Abstract
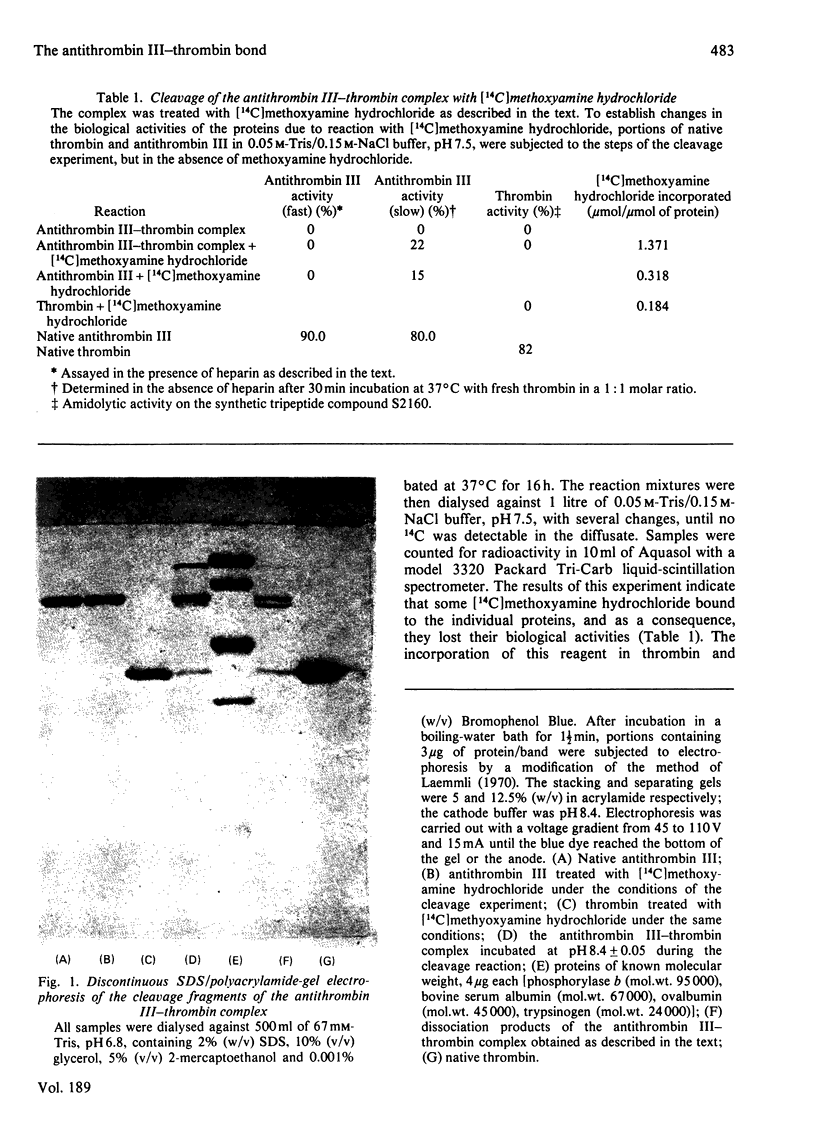
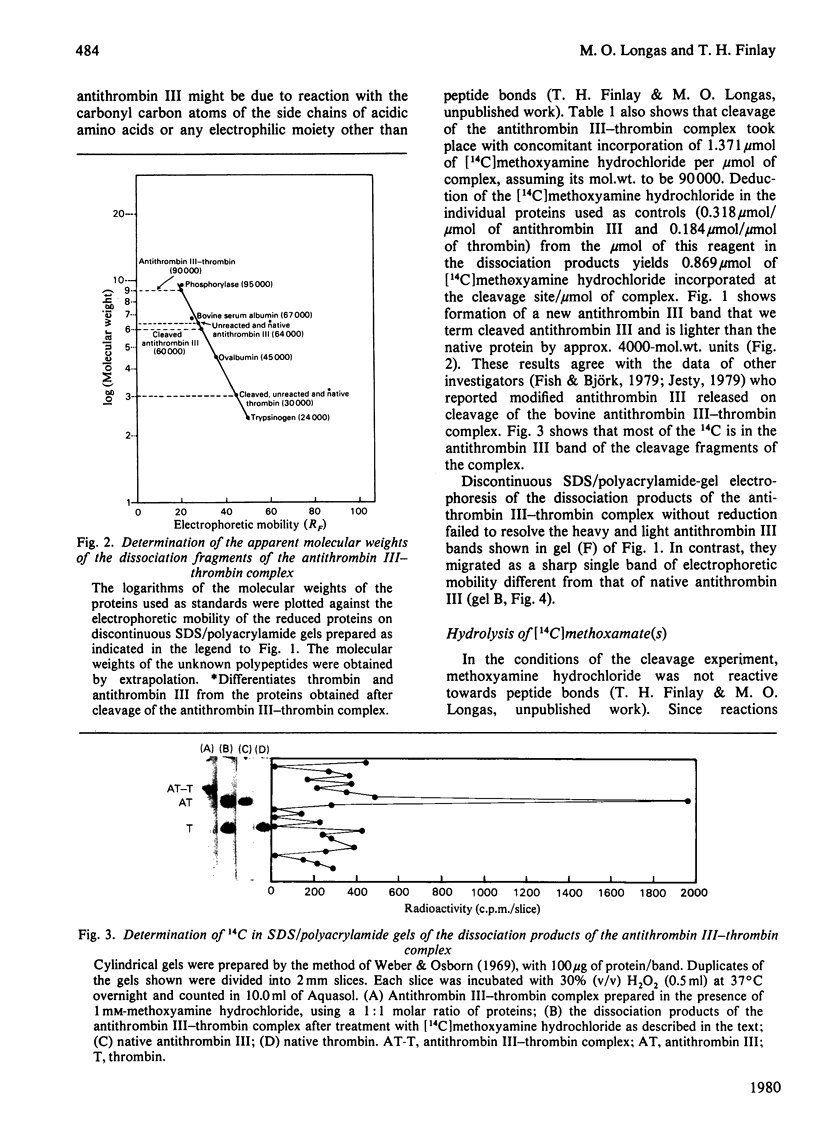
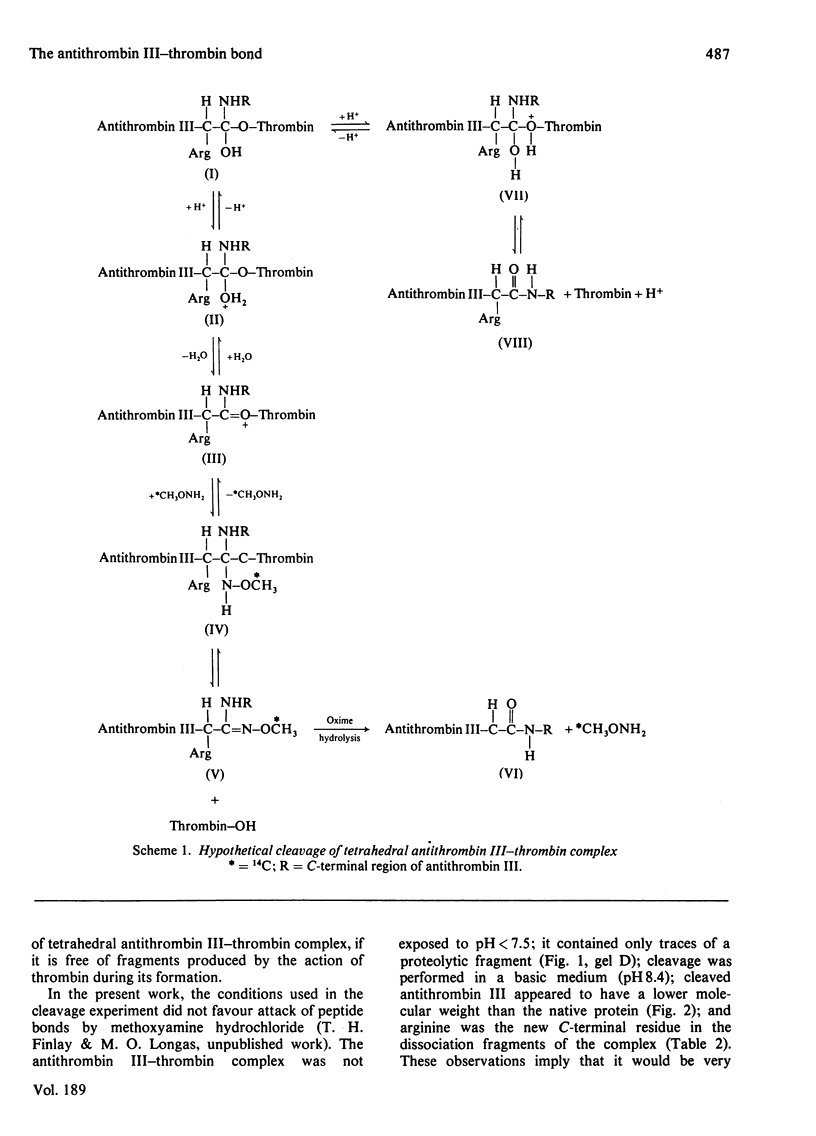
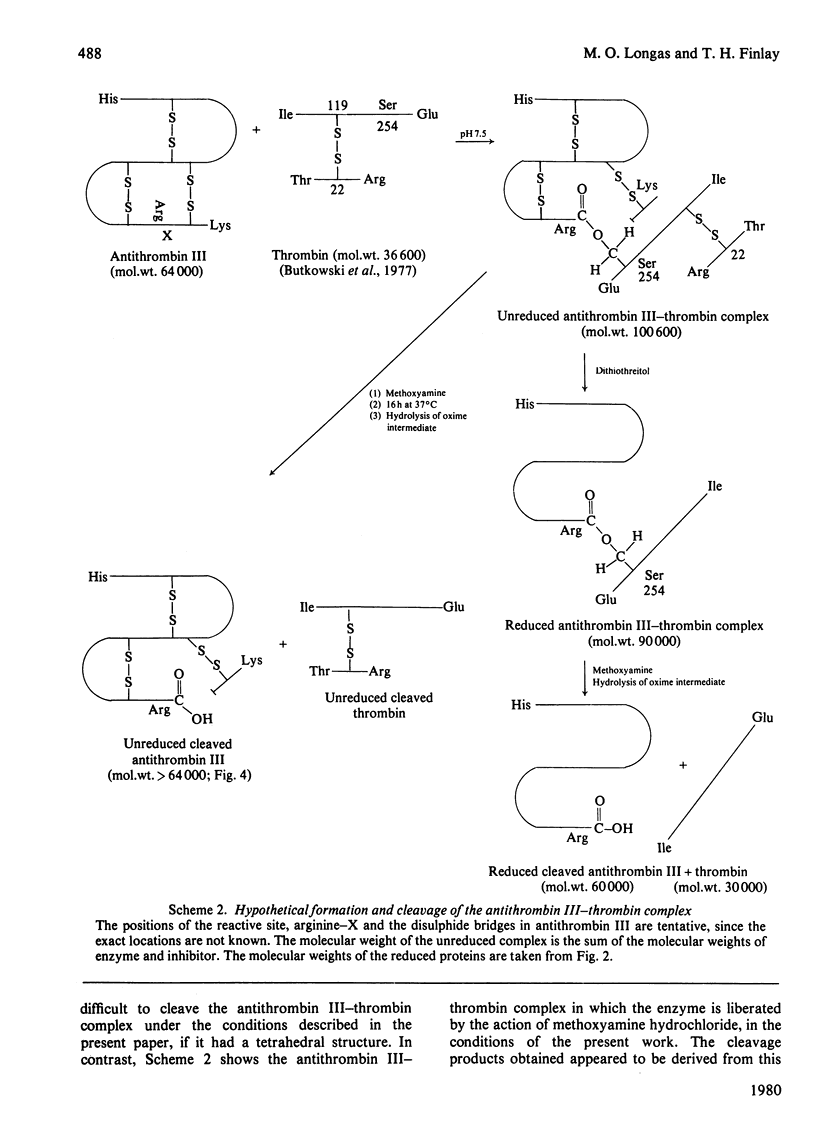
1. Cleavage of the human antithrombin III--thrombin complex with [14C]methoxyamine hydrochloride results in inactive thrombin and 14C-labelled antithrombin III. 2. Discontinuous polyacrylamide-gel electrophoresis of the reduced dissociation fragments of the complex in the presence of sodium dodecyl sulphate reveals two antithrombin III bands that do not resolve during electrophoresis without reduction. The heavy band has the electrophoretic mobility of the native protein. The light band has an apparent mol.wt. that is approx. 4000 less than the molecular weight of native antithrombin III. 3. Treatment of the cleavage products of the complex with carboxypeptidase B yields 1 mumol of arginine, a new C-terminal amino acid, per mumol of thrombin dissociated. The results indicate that during formation of the antithrombin III--thrombin complex, the inhibitor is cleaved at an arginine--X bond; this arginine residue forms a carboxylic ester with the enzyme, while the excised polypeptide remains bound through a disulphide bridge(s).