Abstract
Background
Cerebral blood flow (CBF) is closely regulated by carbon dioxide (CO2). In patients with aneurysmal subarachnoid hemorrhage (aSAH), abnormal arterial partial pressure of CO2 (PaCO2) might deteriorate brain injuries. Nevertheless, the impact of dynamic PaCO2 fluctuations on neurological outcomes in aSAH patients has not been extensively studied. Our study aimed to investigate the association between dynamic PaCO2 levels and unfavorable neurological outcomes in aSAH patients.
Methods
In this retrospective observational study, we consecutively enrolled 159 aSAH patients from December 2019 to July 2021. Arterial blood gas measurements within 10 days after intensive care unit (ICU) admission for each patient were recorded to calculate the time-weighted average (TWA)-PaCO2, an indicator representing the dynamic changes in PaCO2 levels. For the association between TWA-PaCO2 levels and unfavorable neurological outcomes in aSAH patients, multivariable logistic analysis was used to explore TWA-PaCO2 levels as categorical variables, and restricted cubic spline (RCS) was used to explore TWA-PaCO2 levels as continuous variables.
Results
In multivariable logistic analysis, after adjusting confounders, when TWA-PaCO2 35–45 mmHg was as a reference, TWA-PaCO2 < 35 mmHg (odds ratio [OR] 2.15, 95 % confidence interval [CI] 0.83–5.55, P = 0.113) and TWA-PaCO2 > 45 mmHg (OR 8.31, 95 % CI 0.72–96.14, P = 0.090) were not independently associated with unfavorable neurological outcomes (modified Rankin score of 3–6). The RCS shows a “U” shape curve between TWA-PaCO2 levels and unfavorable neurological outcomes, with a nonlinear P-value of 0.023. The lowest ORs of unfavorable neurological outcomes were within PaCO2 32.8–38.1 mmHg.
Conclusions
Both lower and higher PaCO2 levels are harmful to aSAH patients. PaCO2 in the range of 32.8–38.1 mmHg is associated with lowest unfavorable neurological outcomes.
Keywords: Carbon dioxide, Aneurysmal subarachnoid hemorrhage, Neurological outcomes, Restricted cubic spline
1. Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a particularly devastating cerebrovascular event [1]. Neurological impairment and cognitive dysfunction after aSAH remain high and affect relatively young patients at their most productive years in life [2]. Patients with aSAH typically suffer from compromised cerebral perfusion and metabolism, under which circumstances, a dramatic reduction in cerebral blood flow (CBF) triggered by the decrease in arterial partial pressure of arterial carbon dioxide (PaCO2) [3] or elevated intracranial pressure (ICP) due to the increase in PaCO2 [4] would produce additional attacks on the injured brain [5].
Studies have shown that derangements of PaCO2 jeopardize cerebral perfusion and aggravate brain metabolic crises and potential neurological injury in patients with brain injury [[6], [7], [8], [9]]. Nevertheless, in previous studies, there was a notable variation in the threshold values for PaCO2 levels used to identify hypocapnia or hypercapnia. PaCO2 less than 25 mmHg–35 mmHg was the range for hypocapnia, while PaCO2 higher than 45 mmHg–50 mmHg was the range for hypercapnia. In addition, the timepoint of PaCO2 measurement also varied, from one measurement at admission to multiple measurements at any time during the hospital stay, mainly during the first ten days following cerebral injury [10]. Prior publications usually regard one outlier of PaCO2 value at any time as abnormal. However, PaCO2 levels are easily influenced by clinical scenarios, such as fever, pain, and inadequate sedation. The effect of dynamic PaCO2 fluctuations on neurological outcomes in aSAH patients has not been well investigated.
Therefore, this study aims to explore the effect of dynamic carbon dioxide (CO2) exposure on neurological outcomes in SAH patients and to describe the optimal PaCO2 range that might benefit SAH patients.
2. Materials and methods
2.1. Study design
This was a single-center, retrospective, observational study conducted at the intensive care unit (ICU) of Beijing Tiantan Hospital. The study was reviewed and approved by the Institutional Review Board of Beijing Tiantan Hospital Affiliated with Capital Medical University (KY2022-143-01). The study report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines [11].
2.2. Study population
All patients diagnosed with aSAH admitted to the ICU between December 2019 and July 2021 were screened retrospectively and recruited consecutively. Only the data from the first admission was included for patients with multiple ICU admissions.
