.
Appearance of formed aggregates from biomass components D.v-Cu.S (A) and D.v-Cu.R (B) after their homogenization under the same conditions, absorption spectra of components of the water medium after sedimentation of the cell biomass components D.v-Cu.S (C) and D.v- Cu.R(D). To rinse the cells from the salts contained in the culture medium, 10 ml of sterile distilled water was added to cell sediments of equal weight, stirred for 5 min, precipitated again by centrifugation at 3000g, and the spectral characteristics were determined in the water phase.
At the same time, mechanical destruction of D.v-Cu.R cells did not lead to the extraction of these cell components into the water medium (Fig. 8 D). Those, indeed, copper ions produced structural changes in cells, which can be conventionally defined as the formation of supramolecular mechanically stable complexes (Fig. 8 D);
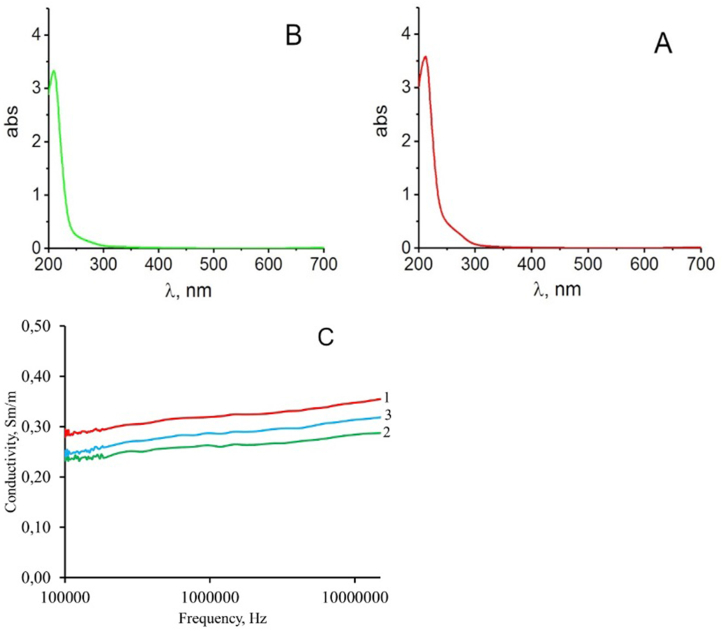
In the next series of experiments, copper sulfate was added to the biomasses of D.v-Cu.S and D.v-Cu.R cells to a final concentration of 7 g/l. After that the cell fragments were transferred to sediment by centrifugation at 15000g, and the number of extractive substances in the water medium was determined. They showed that there were no extractive substances in the aquatic medium, i.e. they were optically “empty” (Fig. 9 A and B). This indicates that after introducing high concentrations of copper ions into the biomass of D.v-Cu.S, aggregates of cell components were also formed, as for D.v-Cu.R. this confirms the fact that copper ions are involved in the formation of complexes with cell components.
Fig. 9.
Absorption spectra of extractive substances from the biomass of cells D.v-Cu.S (A) and D.v-Cu.R (B), which were obtained after supplementary addition of copper sulfate to them at a final concentration of 7 g/l and subsequent sedimentation of the cell biomass by centrifugation at 15,000 g and electrical conductivity of the aquatic medium after sedimentation of the cell biomass components D.v-Cu.S (1) and D.v-Cu.R (2), as well as the electrical conductivity of a solution of copper sulfate (3) at a concentration of 7 g/l (C). Typical results for this series of experiments are presented.
The electrical conductivity of the resulting water solutions after adding 7 g/l copper sulfate and subsequent sedimentation of cell biomass by centrifugation was significantly higher compared to the electrical conductivity of water extracts of the original biomass. At the same time, the electrical conductivity for the D.v-Cu.S and D.v-Cu.R variants did not differ significantly from each other (Fig. 9C). This increase in the electrical conductivity of water phases after the addition of copper sulfate to the biomass of microalgae cells indicates that some of the copper ions remained unbound with the cell components, which increased the electrical conductivity of this medium. It is important to note that the electrical conductivity of a pure copper sulfate solution was intermediate between the D.v-Cu.S and D.v-Cu.R options.