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHER R., CROCKER C. Réactions colorées spécifiques de l'arginine et de la tyrosine réalisées après chromatographie sur papier. Biochim Biophys Acta. 1952 Dec;9(6):704–705. doi: 10.1016/0006-3002(52)90236-9. [DOI] [PubMed] [Google Scholar]
- Binder B. On the complex formation of antithrombin 3 with thrombin. Gelfiltration studies on human plasma and serum. Thromb Diath Haemorrh. 1973 Nov;30(2):280–283. [PubMed] [Google Scholar]
- Butkowski R. J., Elion J., Downing M. R., Mann K. G. Primary structure of human prethrombin 2 and alpha-thrombin. J Biol Chem. 1977 Jul 25;252(14):4942–4957. [PubMed] [Google Scholar]
- Collen D., de Cock F., Verstraete M. Quantitation of thrombin-antithrombin III complexes in human blood. Eur J Clin Invest. 1977 Oct;7(5):407–411. doi: 10.1111/j.1365-2362.1977.tb01627.x. [DOI] [PubMed] [Google Scholar]
- Fenton J. W., 2nd, Fasco M. J., Stackrow A. B. Human thrombins. Production, evaluation, and properties of alpha-thrombin. J Biol Chem. 1977 Jun 10;252(11):3587–3598. [PubMed] [Google Scholar]