2.3. Inclusion and exclusion criteria
The study included adult patients (≥18) whose first diagnosis was "aSAH" confirmed by computed tomography (CT) angiography, digital subtraction angiography, or catheter angiography, and the patients were accepted for neurosurgical clipping within 24 h of hospital admission. Exclusion criteria were as follows: traumatic SAH or arteriovenous malformation, acceptance of endovascular coiling or conservative treatment, an interval longer than 24 h between the end of the surgery and ICU admission, arterial blood gas (ABG) measurements during ICU therapy were unavailable, pregnancy or lactating women, acute respiratory distress syndrome (because a low tidal volume, permissive hypercapnia ventilation strategy was usually performed), brain death (for better controlling the disease severity heterogeneity).
2.4. Data collection
The data included CO2 exposure, baseline characteristics, aneurysm data, ICU therapy data obtained from electronic medical records, neuroimaging, and neuroradiology reports. The neurological outcome at 3 months based on the modified Rankin scale (mRs) was obtained from the neurosurgery's regularly scheduled aneurysm outpatient follow-up records by investigators unknown the patient's CO2 exposure level. In case patients were not attended, the mRs score was assessed by investigators anonymized to the CO2 exposure via telephone interview.
2.4.1. CO2 exposure
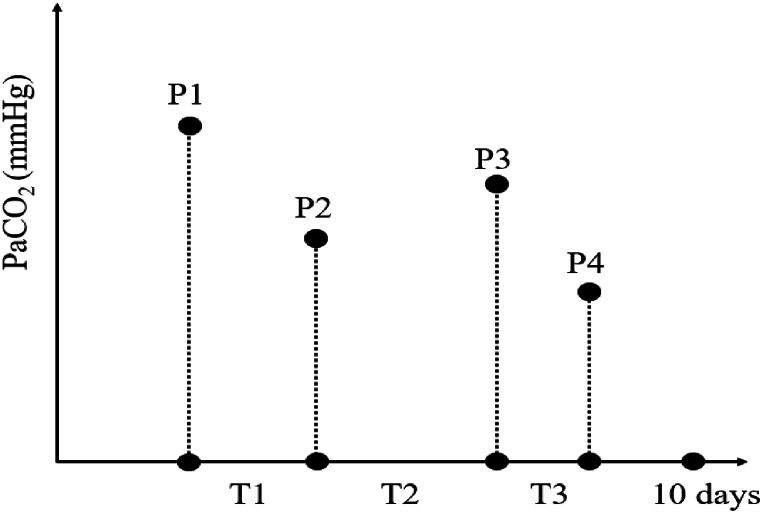
The primary interest in CO2 exposure was dynamic CO2 changes, represented as time-weighted average-arterial partial pressure of carbon dioxide (TWA-PaCO2). The values were obtained from all available arterial blood gas analysis measurements within each patient's initial ten days of ICU admission. The calculation method has been described by Nichol et al. [12]: the mean value of PaCO2 values at two consecutive time points was multiplied by the period between respective time points firstly, then summed the mean values, which the obtained values divided by the total time (Fig. 1).
Fig. 1.
The methods for calculation of TWA-PaCO2. If the patient had 4 ABG measurements within 10 days after ICU admission, and the period between each two PaCO2 measurements varied. The calculation formula was as follows: .
2.4.2. Baseline characteristics
Including 1) Demographic data (age, sex, body mass index (BMI)); 2) Medical history (history of hypertension, diabetes, cardiovascular disease); 3) Personal history (tobacco use, alcohol use); 4) Time from onset to hospital admission.
2.4.3. Aneurysm data
Including 1) Aneurysm characteristics (multiple or single, site and lateral of responsible aneurysm); 2) Hunt-Hess grade ascertained according to the clinical presentation at admission; 3) Modified Fisher scale determined by the amount of blood seen at initial CT scan.
2.4.4. ICU therapy data
Including primary clinical treatment (mechanical ventilation, opioids [including remifentanil and fentanyl]).
2.4.5. Outcomes
The primary outcome was the neurological outcome based on the mRs at 3 months, which ranges from 0 to 6, with 0 representing no symptoms and 6 representing deaths. An unfavorable outcome was defined as an mRs score of 3–6, and a favorable outcome was defined as an mRs score of 0–2.
2.5. Statistical analysis
Continuous variables with normal distribution were shown as mean and standard deviation (SD) and compared with Student t-tests. Continuous variables with skewed distribution were shown as median and interquartile range (IQR) and compared with Wilcoxon rank sum test. Categorical variables were shown as frequency and percentages (%) and compared with chi-square test.
Comparisons were made between groups according to unfavorable and favorable outcomes. The variables with statistically significant (P < 0.05) were included in multivariate logistic regression analysis to calculate the adjusted odds ratios (ORs) and 95 % confidence intervals (CIs) for CO2 exposure indicators. We also performed a restricted cubic spline (RCS) with three knots, adjusted for confounders [13] to show the possible nonlinear relationship between TWA-PaCO2 levels as a continuous variable and unfavorable neurological outcomes.