In conclusion, we note that the greater effect of increasing the antibacterial activity of D.v-Cu.R biomass compared to D.v-Cu.S against S. aureus 124 and P. aeruginosa 18, after supplementary adding 7 g/l copper sulfate to the algae biomass, can be explained by the fact that in D.v-Cu.R the ratio between the ionic form and the chelate form of copper is shifted towards the ionic form, and the part of copper that is associated “nonspecifically” with cell components, which occurs at saturating concentrations of copper, relatively easily passes into the ionic form and exhibits antibacterial effect. We believe that the D.v-Cu.R strain can be considered as a “carrier” or “depot” for copper, which exhibits antibacterial effects and, unlike antibiotics, does not show species specificity to bacteria along with copper applied to the tissue. It is important to consider that copper deposited on tissue and copper in the cell biomass of D.v-Cu.R can have different biological effects on the body, which is the goal of our further research.
3. Discussion
It has been known for a long time that copper ions, like silver ions, have a pronounced antibacterial effect. But only nowadays there is a growing interest in the use of copper ions in a wide range of medical problems, including the fight against infections [[15], [16], [17]]. Copper is shown to be associated with pathologies such as cancer [18], neurodegeneration [19], and angiogenic dysfunction [20]. Copper ions became an attractive drug target as well as a potential tool for the development of novel probes for medical interventions [[21], [22], [23]]. The antimicrobial effects of copper complexes on Gram-negative [24], Gram-positive [25], and mycobacteria [26,27] have been described.
However, the use of copper as an antibacterial agent has some organicities and peculiarities.
Firstly, copper ions are vital (essential) trace elements for all life forms and at the same time, they are extremely toxic. This is due to the fact that in biological systems copper can be in different states: Cu0 (nanoparticles), Cu1+; Cu2+ and Cu-ligand (chelate form). Moreover, copper ions form complexes with different types of macromolecules and its biological activity including antibacterial activity may depend on the balance between ionic forms, chelate forms, and nanoparticles in the organism and copper concentration.
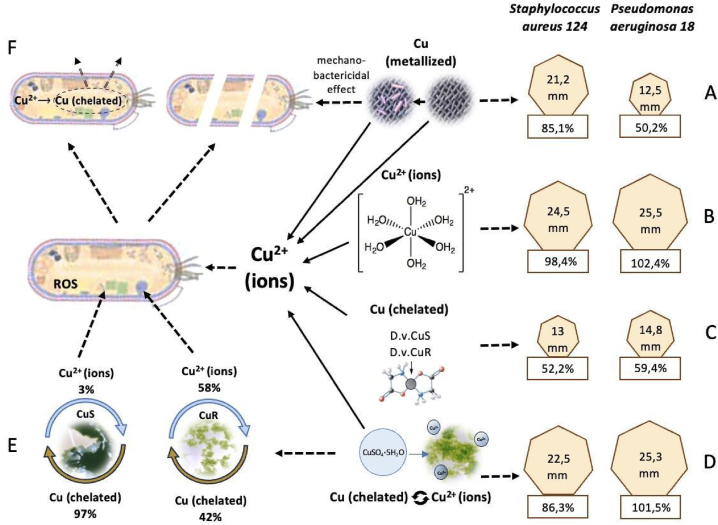
Secondly, in the process of evolution, which probably began about 2,7 billion years ago, when the oxygen level on the planet began to increase and, in such conditions, copper had “advantages" over iron, as in the oxidized form (Fe3+) tends to form insoluble minerals. In changing conditions living systems “learned" to use copper in metabolic processes, and in case it reaches high concentrations formed defense mechanisms against toxic effects of copper. Adaptive mechanisms to the toxic effects of copper are extremely diverse [[28], [29], [30]]. Microorganisms are able to bind “excess" copper with intracellular ligands and remove it from the cell (Fig. 10F). Moreover, it cannot be excluded that the use of copper as an antibacterial agent will also lead as in the case of antibiotics to the formation of resistant forms of microorganisms.
Fig. 10.
Scheme that demonstrates the effectiveness of inhibiting the growth of microorganisms S. aureus 124 and P. aeruginosa 18 when copper in metalized form - A, ionic form - B, was introduced into the bacterial cultures, the effect of components of microalgae D.v-Cu.S and D.v-Cu.R, which contained a small amount of copper (32.2 μg/106 cells) in chelate form - C, the effect of components of the biomass of these microalgae with supplementary added copper sulfate at a concentration of 7 g/l – D, the ratios between the chelate and ionic forms of copper at D.v-Cu.S and D.v-Cu.R - E, as well as a hypothetical mechanism of antibacterial action of copper ions, which demonstrates both the death of most bacteria due to the action on their cell walls and pro-oxidant action of copper ions and possible resistance in a small part of bacteria, which are able to provide effective chelation and its excretion from cells - F.
