Abstract
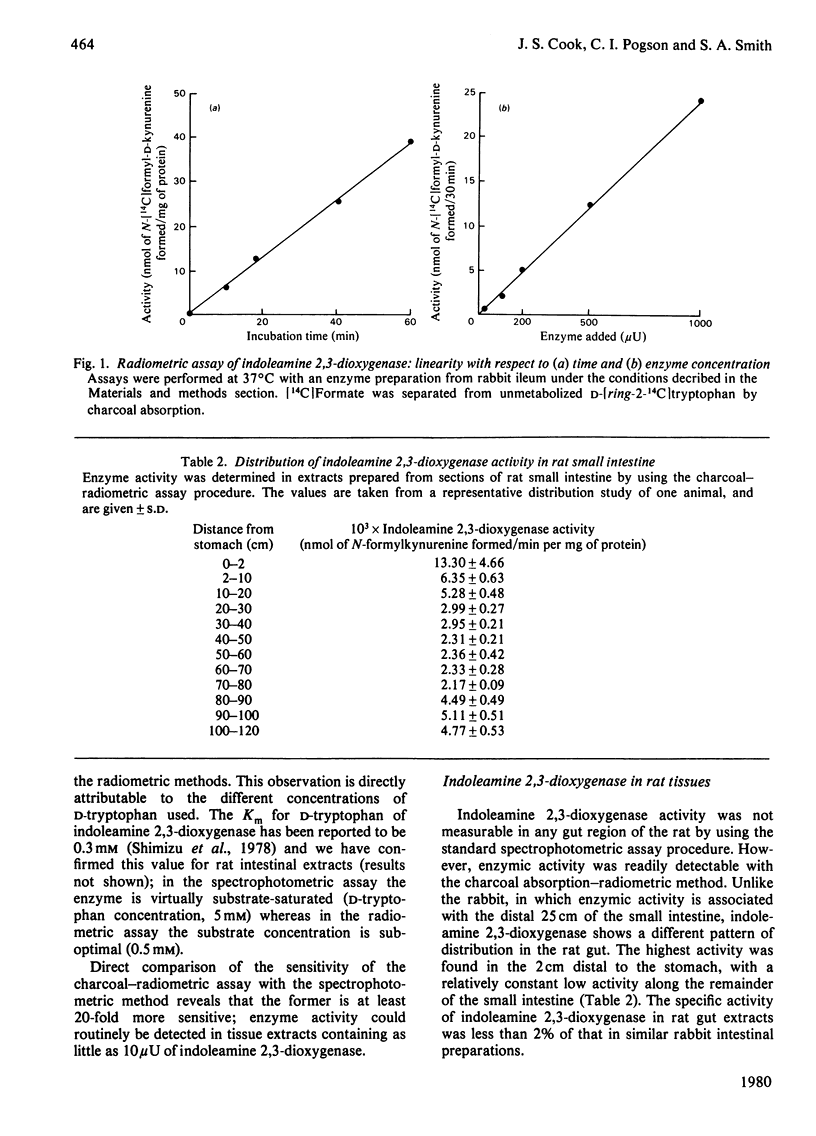
A simple and convenient assay for indoleamine 2,3-dioxygenase has been developed. This depends on the conversion of D-[ring-2-14C]tryptophan to [14C]formate, excess substrate is removed by adsorption onto charcoal. This assay, which is 20-fold more sensitive than previous procedures, is applicable both to crude extracts and to large numbers of samples. Activity in rat tissues is very much lower than in those of the rabbit; measureable activity is found only in the stomach, spleen, intestine and kidney. Enzyme activity in the rat intestine was increased by 50% in rats pretreated with L-tryptophan.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fujiwara M., Shibata M., Nomiyama Y., Sugimoto T., Hirata F., Tokuyama T., Senoh S., Hayaishi O. Formation of 5-hydroxykynurenine and 5-hydroxykynurenamine from 5-hydroxytryptophan in rabbit small intestine. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1145–1149. doi: 10.1073/pnas.76.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi K., Hayaishi O. Enzymic formation of D-kynurenine from D-tryptophan. Arch Biochem Biophys. 1967 May;120(2):397–403. doi: 10.1016/0003-9861(67)90256-1. [DOI] [PubMed] [Google Scholar]
- Hirata F., Hayaishi O. Studies on indoleamine 2,3-dioxygenase. I. Superoxide anion as substrate. J Biol Chem. 1975 Aug 10;250(15):5960–5966. [PubMed] [Google Scholar]
- Hirata F., Hayaishi O., Tokuyama T., Seno S. In vitro and in vivo formation of two new metabolites of melatonin. J Biol Chem. 1974 Feb 25;249(4):1311–1313. [PubMed] [Google Scholar]
- Hirata F., Ohnishi T., Hayaishi O. Indoleamine 2,3-dioxygenase. Characterization and properties of enzyme. O2- complex. J Biol Chem. 1977 Jul 10;252(13):4637–4642. [PubMed] [Google Scholar]
- Konishi N., Noguchì T., Kido R. The pyrrole ring cleavaging enzyme of 5-hydroxytryptophan. Life Sci II. 1971 Apr 22;10(8):431–436. doi: 10.1016/0024-3205(71)90304-3. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Hirata F., Hayaish O. Indoleamine 2,3-dioxygenase. Potassium superoxide as substrate. J Biol Chem. 1977 Jul 10;252(13):4643–4647. [PubMed] [Google Scholar]
- Peterkofsky B. Use of a new radioassay for tryptophan oxygenase to study the development of the enzyme in chick embryos. Arch Biochem Biophys. 1968 Dec;128(3):637–645. doi: 10.1016/0003-9861(68)90073-8. [DOI] [PubMed] [Google Scholar]
- Pogson C. I., Smith S. A. The activity of phosphoenolpyruvate carboxykinase in rat tissues. Assay techniques and effects of dietary and hormonal changes. Biochem J. 1975 Nov;152(2):401–408. doi: 10.1042/bj1520401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIMKE R. T., SWEENEY E. W., BERLIN C. M. THE ROLES OF SYNTHESIS AND DEGRADATION IN THE CONTROL OF RAT LIVER TRYPTOPHAN PYRROLASE. J Biol Chem. 1965 Jan;240:322–331. [PubMed] [Google Scholar]
- Shimizu T., Nomiyama S., Hirata F., Hayaishi O. Indoleamine 2,3-dioxygenase. Purification and some properties. J Biol Chem. 1978 Jul 10;253(13):4700–4706. [PubMed] [Google Scholar]
- Smith S. A., Pogson C. I. The metabolism of L-tryptophan by isolated rat liver cells. Effect of albumin binding and amino acid competition on oxidatin of tryptophan by tryptophan 2,3-dioxygenase. Biochem J. 1980 Mar 15;186(3):977–986. doi: 10.1042/bj1860977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S. A., Pogson C. L. Tryptophan and the control of plasma glucose concentrations in the rat. Biochem J. 1977 Dec 15;168(3):495–506. doi: 10.1042/bj1680495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart K. K., Doherty R. F. Resolution of DL-tryptophan by affinity chromatography on bovine-serum albumin-agarose columns. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2850–2852. doi: 10.1073/pnas.70.10.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S., Hayaishi O. Tryptophan pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme or enzymes. J Biol Chem. 1967 Nov 25;242(22):5260–5266. [PubMed] [Google Scholar]