All variables in our study were complete except for BMI (11.3 % missing, 18 in 159). To minimize bias caused by missing data, we applied mean value imputation to fill the data. Two-sided P < 0.05 was considered statistically significant. Statistical analysis was performed using SPSS software (version 26.0; IBM) and R software (version 4.1.2, www.r-project.org).
3. Results
3.1. Flow chart for enrollment and baseline characteristics
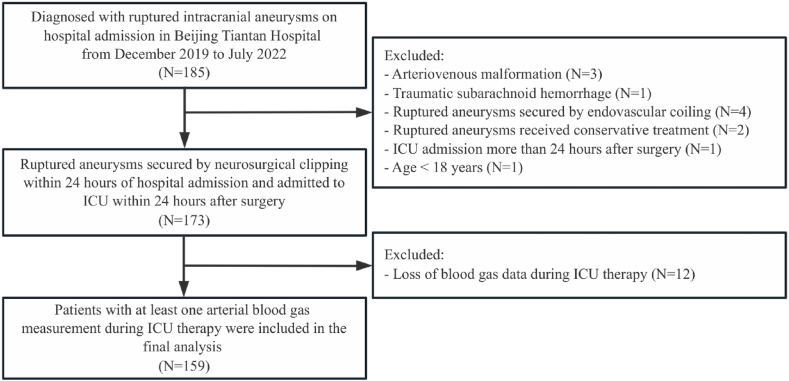
The flow chart for patient screening is shown in Fig. 2. In the time window for screening, 185 patients diagnosed with "aSAH" were admitted to the ICU, 26 patients were excluded according to the exclusion criteria, and 159 patients were included in the final analysis.
Fig. 2.
The flow chart for patient screening.
Clinical characteristics for 159 patients are shown in Table 1. 49 patients suffered from unfavorable neurological outcomes (defined as an mRs score of 3–6). The mean age was significantly higher in the unfavorable neurological outcomes group compared with the favorable outcome group (57.7 vs. 50.8, P < 0.001). A significantly higher proportion of Hunt-Hess grade 3–5 (69.4 % vs. 31.8 %, P < 0.001) and modified Fisher scale 3–4 (83.7 % vs. 51.8 %, P < 0.001) in the unfavorable neurological outcomes group. As for ICU therapies, there was more use of opioids (61.2 % vs. 36.4 %, P = 0.004) and mechanical ventilation (69.4 % vs. 17.3 %, P < 0.001) in the unfavorable outcomes group.
Table 1.
Clinical characteristics.
| Characteristic | mRs 0–2 (n = 110) | mRs 3–6 (n = 49) | P |
|---|---|---|---|
| Age, year | 50.8 ± 9.6 | 57.7 ± 10.4 | <0.001 |
| Sex, female | 58(52.7 %) | 27(55.1 %) | 0.782 |
| BMI, kg/m2 | 24.7[22.5–26.1] | 24.7[23.3–27.3] | 0.392 |
| History of hypertension | 60(54.5 %) | 27(55.1 %) | 0.948 |
| History of diabetes | 5(4.5 %) | 6(12.2 %) | 0.077 |
| History of cardiovascular disease | 4(3.6 %) | 2(4.1 %) | 1.000 |
| Tobacco use | 16(14.5 %) | 7(14.3 %) | 0.966 |
| Alcohol use | 13(11.8 %) | 8(16.3 %) | 0.438 |
| Location of responsible aneurysm | 0.816 | ||
| Middle cerebral artery | 38(34.5 %) | 17(34.7 %) | |
| Anterior communication artery | 38(34.5 %) | 16(32.7 %) | |
| Internal carotid artery | 13(11.8 %) | 9(18.4 %) | |
| Posterior communication artery | 10(9.1 %) | 5(10.2 %) | |
| Anterior cerebral artery | 6(5.5 %) | 1(2.0 %) | |
| Other | 5(4.5 %) | 1(2.0 %) | |
| Time from onset to admission, day | 2 [[1], [2], [3]] | 2 [[1], [2], [3]] | 0.653 |
| Left lateral of responsible aneurysm | 51(46.4 %) | 26(53.1 %) | 0.435 |
| More than one aneurysm | 15(13.6 %) | 8(16.7) | 0.619 |
| Hunt-Hess grade 3-5 | 35(31.8 %) | 34(69.4 %) | <0.001 |
| Modified Fisher scale 3-4 | 57(51.8 %) | 41(83.7 %) | <0.001 |
| Opioids | 40(36.4 %) | 30(61.2 %) | 0.004 |
| Mechanical Ventilation | 19(17.3 %) | 34(69.4 %) | <0.001 |
| TWA-PaCO2 | 0.024 | ||
| TWA-PaCO2 < 35 mmHg | 47(42.7 %) | 25(51.0 %) | |
| TWA-PaCO2 35–45 mmHg | 62(56.4 %) | 20(40.8 %) | |
| TWA-PaCO2 > 45 mmHg | 1(0.9 %) | 4(8.2 %) | |
Data are shown as mean ± standard deviation or median [interquartile range] or number (percentage); mRs, modified Rankin scale; BMI, body mass index; TWA-PaCO2, time-weighted average-arterial partial pressure of carbon dioxide.