Thirdly, copper shows toxicity not only in relation to microorganisms, but also in relation to the host organism, i.e., it is not species-specific including in relation to bacteria, which is shown in the present work for Staphylococcus aureus 124 и Pseudomonas aeruginosa 18.
Proceeding from these features, it is necessary to divide the primary response of organisms to copper action and remote consequences or prolonged actions, as well as the response of the organism that will be formed on multiple consecutive actions of copper.
In the present work, it was determined a primary response of two bacterial cultures, S. aureus 124 and P. aeruginosa 18, to the action of copper, which was in three different forms: applied as a thin layer of 3 μm on cotton cloth; in ionic form and chelate form bound to components of the cells of microalgae D. viridis.
There are some works indicating that copper metal complexes can overcome bacterial resistance mechanisms [26] and this is because the ligand can broaden the spectrum of toxicity of copper ions against microorganisms, compared to free copper.
The results of the present work showed:
-
1
- a thin layer of copper applied to the tissue showed a pronounced antibacterial activity against Staphylococcus and less pronounced antibacterial activity against Pseudomonas. Thus, after the first contact of S. aureus 124 with copper on fabric, there was an inhibition of culture growth, which was about 85 % compared to the effect of the antibiotic meropenem. Copper fabric also inhibited the growth of P. aeruginosa 18, which was resistant to the antibiotic, but with half the intensity compared to S. aureus 124 (Fig. 10A).
The antibacterial action of a thin layer of copper applied on fabric can be achieved in at least two ways: by action of copper ions, which may be present in such material or formed when tested in liquid media and by mechano-bactericidal inactivation (Fig. 10A). It has been shown that the formation of three-dimensional (3D) hierarchically complex surfaces in titanium (or other materials) provided capture of S. aureus and P. aeruginosa cells and this was accompanied by their inactivation [31]. The authors of this work suggest that the development of intelligent mechano-bactericidal surfaces may be particularly effective in inactivating cocci such as S. aureus. Perhaps this explains the greater growth inhibition effect of S. aureus compared to P. aeruginosa by a thin layer of copper applied to the fabric (Fig. 2). We cannot exclude the combined action (ionic and mechano-bactericidal) of metalized copper on tissues, which provides the formation of complex surfaces (Fig. 1, Fig. 10).
-
2
- copper in ionic form inhibited the growth of S. aureus 124 as well as the antibiotic, while the ionic form of copper also effectively inhibited the growth of P. aeruginosa 18, which was resistant to the antibiotic (Fig. 10 B). The effectiveness of growth inhibition of bacterial cultures depended on the copper ion concentration and this dependence was saturating in nature, the yield of the dose curve to plateau (reaching the maximum inhibition for P. aeruginosa 18 was 7 g/l copper sulfate, and 20 g/l for S. aureus 124 during using disk-diffusion method. While using the agar well method, the steady level of growth inhibition was reached at 7 g/l for both species (Fig. 3). Differences in the effectiveness of antibacterial action in these two methods of testing may be due to the fact that copper ions can form complexes with cellulose, which slows down the rate of diffusion of ions into the agarized medium as opposed to direct application in wells.
-
3
- the presence of copper in chelated form in D. viridis cells, (32,3 μg in 106 cells), had no inhibitory effect on the studied bacterial species. At the same time, the components of microalgae cells after their homogenization had a weakly pronounced antibacterial effect, which was manifested to the same extent for the two strains of microalgae and was at least 50 % compared to the antibiotic for Staphylococcus aureus 124 (Fig. 10 C).
-
4
- supplementary addition of copper sulfate to D.v-Cu.S and D.v-Cu.R cell biomasses, which were obtained by homogenization, increased their antibacterial activity against the tested bacterial strains, and this was more pronounced for D.v-Cu.R than for D.v-Cu.S. There was no direct correlation between the increase in the dose of copper sulfate added to the cell biomass and antibacterial activity, and the rate of growth inhibition of S. aureus 124 and P. aeruginosa 18 by microalgae cell biomass to which copper sulfate was supplementary added did not exceed the antibacterial activity of pure copper sulfate solution and copper tissue (Fig. 10 D).
