Dilated cardiomyopathy (DCM) is often driven by myocarditis, a condition known as inflammatory DCM (infl-DCM). Infl-DCM classifies as an immune-mediated disease according to the Rose-Witebsky criteria. To date, most evidence points to IL (interleukin)-17 expressing helper T cells (Th17 cells) as drivers of the progression from acute myocarditis to infl-DCM (eg, the study by Myers et al1). Research on the role of cytotoxic T cells (Tc cells) is lacking.
To investigate the adaptive T cell–mediated immune responses in infl-DCM, comprehensive immunophenotyping was performed in peripheral blood mononuclear cells from 125 patients with nonischemic, nonvalvular DCM undergoing endomyocardial biopsy (EMB; median time since DCM diagnosis, 9 [4–26] months), excluding patients with systemic immune diseases (n=6). Flow cytometry was performed on T cells and innate immune cells. Plasma levels of IL-6 and TGF (tumor growth factor)-β, which drive Th17-differentiation (eg, the study by Myers et al1), were measured by ELISA. Patients were allocated to 3 groups: infl-DCM (≥7 T cells/mm2 or ≥14 leukocytes/mm2 in EMB; n=20), non-infl-DCM (<3 T cells/mm2 and <5 leukocytes/mm2; n=37), and patients not fulfilling these criteria (n=68). A combined outcome of all-cause mortality, heart failure hospitalization, and life-threatening arrhythmia was assessed over a 2-year period (median duration, 8.5 [4.3–25.7] months). Findings were correlated with RNA sequencing of EMBs in a cohort of 41 patients. Normality was assessed by using Q-Q plots and histograms. All analyses were performed with R (version 4.0.4) using the DESeq2 and biomaRt packages. This study adhered to the Helsinki Declaration and obtained ethics approval and patient consent.
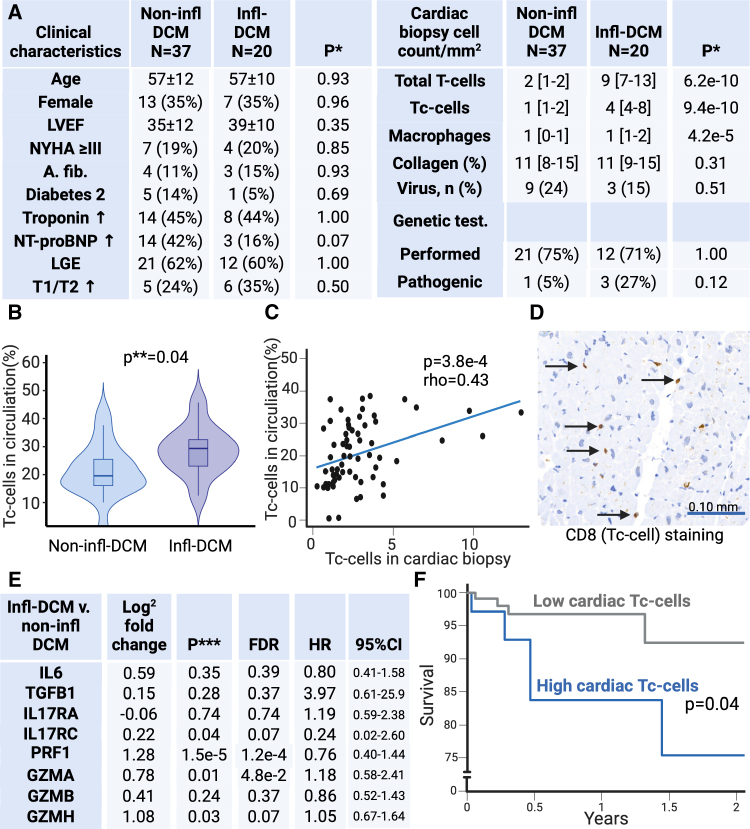
Clinical characteristics were similar between infl-DCM and non-infl-DCM (Figure [A]). Helper T cells more often expressed IL-17A in infl-DCM compared with non-infl-DCM (17.8±5.1% versus 13.5±5.4%; P=0.04). Interestingly, the cardiac transcript of IL-17RC, part of the IL-17A receptor complex, was increased in infl-DCM compared with non-infl-DCM (Figure [E]). However, the expression of IL-6 or TGF-β did not significantly differ, when measured in plasma (protein), circulating monocytes and dendritic cells (RNA), or EMBs (RNA; Figure [E]).
Figure.