TWA-PaCO2 values were shown as categorical variables and compared between the unfavorable and favorable outcomes groups. When TWA-PaCO2 levels were categorized into three ranges: <35, 35–45, and >45 mmHg, there were higher proportions of TWA-PaCO2 < 35 mmHg (51.0 % vs. 42.7 %) and lower proportions of TWA-PaCO2 35–45 mmHg (40.8 % vs. 56.4 %) in unfavorable outcome group compared with favorable outcome group, the sample of TWA-PaCO2 > 45 mmHg was small. The overall difference in P value was 0.024. There were no significant differences in other variables.
3.2. Multivariate logistic regression analysis for unfavorable neurological outcomes
The variables that show significant differences between unfavorable and favorable outcomes groups were included in multivariate logistic regression analysis: age, Hunt-Hess grade, modified Fisher scale, opioids, mechanical ventilation, and TWA-PaCO2 levels (Table 2). Based on multivariate logistic regression analysis, after adjusting the confounders, when TWA-PaCO2 35–45 mmHg was as a reference, TWA-PaCO2 < 35 mmHg (OR 2.15[95 % CI 0.83–5.55], P = 0.113) and TWA-PaCO2 > 45 mmHg (OR 8.31[95%CI 0.72–96.14], P = 0.090) were not shown to be an independent risk factor for unfavorable neurological outcomes.
Table 2.
Multivariate logistic regression analysis for unfavorable neurological outcome.
| Variables | Adjusted OR [95 % CI] | P |
|---|---|---|
| Age | 1.06[1.02–1.11] | 0.008 |
| Hunt-Hess grade 3-5 | 1.55[0.61–3.94] | 0.360 |
| Modified Fisher scale 3-4 | 4.02[1.39–11.66] | 0.010 |
| Opioids | 1.47[0.50–4.29] | 0.482 |
| Mechanical ventilation | 7.23[2.61–20.00] | <0.001 |
| TWAPaCO2 | 0.116 | |
| TWA-PaCO2 < 35 mmHg | 2.15[0.83–5.55] | 0.113 |
| TWA-PaCO2 35–45 mmHg | Reference | |
| TWA-PaCO2 > 45 mmHg | 8.31[0.72–96.14] | 0.090 |
OR, odds ratio; CI, confidence interval; TWA-PaCO2, time-weighted average-arterial partial pressure of carbon dioxide.
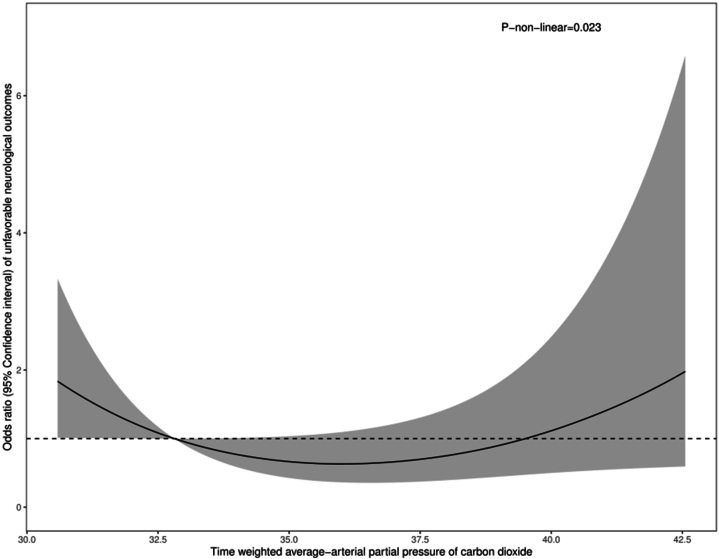
3.3. Nonlinear relationship between PaCO2 and unfavorable neurological outcomes
We conducted a restricted cubic spline to show the nonlinear relationship between TWA-PaCO2 as a continuous variable and unfavorable neurological outcome (defined as an mRs score of 3–6) (Fig. 3). After adjusting the confounders, including age, Hunt-Hess grade, modified Fisher scale, mechanical ventilation, and opioids, the relationship was shown as a “U” shape curve, with the P value 0.023 indicating a nonlinear relationship between PaCO2 levels and unfavorable neurological outcomes, and the lowest range was within 32.8–38.1 mmHg. TWA-PaCO2 levels lower than 32.8 mmHg or higher than 38.1 mmHg were associated with an increasing odds ratio of unfavorable neurological outcomes.