-
5
- in the biomass of D.v-Cu.R cells the ratio between the ionic form and chelate form of copper is shifted towards ionic form (Fig. 10 E) and the “degree of binding" of copper to the components of microalgae cells D.v-Cu.R is less strong compared to D.v-Cu.S (Fig. 6 stage III) and their biomass showed greater antibacterial activity. This feature is explained by the fact that the microalgae strain D.v-Cu.R is adapted to grow on medium with high copper content and it contained 215 times more copper before additional copper application compared to the natural strain - D.v-Cu.S (Fig. 6, stage I). The obtained results showed that in D.v-Cu.R cells there was a “saturation" of possible copper ion binding centers and the biomass of these cells contained more copper ions compared to the chelate forms of copper (Fig. 10 E), which provided greater antibacterial activity compared to the biomass of D.v-Cu.S, where the same amount of copper sulfate was added.
-
6
- the additional introduction of copper sulfate to microalgae biomasses up to concentrations of 7 g/l and more, was accompanied by a significant decrease in the extractivity of microalgae cell components into the aqueous medium (Fig. 9). This is due to the formation of copper-containing biocomplexes, which significantly reduced the degree of extractivity and probably led to the inhibition of antibacterial activity of cellular components that are part of microalgae cells. Such an effect of copper ions on microalgae cell components explains the absence of an additive effect between the antibacterial action of microalgae components and the addition of copper ions to the cell biomass.
The mechanisms of the antibacterial action of copper are rather complex and have not been definitively established. As already mentioned, it is necessary to distinguish between the primary effects of copper action on bacterial cells and prolonged (subsequent) effects. The results of the present work suggest that in the primary action of copper, which may be in different states, its mechanisms of antibacterial action are associated with the action of copper ions. As it is known copper ions are able to disrupt the function of bacterial cell walls, induce oxidative stress, and inhibit the activity of primarily antioxidant enzymes [32]. Unlike many antibiotics, copper ions are capable of exerting a variety of non-specific effects on the structural and functional organization of cells, and these actions are not as species-specific as those of some antibiotics. At the same time, in the case of relatively low concentrations of copper ions there are also small species differences in sensitivity, at least for S. aureus 124 and P. aeruginosa 18. This may be due to the fact that these species differed in cell wall structure and some metabolic features possibly and different efficiency of copper excretion from cells.
It cannot be excluded that after the initial (rapid) effect of copper ions, some bacterial cells remained resistant to them (Fig. 10 F) and could form resistance to the subsequent (prolonged) action of copper ions), it can be expected that they may be influenced by chelated copper ions, which was written about by a number of researchers [[24], [25], [26], [27]]. Experimental studies of the possible prolonged action of ionic complexes with a variety of ligands is the aim of our new work.
4. Conclusion
The antibacterial effect of copper depends on its aggregation states. In different states, different mechanisms of bacterial growth inhibition can be realized. The use of copper ion-resistant microalgae D.v-Cu.R, which has a balance between ionic and chelate forms of copper, can be a promising antibacterial material, as well as the application of copper on tissue.
CRediT authorship contribution statement
A.I. Bozhkov: Writing – review & editing, Writing – original draft, Methodology. V.V. Bobkov: Project administration, Methodology, Investigation. T.P. Osolodchenko: Investigation. O.I. Yurchenko: Investigation. V.Y. Ganin: Investigation. E.G. Ivanov: Investigation. Y.D. Batuieva: Visualization, Investigation. V.V. Minukhin: Project administration. A.V. Goltvyanskiy: Investigation. V.A. Kozheshkurt: Investigation. S.V. Ponomarenko: Investigation.
Data availability statement
Data will be made available on request.
Bioethics
All experiments were carried out in agreement with the bioethical committee of V.N. Karazin Kharkiv National University (Protocol of the Bioethical Commission of Kharkiv National University No. 1 of 04.10.23).
