Abstract
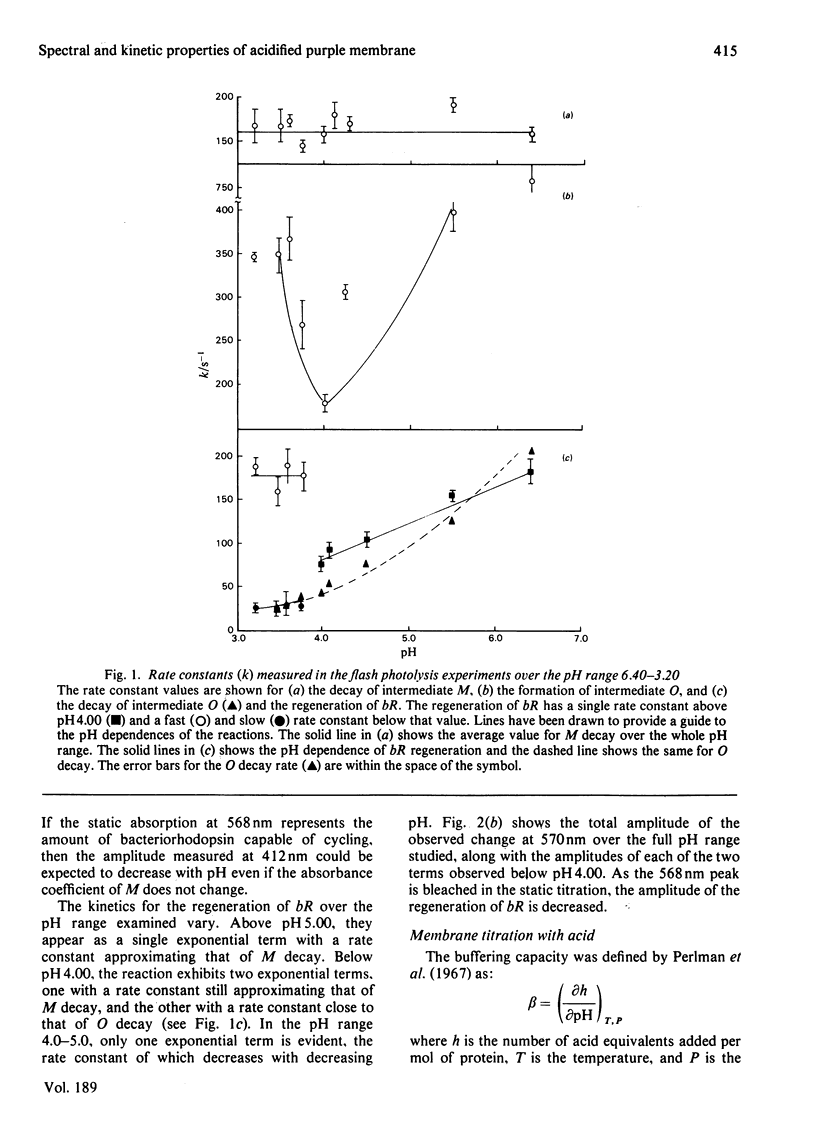
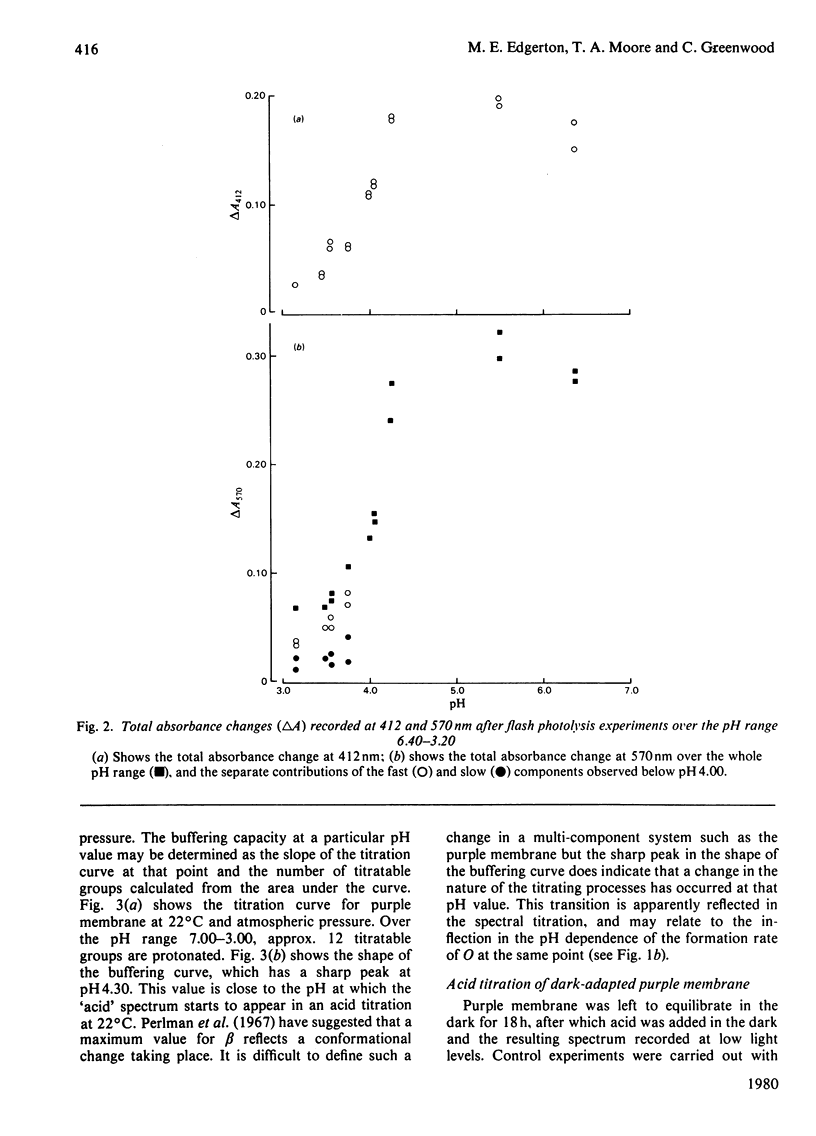
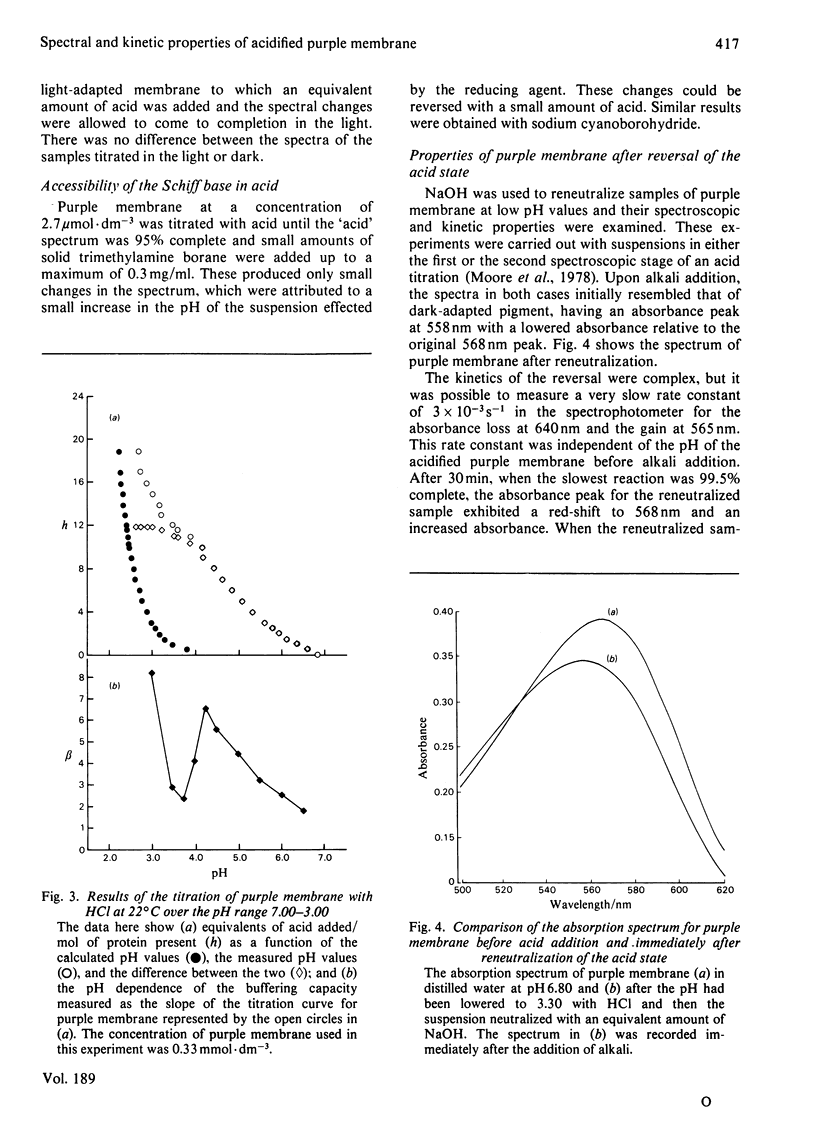
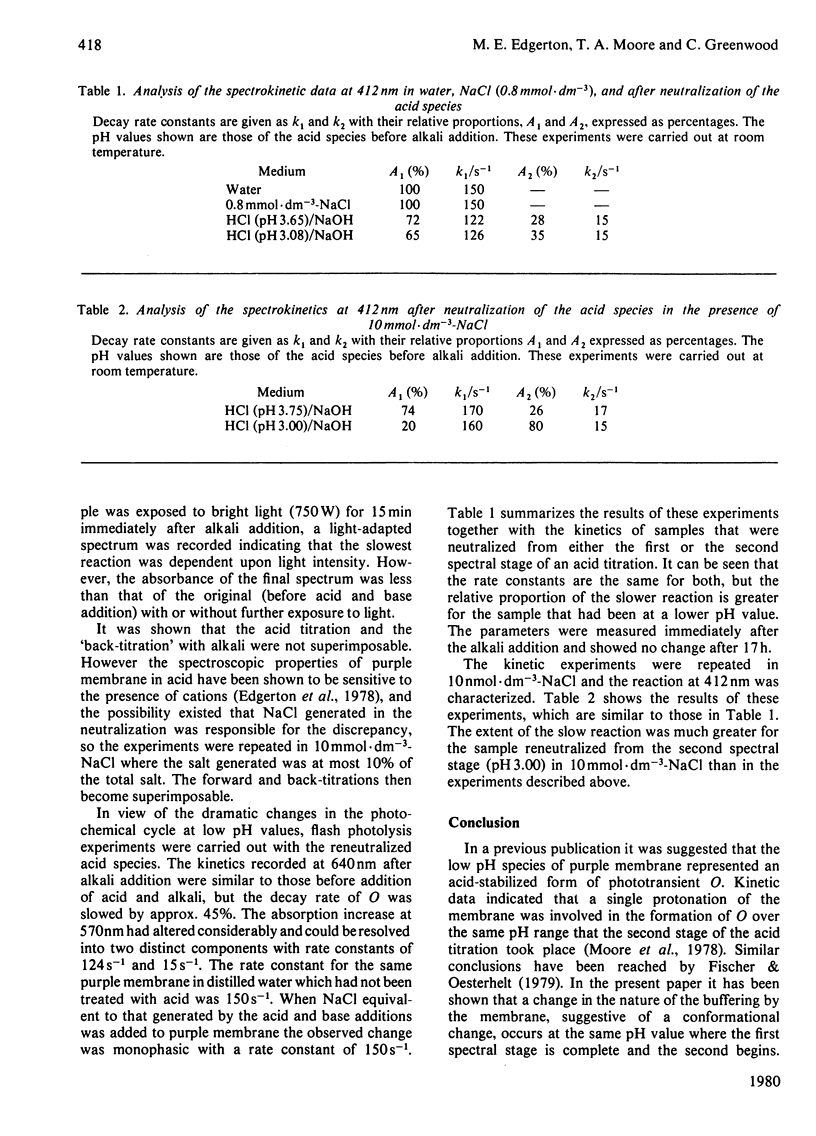
The formation and reversal of the acid species of purple membrane generated below pH 4.00 (22 degrees C) is studied together with the photochemical cycle over the pH range 6.40--3.20. The buffering capacity of the membrane reaches a peak at pH 4.30, indicating the possibility of a conformational change taking place. The generation of the new spectral species can take place in the dark and is unaffected by the addition of reducing agents. Kinetic parameters measured indicate that the group being titrated below pH 4.00 could be the same as that protonated in the formation of intermediate O. The temporal placement of intermediate O after M in the photochemical cycle is shown to be incompatible with the data presented here. Reneutralization of acidified purple membrane shows that the spectral changes in acid are reversible but the phototransient properties are altered.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridgen J., Walker I. D. Photoreceptor protein from the purple membrane of Halobacterium halobium. Molecular weight and retinal binding site. Biochemistry. 1976 Feb 24;15(4):792–798. doi: 10.1021/bi00649a010. [DOI] [PubMed] [Google Scholar]
- Edgerton M. E., Moore T. A., Greenwood C. Salt reversal of the acid-induced changes in purple membrane from Halobacterium halobium. FEBS Lett. 1978 Nov 1;95(1):35–39. doi: 10.1016/0014-5793(78)80046-5. [DOI] [PubMed] [Google Scholar]