Fig. 3.
The restricted cubic spline shows the nonlinear relationship between TWA-PaCO2 levels and unfavorable neurological outcomes in aSAH patients. The nonlinear P-value is 0.023.
4. Discussion
This study explores the associations between dynamic PaCO2 levels and neurological outcomes in aSAH patients. We found that both lower and higher PaCO2 levels are harmful to aSAH patients. The optimal PaCO2 range was within a narrower range (32.8–38.1 mmHg) for aSAH patients sampled in our study.
In physiology, PaCO2 levels mainly depend on production and elimination [6] and fluctuate within 35–45 mmHg [14]. Nevertheless, higher and lower PaCO2 levels were quite common in clinical scenarios. In addition, CO2 can freely diffuse across the blood-brain barrier [15] to affect the perivascular pH in the brain.
When too much CO2 is eliminated, PaCO2 levels will decrease. The changes may constrict cerebral small vessels and reduce CBF, accompanied by decreased ICP [16]. According to this, induced hyperventilation was used to interfere with the elevated ICP [17]. Additionally, vasoconstriction has been shown to help restore cerebral autoregulation [18]. However, when CBF reduction reaches the threshold value (3 % for every one mmHg decrease in PaCO2) [3], cerebral ischemic injury worsens [19]. Thus, prophylactic hyperventilation was not advised in cases of brain injury [20]. Vasodilation occurs with increasing PaCO2 levels, with an approximate 2–4% increment in CBF for every unit increase in PaCO2 [21]. Studies have found that CBF increased gradually without raising ICP as PaCO2 levels rose from 30 mmHg to 40, 50, and 60 mmHg [22].
As for the probable mechanisms for CO2-mediated changes in cerebral vascular tone: altered extracellular pH secondary to PaCO2 changes is the initial step, then neuronal isoform of nitric oxide synthase (nNOS) activates which increases the NO production and cyclic guanosine monophosphate (cGMP) concentration in vascular smooth muscle (VSM). Both NO and cGMP can activate potassium channels, which hyperpolarize VSM. Membrane hyperpolarization inhibits voltage-gated calcium channels, which reduces VSM intracellular calcium concentrations and causes vascular relation [23]. CVS (cerebral vasospasm) can be regarded as an abnormal and prolonged contraction of VSM, which intracellular free calcium level plays a pivotal role in the regulation of smooth muscle contractility [24]. According to Lucke-Wold B et al., a crucial component in the development of vasospasm and subsequent DCI is an increased inflammatory cascade mediated by interleukin (IL-6) [25]. It is interesting how IL-6 levels change in response to CO2 fluctuations. However, little is known about the association between CO2 and inflammatory cascade, primarily concerning IL-6, which needs to be investigated in the future. Total expression of NOS enzymes decreases after SAH [26] and free heme molecules from extravasated blood affect NO bioavailability according to Motwani K et al. [27] The pathological changes above will affect the way in which CO2 dilates cerebral arterioles. Recent data is emerging that glymphatic blockage can increase the inflammatory milieu and cause microspasm. However, little is known about how glymphatic blockage would affect the effect of CO2 on cerebral vascular tone, which needs further investigation.
Increased or decreased PaCO2 levels affect brain physiology by the mechanisms described above. As for clinical outcomes, some retrospective studies have demonstrated an association between decreased PaCO2 levels and poor neurological outcomes after brain injury. As Williamson et al. [7] reported, PaCO2 < 35 mmHg and pH > 7.45 were associated with poor neurological outcomes at discharge. Additionally, several studies found that increased PaCO2 levels were associated with unfavorable outcomes in SAH patients [4,20,28]. According to those, we could learn that decreased or increased PaCO2 levels are not beneficial to clinical outcomes, and the definition of “abnormal” PaCO2 levels among studies varied widely. As of yet, no “optimum PaCO2 levels” have been established for SAH patients in the prospective study, and it has also not been confirmed that maintenance within the range of “optimum PaCO2 levels” can benefit clinical outcomes.
Furthermore, Solaiman et al. found that a longer-lasting hypocapnia was independently associated with poor neurological outcome [9], suggesting that the duration time of abnormal PaCO2 levels should be considered. Clinicians routinely adjust the ventilator settings or sedation levels when PaCO2 values are outside the desired range. Considering only one abnormal PaCO2 value would ignore the effect of duration time of “abnormal” PaCO2 levels and underestimate the impact of clinical interventions. To provide a dynamic CO2 exposure indicator that takes the duration time of abnormal CO2 levels into consideration, we calculated the TWA-PaCO2.