Declaration of competing interest
The work was carried out within the framework of the project of the Ministry of Education and Science of Ukraine No. 0123U101860. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhu Y., Huang W.E., Yang Q. Clinical perspective of antimicrobial resistance in bacteria. Infect. Drug Resist. 2022:735–746. doi: 10.2147/IDR.S345574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Årdal C., Balasegaram M., et al. Antibiotic development—economic, regulatory and societal challenges. Nat. Rev. Microbiol. 2020;18(5):267–274. doi: 10.1038/s41579-019-0293-3. [DOI] [PubMed] [Google Scholar]
- 3.Durand G.A., Raoult D., Dubourg G. Antibiotic discovery: history, methods and perspectives. Int. J. Antimicrob. Agents. 2019;53(4):371–382. doi: 10.1016/j.ijantimicag.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Wohlleben W., Mast Y., et al. Antibiotic drug discovery. Microb. Biotechnol. 2016;9(5):541–548. doi: 10.1111/1751-7915.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Godoy-Gallardo M., Eckhard U., Delgado L.M., et al. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: from mechanisms to applications. Bioact. Mater. 2021;6(12):4470–4490. doi: 10.1016/j.bioactmat.2021.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mittapally S., Taranum R., Parveen S. Metal ions as antibacterial agents. J. Drug Deliv. Therapeut. 2018;8(6-s):411–419. doi: 10.22270/jddt.v8i6-s.2063. [DOI] [Google Scholar]
- 7.Wyszogrodzka G., Marszałek B., Gil B., Dorożyński P. Metal-organic frameworks: mechanisms of antibacterial action and potential applications. Drug Discov. Today. 2016;21(6):1009–1018. doi: 10.1016/j.drudis.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Mendes C.R., Dilarri G., Forsan, et al. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022;12(1):2658. doi: 10.1038/s41598-022-06657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kędziora A., Speruda M., Krzyżewska E., Rybka J., et al. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int. J. Mol. Sci. 2018;19(2):444. doi: 10.3390/ijms19020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee A.K., Chakraborty R., Basu T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology. 2014;25(13) doi: 10.1088/0957-4484/25/13/135101. [DOI] [PubMed] [Google Scholar]
- 11.Bobkov V.V., Alimov S.S., Andreiev V.V., Onischenko A.V., Starovoitov R.I. 29th EPS Conference in Plasma Phys. And Contr. Fusion. Montreux; June: 2002. Transitional phenomena in magnetron discharge; pp. 17–21. [Google Scholar]
- 12.Kovaleva M.K., Menzyanova N.G., Jain A., Yadav A., Flora S., Bozhkov A.I. Effect of hormesis in Dunaliella viridis Teodor. (Chlorophyta) under the influence of copper sulfate. Int. J. Algae. 2012;14(1) doi: 10.1615/InterJAlgae.v14.i1.40. [DOI] [Google Scholar]
- 13.Yurchenko O.I., Chernozhuk T.V., Nikolenko M.V., Baklanov O.M., Kravchenko O.A. vol. 1. Issues of Chemistry & Chemical Technology/Voprosy Khimii & Khimicheskoi Tekhnologii; 2024. (Atomic Absorption Determination of Copper and Zinc in Pharmaceuticals). [DOI] [Google Scholar]
- 14.Bozhkov A.I., Sidorov V.I., Alboqai O.K., Akzhyhitov R.A., Kurguzova N.I., Malyshev A.B.…Gromovoi T.Y. The role of metallothioneins in the formation of hierarchical mechanisms of resistance to toxic compounds in young and old animals on the example of copper sulfate. Translational Medicine of Aging. 2021;5:62–74. doi: 10.1016/j.tma.2021.11.001. [DOI] [Google Scholar]
- 15.Becker K.W., Skaar E.P. Metal limitation and toxicity at the interface between host and pathogen. FEMS Microbiol. Rev. 2014;38(6):1235–1249. doi: 10.1111/1574-6976.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Djoko K.Y., Ong C. The role of copper and zinc toxicity in innate immune defense against bacterial pathogens. J. Biol. Chem. 2015;290(31):18954–18961. doi: 10.1074/jbc.R115.647099. lynn Y, Walker MJ, McEwan AG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ladomersky E., Petris M.J. Copper tolerance and virulence in bacteria. Metallomics. 2015;7(6):957–964. doi: 10.1039/c4mt00327f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biomedicine Garber K. Targeting copper to treat breast cancer. Science. 2015 Jul 10;349(6244):128–129. doi: 10.1126/science.349.6244.128. [DOI] [PubMed] [Google Scholar]