- Fischer U., Oesterhelt D. Chromophore equilibria in bacteriorhodopsin. Biophys J. 1979 Nov;28(2):211–230. doi: 10.1016/S0006-3495(79)85172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBSON Q. H., GREENWOOD C. KINETIC OBSERVATIONS ON THE NEAR INFRARED BAND OF CYTOCHROME C OXIDASE. J Biol Chem. 1965 Jun;240:2694–2698. [PubMed] [Google Scholar]
- Kaufmann K. J., Rentzepis P. M., Stoeckenius W., Lewis A. Primary photochemical processes in bacteriorhodopsin. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1109–1115. doi: 10.1016/0006-291x(76)90310-7. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Hydration effects on cis--trans isomerization of bacteriorhodopsin. FEBS Lett. 1977 Oct 1;82(1):7–11. doi: 10.1016/0014-5793(77)80874-0. [DOI] [PubMed] [Google Scholar]
- Korenstein R., Hess B. Hydration effects on the photocycle of bacteriorhodopsin in thin layers of purple membrane. Nature. 1977 Nov 10;270(5633):184–186. doi: 10.1038/270184a0. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Martin W. G. Characterization and composition of the purple and red membrane from Halobacterium cutirubrum;. Can J Biochem. 1975 Mar;53(3):284–292. doi: 10.1139/o75-040. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Stoeckenius W. Comparison of purple membrane from Halobacterium cutirubrum and Halobacterium halabium. Biochim Biophys Acta. 1976 Apr 5;426(4):703–710. doi: 10.1016/0005-2736(76)90135-8. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W., Bogomolni R. A., Hwang S., Stoeckenius W. Kinetics and stoichiometry of light-induced proton release and uptake from purple membrane fragments, Halobacterium halobium cell envelopes, and phospholipid vesicles containing oriented purple membrane. Biochim Biophys Acta. 1976 Sep 13;440(3):545–556. doi: 10.1016/0005-2728(76)90041-4. [DOI] [PubMed] [Google Scholar]
- Lozier R. H., Niederberger W. The photochemical cycle of bacteriorhodopsin. Fed Proc. 1977 May;36(6):1805–1809. [PubMed] [Google Scholar]
- Moore T. A., Edgerton M. E., Parr G., Greenwood C., Perham R. N. Studies of an acid-induced species of purple membrane from Halobacterium halobium. Biochem J. 1978 May 1;171(2):469–476. doi: 10.1042/bj1710469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowery P. C., Lozier R. H., Chae Q., Tseng Y. W., Taylor M., Stoeckenius W. Effect of acid pH on the absorption spectra and photoreactions of bacteriorhodopsin. Biochemistry. 1979 Sep 18;18(19):4100–4107. doi: 10.1021/bi00586a007. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971 Sep 29;233(39):149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- Perlmann G. E., Oplatka A., Katchalsky A. Potentiometric titration and conformational change in pepsinogen. J Biol Chem. 1967 Nov 25;242(22):5163–5168. [PubMed] [Google Scholar]
- Racker E., Stoeckenius W. Reconstitution of purple membrane vesicles catalyzing light-driven proton uptake and adenosine triphosphate formation. J Biol Chem. 1974 Jan 25;249(2):662–663. [PubMed] [Google Scholar]