Bedside physiology is crucial in the ICU in guiding clinical decision-making [29,30]. Deranged physiology is frequently associated with poor clinical outcomes. Therefore, it is tempting to assume that intervening in physiological parameters might improve patient outcomes [31]. We found the optimal PaCO2 levels for aSAH patients sampled in our study were within a narrow range of 32.8–38.1 mmHg. If we set the target range of 35–45 mmHg, which is a physiological range that may not be ideal for SAH patients. Furthermore, whether fluctuations in PaCO2 levels adaptive or maladaptive in critically ill patients? Further studies should determine if “hypocapnia” or “hypercapnia” represents a disease that requires treatment or if it is a beneficial compensatory response orchestrated by the human body to optimize chances of survival should not be intervened.
Opioids, including fentanyl or remifentanil, were used in 70 (44.0 %) patients in our study. Studies have indicated that opioids have the potential to reduce respiratory rate and minute ventilation in a dose-dependent manner [32,33], leading to an increase in PaCO2 levels. As part of our unit's clinical routine, opioids were administered to relieve pain or intervene in abnormal PaCO2 levels. Accordingly, the use of opioids may be a therapeutic intervention for abnormal PaCO2 levels. Nevertheless, as a retrospective study, we are unable to verify that the purpose of opioid administration or dose adjustments was to control PaCO2 levels. Prospective studies are needed to investigate the effect of opioid usage on abnormal PaCO2 levels and clinical outcomes.
Cerebral injury is one of the most prevalent causes of mechanical ventilation in critically ill patients [34], and ventilatory support is often titrated based on physiological measurements, such as PaCO2 levels. Our study shows that patients with unfavorable outcomes are more likely to require mechanical ventilation (69.4 % vs. 17.3 %). Still, prospective research needs to investigate if mechanical ventilation in aSAH patients significantly influences PaCO2 levels and clinical outcomes.
There were several limitations in our study. First, studies have shown that patients with cerebral injuries start artificial ventilation in pre-hospital or emergency department settings [35] which time abnormal PaCO2 levels relate to higher mortality [36]. Nevertheless, before ICU admission, PaCO2 measurements could not be obtained for our study. Second, we only enrolled patients who had undergone neurosurgical clipping for intracranial ruptured aneurysms within 24 h after hospital admission. Therefore, generalizing our results to all patients, including elective neurosurgical clipping and neurosurgical clipping for unruptured aneurysms, may be challenging. Third, clinicians might adjust ventilator settings, sedation levels, or other therapies in response to abnormal PaCO2 levels, whether achievement of target PaCO2 levels might be ensured by repeat blood gas analysis. However, ABG data following relevant interventions could not be documented due to the retrospective nature of our study.
5. Conclusion
Our study demonstrates that both lower and higher PaCO2 levels are associated with unfavorable neurological outcomes in aSAH patients. PaCO2 levels within 32.8–38.1 mmHg might be optimal for SAH patients sampled in our study. Prospective studies are still needed to determine an optimal PaCO2 range for SAH patients. Whether an intervention to control PaCO2 levels in aSAH patients benefits clinical outcomes should be explored.
CRediT authorship contribution statement
Rui Su: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Hong-Liang Li: Methodology, Conceptualization. Yu-Mei Wang: Methodology, Conceptualization. Linlin Zhang: Writing – review & editing, Methodology, Conceptualization. Jian-Xin Zhou: Methodology, Funding acquisition, Conceptualization.
Ethics approval and consent to participate
The study was reviewed and approved by the Institutional Review Board of Beijing Tiantan Hospital Affiliated with Capital Medical University (KY2022-143-01). Because the study was a retrospective nature study. It was allowed to be conducted by board without patients’ consent.
Data availability statement
The data supporting the findings of this study are available from the corresponding author on reasonable request.
Funding
This study was supported by Capital's Funds for Health Improvement and Research (CFH 2024-1-2081) and a grant from the Clinical and Research Center program of Capital Medical University (CMU-2023-45).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Contributor Information
Hong-Liang Li, Email: arnold_lhl@126.com.
Linlin Zhang, Email: abluelemon@163.com.
Jian-Xin Zhou, Email: zhoujx.cn@icloud.com.