- 19.White A.R., Kanninen K.M., Crouch P.J. Metals and neurodegeneration: restoring the balance. Front. Aging Neurosci. 2015;7:127. doi: 10.3389/fnagi.2015.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urso E., Maffia M. Behind the link between copper and angiogenesis: established mechanisms and an overview on the role of vascular copper transport systems. J. Vasc. Res. 2016;52(3):172–196. doi: 10.1159/000438485. [DOI] [PubMed] [Google Scholar]
- 21.Denoyer D., Pearson H.B., Clatworthy S.A., Smith Z.M., Francis P.S., Llanos R.M.…Cater M.A. Copper as a target for prostate cancer therapeutics: copper-ionophore pharmacology and altering systemic copper distribution. Oncotarget. 2016;7(24) doi: 10.18632/oncotarget.9245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzano C., Pellei M., Tisato F., Santini C. Copper complexes as anticancer agents. Anti Cancer Agents Med. Chem. 2009;9(2):185–211. doi: 10.2174/187152009787313837. [DOI] [PubMed] [Google Scholar]
- 23.Neyrolles O., Wolschendorf F., Mitra A., Niederweis M. Mycobacteria, metals, and the macrophage. Immunol. Rev. 2015;264(1):249–263. doi: 10.1111/imr.12265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Djoko K.Y., Paterson B.M., Donnelly P.S., McEwan A.G. Antimicrobial effects of copper (II) bis (thiosemicarbazonato) complexes provide new insight into their biochemical mode of action. Metallomics. 2014;6(4):854–863. doi: 10.1039/c3mt00348e. [DOI] [PubMed] [Google Scholar]
- 25.Haeili M., Moore C., Davis C.J., Cochran J.B., Shah S., Shrestha T.B.…Wolschendorf F. Copper complexation screen reveals compounds with potent antibiotic properties against methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2014;58(7):3727–3736. doi: 10.1128/aac.02316-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dalecki A.G., Haeili M., Shah S., Speer A., Niederweis M., Kutsch O., Wolschendorf F. Disulfiram and copper ions kill Mycobacterium tuberculosis in a synergistic manner. Antimicrob. Agents Chemother. 2015;59(8):4835–4844. doi: 10.1128/aac.00692-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah S., Dalecki A.G., Malalasekera A.P., Crawford C.L., Michalek S.M., Kutsch O.…Wolschendorf F. 8-Hydroxyquinolines are boosting agents of copper-related toxicity in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2016;60(10):5765–5776. doi: 10.1128/aac.00325-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bozhkov A.I., Kovaleva M.K., Goltvyanskiy A.V., Ushakova E.O., Tsapko H.Y., Gavrish A.O. Preliminary adaptation of Dunaliella viridis strains to copper sulfate affects the thermal stability of the culture. Int. J. Algae. 2020;22(1) doi: 10.1615/InterJAlgae.v22.i1.50. [DOI] [Google Scholar]
- 29.Bozhkov A.I., Goltvyanskiy A.V., Kovaleva M.K., Menzyanova N.G. On the inheritance of induced resistance to toxic concentrations of sulfur acid of copper by subsequent cell generations of Dunaliella viridis teodoresco. Int. J. Algae. 2018;20(4) doi: 10.1615/InterJAlgae.v20.i4.20. [DOI] [Google Scholar]
- 30.Rostama S., Bozhkov A.I., Goltvyanskiy A.V. The effect of copper, lead, and cadmium ions on induced aggregation in cells of Dunaliella viridis (Teodor.)(Chlorophyta) Int. J. Algae. 2012;14(2) doi: 10.1615/InterJAlgae.v14.i2.30. [DOI] [Google Scholar]
- 31.Linklater D.P., Juodkazis S., Crawford R.J., Ivanova E.P. Mechanical inactivation of Staphylococcus aureus and Pseudomonas aeruginosa by titanium substrata with hierarchical surface structures. Materialia. 2019;5 doi: 10.1016/j.mtla.2018.100197. [DOI] [Google Scholar]
- 32.Giachino A., Waldron K.J. Copper tolerance in bacteria requires the activation of multiple accessory pathways. Mol. Microbiol. 2020;114(3):377–390. doi: 10.1111/mmi.14522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.