- Schreckenbach T., Walckhoff B., Oesterhelt D. Specificity of the retinal binding site of bacteriorhodopsin: chemical and stereochemical requirements for the binding of retinol and retinal. Biochemistry. 1978 Dec 12;17(25):5353–5359. doi: 10.1021/bi00618a005. [DOI] [PubMed] [Google Scholar]
- Schreckenbach T., Walckhoff B., Oesterhelt D. Studies on the retinal-protein interaction in bacteriorhodopsin. Eur J Biochem. 1977 Jun 15;76(2):499–511. doi: 10.1111/j.1432-1033.1977.tb11620.x. [DOI] [PubMed] [Google Scholar]
- Sherman W. V., Korenstein R., Caplan S. R. Energetics and chronology of phototransients in the light response of the purple membrane of Halobacterium halobium. Biochim Biophys Acta. 1976 Jun 8;430(3):454–458. doi: 10.1016/0005-2728(76)90021-9. [DOI] [PubMed] [Google Scholar]
- Sherman W. V., Slifkin M. A., Caplan S. R. Kinetic studies of phototransients in bacteriorhodopsin. Biochim Biophys Acta. 1976 Feb 16;423(2):238–248. doi: 10.1016/0005-2728(76)90182-1. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Lozier R. H., Bogomolni R. A. Bacteriorhodopsin and the purple membrane of halobacteria. Biochim Biophys Acta. 1979 Mar 14;505(3-4):215–278. doi: 10.1016/0304-4173(79)90006-5. [DOI] [PubMed] [Google Scholar]
- Tsuji K., Rosenheck K. The low pH species of bacteriorhodopsin. Structure and proton pump activity. FEBS Lett. 1979 Feb 15;98(2):368–372. doi: 10.1016/0014-5793(79)80219-7. [DOI] [PubMed] [Google Scholar]