References
- 1.Claassen J., Park S. Spontaneous subarachnoid haemorrhage. Lancet (London, England) 2022;400(10355):846–862. doi: 10.1016/S0140-6736(22)00938-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nieuwkamp D.J., Setz L.E., Algra A., Linn F.H., de Rooij N.K., Rinkel G.J. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8(7):635–642. doi: 10.1016/S1474-4422(09)70126-7. [DOI] [PubMed] [Google Scholar]
- 3.Curley G., Kavanagh B.P., Laffey J.G. Hypocapnia and the injured brain: more harm than benefit. Crit. Care Med. 2010;38(5):1348–1359. doi: 10.1097/CCM.0b013e3181d8cf2b. [DOI] [PubMed] [Google Scholar]
- 4.Reiff T., Barthel O., Schönenberger S., Mundiyanapurath S. High-normal P(a)CO(2) values might be associated with worse outcome in patients with subarachnoid hemorrhage - a retrospective cohort study. BMC Neurol. 2020;20(1):31. doi: 10.1186/s12883-020-1603-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi T., Suzuki A., Hatazawa J., et al. Cerebral circulation and metabolism in the acute stage of subarachnoid hemorrhage. J. Neurosurg. 2000;93(6):1014–1018. doi: 10.3171/jns.2000.93.6.1014. [DOI] [PubMed] [Google Scholar]
- 6.Godoy D.A., Rovegno M., Lazaridis C., Badenes R. The effects of arterial CO(2) on the injured brain: two faces of the same coin. J. Crit. Care. 2021;61:207–215. doi: 10.1016/j.jcrc.2020.10.028. [DOI] [PubMed] [Google Scholar]
- 7.Williamson C.A., Sheehan K.M., Tipirneni R., et al. The association between spontaneous hyperventilation, delayed cerebral ischemia, and poor neurological outcome in patients with subarachnoid hemorrhage. Neurocritical Care. 2015;23(3):330–338. doi: 10.1007/s12028-015-0138-5. [DOI] [PubMed] [Google Scholar]
- 8.Li K.C., Tam C.W.Y., Shum H.P., Yan W.W. Impact of hyperoxia and hypocapnia on neurological outcomes in patients with aneurysmal subarachnoid hemorrhage: a retrospective study. Critical care research and practice. 2019;2019 doi: 10.1155/2019/7584573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Solaiman O., Singh J.M. Hypocapnia in aneurysmal subarachnoid hemorrhage: incidence and association with poor clinical outcomes. J. Neurosurg. Anesthesiol. 2013;25(3):254–261. doi: 10.1097/ANA.0b013e3182806465. [DOI] [PubMed] [Google Scholar]
- 10.Roberts B.W., Karagiannis P., Coletta M., Kilgannon J.H., Chansky M.E., Trzeciak S. Effects of PaCO2 derangements on clinical outcomes after cerebral injury: a systematic review. Resuscitation. 2015;91:32–41. doi: 10.1016/j.resuscitation.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 11.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Annals of internal medicine. 2007;147(8):573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 12.Nichol A., Bailey M., Egi M., et al. Dynamic lactate indices as predictors of outcome in critically ill patients. Crit. Care. 2011;15(5):R242. doi: 10.1186/cc10497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desquilbet L., Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010;29(9):1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 14.Akça O. Optimizing the intraoperative management of carbon dioxide concentration. Curr. Opin. Anaesthesiol. 2006;19(1):19–25. doi: 10.1097/01.aco.0000192776.32398.5c. [DOI] [PubMed] [Google Scholar]
- 15.Caldwell H.G., Howe C.A., Chalifoux C.J., et al. Arterial carbon dioxide and bicarbonate rather than pH regulate cerebral blood flow in the setting of acute experimental metabolic alkalosis. The Journal of physiology. 2021;599(5):1439–1457. doi: 10.1113/JP280682. [DOI] [PubMed] [Google Scholar]
- 16.Boron W.F. Evaluating the role of carbonic anhydrases in the transport of HCO3--related species. Biochim. Biophys. Acta. 2010;1804(2):410–421. doi: 10.1016/j.bbapap.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neumann J.O., Chambers I.R., Citerio G., et al. The use of hyperventilation therapy after traumatic brain injury in Europe: an analysis of the BrainIT database. Intensive Care Med. 2008;34(9):1676–1682. doi: 10.1007/s00134-008-1123-7. [DOI] [PubMed] [Google Scholar]
- 18.Paulson O.B., Olesen J., Christensen M.S. Restoration of autoregulation of cerebral blood flow by hypocapnia. Neurology. 1972;22(3):286–293. doi: 10.1212/wnl.22.3.286. [DOI] [PubMed] [Google Scholar]
- 19.Coles J.P., Fryer T.D., Coleman M.R., et al. Hyperventilation following head injury: effect on ischemic burden and cerebral oxidative metabolism. Crit. Care Med. 2007;35(2):568–578. doi: 10.1097/01.CCM.0000254066.37187.88. [DOI] [PubMed] [Google Scholar]