- Fish W. W., Björk I. Release of a two-chain form of antithrombin from the antithrombin-thrombin complex. Eur J Biochem. 1979 Nov 1;101(1):31–38. doi: 10.1111/j.1432-1033.1979.tb04212.x. [DOI] [PubMed] [Google Scholar]
- Fish W. W., Orre K., Björk I. Routes of thrombin action in the production of proteolytically modified, secondary forms of antithrombin-thrombin complex. Eur J Biochem. 1979 Nov 1;101(1):39–44. doi: 10.1111/j.1432-1033.1979.tb04213.x. [DOI] [PubMed] [Google Scholar]
- GUIDOTTI G. The action of carboxypeptidases A and B on the separated alpha and beta chains of normal adult human hemoglobin. Biochim Biophys Acta. 1960 Jul 29;42:177–179. doi: 10.1016/0006-3002(60)90774-5. [DOI] [PubMed] [Google Scholar]
- Jesty J. Dissociation of complexes and their derivatives formed during inhibition of bovine thrombin and activated factor X by antithrombin III. J Biol Chem. 1979 Feb 25;254(4):1044–1049. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Longas M. O., Ferguson W. S., Finlay T. H. Studies on the interaction of heparin with thrombin, antithrombin, and other plasma proteins. Arch Biochem Biophys. 1980 Apr 1;200(2):505–602. doi: 10.1016/0003-9861(80)90392-6. [DOI] [PubMed] [Google Scholar]
- Moore S. Amino acid analysis: aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J Biol Chem. 1968 Dec 10;243(23):6281–6283. [PubMed] [Google Scholar]
- Odegard O. R., Lie M., Abildgaard U. Heparin cofactor activity measured with an amidolytic method. Thromb Res. 1975 Apr;6(4):287–294. doi: 10.1016/0049-3848(75)90078-x. [DOI] [PubMed] [Google Scholar]
- Owen W. G. Evidence for the formation of an ester between thrombin and heparin cofactor. Biochim Biophys Acta. 1975 Oct 20;405(2):380–387. doi: 10.1016/0005-2795(75)90103-8. [DOI] [PubMed] [Google Scholar]
- Pepper D. S., Banhegyi D., Cash J. D. The different forms of antithrombin II in serum. Thromb Haemost. 1977 Aug 31;38(2):494–503. [PubMed] [Google Scholar]
- Rosenberg R. D., Damus P. S. The purification and mechanism of action of human antithrombin-heparin cofactor. J Biol Chem. 1973 Sep 25;248(18):6490–6505. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]