- 20.Cai G., Zhang X., Ou Q., et al. Optimal targets of the first 24-h partial pressure of carbon dioxide in patients with cerebral injury: data from the MIMIC-III and IV database. Neurocritical Care. 2022;36(2):412–420. doi: 10.1007/s12028-021-01312-2. [DOI] [PubMed] [Google Scholar]
- 21.Curley G., Laffey J.G., Kavanagh B.P. Bench-to-bedside review: carbon dioxide. Crit. Care. 2010;14(2):220. doi: 10.1186/cc8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westermaier T., Stetter C., Kunze E., et al. Controlled hypercapnia enhances cerebral blood flow and brain tissue oxygenation after aneurysmal subarachnoid hemorrhage: results of a phase 1 study. Neurocritical Care. 2016;25(2):205–214. doi: 10.1007/s12028-016-0246-x. [DOI] [PubMed] [Google Scholar]
- 23.Brian J.E., Jr. Carbon dioxide and the cerebral circulation. Anesthesiology. 1998;88(5):1365–1386. doi: 10.1097/00000542-199805000-00029. [DOI] [PubMed] [Google Scholar]
- 24.Horowitz A., Menice C.B., Laporte R., Morgan K.G. Mechanisms of smooth muscle contraction. Physiol. Rev. 1996;76(4):967–1003. doi: 10.1152/physrev.1996.76.4.967. [DOI] [PubMed] [Google Scholar]
- 25.Lucke-Wold B., Hosaka K., Dodd W., et al. Interleukin-6: important mediator of vasospasm following subarachnoid hemorrhage. Curr. Neurovascular Res. 2021;18(3):364–369. doi: 10.2174/1567202618666211104122408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pluta R.M. Dysfunction of nitric oxide synthases as a cause and therapeutic target in delayed cerebral vasospasm after SAH. Acta Neurochir. Suppl. 2008;104:139–147. doi: 10.1007/978-3-211-75718-5_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motwani K., Dodd W.S., Laurent D., Lucke-Wold B., Chalouhi N. Delayed cerebral ischemia: a look at the role of endothelial dysfunction, emerging endovascular management, and glymphatic clearance. Clin. Neurol. Neurosurg. 2022;218 doi: 10.1016/j.clineuro.2022.107273. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama S., Hifumi T., Okazaki T., et al. Association of abnormal carbon dioxide levels with poor neurological outcomes in aneurysmal subarachnoid hemorrhage: a retrospective observational study. Journal of intensive care. 2018;6:83. doi: 10.1186/s40560-018-0353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laffey J.G., Kavanagh B.P. Fifty years of research in ARDS. Insight into acute respiratory distress syndrome. From models to patients. Am. J. Respir. Crit. Care Med. 2017;196(1):18–28. doi: 10.1164/rccm.201612-2415CI. [DOI] [PubMed] [Google Scholar]
- 30.Dianti J., Morris I.S., Urner M., et al. Linking acute physiology to outcomes in the ICU: challenges and solutions for research. Am. J. Respir. Crit. Care Med. 2023;207(11):1441–1450. doi: 10.1164/rccm.202206-1216CI. [DOI] [PubMed] [Google Scholar]
- 31.Slutsky A.S. Improving outcomes in critically ill patients: the seduction of physiology. JAMA. 2009;302(18):2030–2032. doi: 10.1001/jama.2009.1653. [DOI] [PubMed] [Google Scholar]
- 32.Natalini G., Di Maio A., Rosano A., Ferretti P., Bertelli M., Bernardini A. Remifentanil improves breathing pattern and reduces inspiratory workload in tachypneic patients. Respir. Care. 2011;56(6):827–833. doi: 10.4187/respcare.01014. [DOI] [PubMed] [Google Scholar]
- 33.Cavaliere F., Antonelli M., Arcangeli A., et al. A low-dose remifentanil infusion is well tolerated for sedation in mechanically ventilated, critically-ill patients. Canadian journal of anaesthesia = Journal canadien d'anesthesie. 2002;49(10):1088–1094. doi: 10.1007/BF03017909. [DOI] [PubMed] [Google Scholar]
- 34.Pelosi P., Ferguson N.D., Frutos-Vivar F., et al. Management and outcome of mechanically ventilated neurologic patients. Crit. Care Med. 2011;39(6):1482–1492. doi: 10.1097/CCM.0b013e31821209a8. [DOI] [PubMed] [Google Scholar]
- 35.Davis D.P., Dunford J.V., Poste J.C., et al. The impact of hypoxia and hyperventilation on outcome after paramedic rapid sequence intubation of severely head-injured patients. J. Trauma. 2004;57(1):1–8. doi: 10.1097/01.ta.0000135503.71684.c8. ; discussion 8-10. [DOI] [PubMed] [Google Scholar]
- 36.Caulfield E.V., Dutton R.P., Floccare D.J., Stansbury L.G., Scalea T.M. Prehospital hypocapnia and poor outcome after severe traumatic brain injury. J. Trauma. 2009;66(6):1577–1582. doi: 10.1097/TA.0b013e3181a3931d. ; discussion 1583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author on reasonable request.