Circulating and cardiac cytotoxic T cells (Tc cells) drive poor outcomes in inflammatory dilated cardiomyopathy. A, Clinical characteristics comparison between non-inflammatory DCM (non-infl-DCM) and inflammatory DCM (infl-DCM) shows no between-group differences. However, cardiac T cells and Tc cells and macrophages are increased in infl-DCM compared with non-infl-DCM (P=6.2×10−10, P=9.4×10−10, and P=4.2×10−5, respectively). Genetic mutations were found in TTN, FLNC, and MYBPC3 in infl-DCM and MYH7 in non-infl-DCM. B, The proportion of circulating Tc cells in infl-DCM was increased compared with non-infl-DCM (P=0.04). C, In the total study cohort (n=125), the proportion of circulating Tc cells correlated with the density of Tc cells in endomyocardial biopsy (EMB; Spearmanρ=0.43; P=3.8×10−4). D, An example of Tc cell staining of an EMB of infl-DCM. E, Comparison of cardiac gene expression by transcriptomics of EMBs of non-infl-DCM and infl-DCM patients shows higher expression of PRF1 (perforin-1), GZMA (granzyme-A), GZMH (granzyme-H), and IL17RC (interleukin-17 receptor-C), after correcting for multiple testing (false discovery rate [FDR] <0.1 as per Benjamini-Hochberg procedure). Expression of these genes was not associated with prognosis. F, In the total study cohort (n=125), high cardiac Tc cells (≥3 Tc cells/mm2 corresponding with the third quartile of cardiac Tc cells) correlated with a worse prognosis compared with low cardiac Tc cells (<3 Tc cells/mm2) as tested by log-rank test (P=0.04). *Fisher exact or Mann-Whitney U test; **Mann-Whitney U test; and ***2-sample t tests. a. fib indicates atrial fibrillation; CD, cluster of differentiation; GZMB, granzyme-B; IL17RA, interleukin-17 receptor-A; IL-6, interleukin-6; LGE, late-gadolinium enhancement; LVEF, left ventricle ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association dyspnea classification; T1/T2, T1- or T2-weighted imaging; and TGFB1, tumor growth factor-β1.
In infl-DCM, a Tc cell–driven immune reaction stood out as a dominant feature of the ongoing immune response (Figure [B–F]). Tc cells were increased in EMBs (Figure [A, C, and D]) and in the circulation (Figure [B]) of patients with infl-DCM compared with non-infl-DCM. Crucially, a higher proportion of Tc cells expressed granzyme-B in infl-DCM (50.7% [40.8–73.0] versus 33.1% [16.6–57.8] in non-infl-DCM; P=0.01), indicating enhanced cytolytic activity. These Tc cells also shifted towards an exhausted phenotype and displayed markers associated with CD45RA re-expression (TEMRA [cytotoxic T-cells with markers associated with CD45RA re-expression] cells; CD8+/CCR7-/CD45RO-/CD57+/KLRG-1+/PD-1+; 0.6% [0.4–1.7] versus 0.3% [0.1–0.6]; P=0.02). The frequency of circulating Tc cells directly correlated with their density in EMBs (Figure [C]), suggesting migration of these cells to the myocardium. This conclusion was reinforced by increased Tc-cell gene transcription in infl-DCM compared with non-infl-DCM EMBs (Figure [E]). Cardiac T cell infiltration predicted poor patient outcomes (Figure [F]), highlighting their role in infl-DCM progression.
Our study reveals Tc cell–mediated immunity as a dominant pathway in infl-DCM, possibly explained by an upregulated Th17 response. Heart-infiltrating Tc cells induce cardiomyocyte apoptosis and adverse remodeling via granzyme release.2 Th17 cells promote T cell exhaustion and enhance cellular cytotoxicity.3 Thus, Th17-mediated immunity may enhance T cell activity and subsequent adverse cardiac remodeling. Based on these findings, downregulating T cell activity by targeted therapy may hold promise to halt infl-DCM progression.
This study could not confirm the upregulation of the IL-6/TGF-β pathway as the main driver behind the observed increase in IL-17A response. While IL-6 and TGF-β are important for the differentiation of Th17 cells, IL-23 may sustain preexisting Th17 responses in the absence of IL-6 and TGF-β.1 Finally, the drivers of the T cell response remain yet to be elucidated. A viral cause of the increased T cell response was not found.
The strength of this study includes the performance of comprehensive immune phenotyping and follow-up of patients with biopsy-confirmed infl-DCM and non-infl-DCM. Limitations include the relatively short follow-up period and the lack of a validation cohort. Despite this, our study reveals a key role for Tc cell–driven immune response as a key pathogenic feature in infl-DCM. Therefore, Tc cell–targeting immunotherapies may, therefore, be considered in infl-DCM.
ARTICLE INFORMATION
Acknowledgments
Figure was created by M.A.S. via BioRender.com.
Sources of Funding
The authors acknowledge the Double Dose Program 2020-B005, CVONArena-PRIME-2017-18, HORIZON-EIC-2022-PATHFINDERCHALLENGES, ZonMW-Metacor, and British Heart Foundation grants FS/CRTF/20/24058, RG/20/8/34995, CH/15/2/32064, and AA/18/5/34222.
Disclosures
S.R.B. Heymans receives fees for scientific advice for AstraZeneca, Ribocure, and CSL Behring and receives research support from AstraZeneca and CSL Behring. The other authors report no conflicts.
Data Availability
Data are available in the Major Resources Table in the Supplemental Material.
Supplementary Material
Footnotes
F.M. Marelli-Berg and S.R.B. Heymans contributed equally.
For Sources of Funding and Disclosures, see page 1195.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.124.325183.
References
- 1.Myers JM, Cooper LT, Kem DC, Stavrakis S, Kosanke SD, Shevach EM, Fairweather D, Stoner JA, Cox CJ, Cunningham MW. Cardiac myosin-Th17 responses promote heart failure in human myocarditis. JCI Insight. 2016;1:e85851. doi: 10.1172/jci.insight.85851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santos-Zas I, Lemarié J, Zlatanova I, Cachanado M, Seghezzi JC, Benamer H, Goube P, Vandestienne M, Cohen R, Ezzo M, et al. Cytotoxic CD8+ T cells promote granzyme B-dependent adverse post-ischemic cardiac remodeling. Nat Commun. 2021;12:1483. doi: 10.1038/s41467-021-21737-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim BS, Kuen DS, Koh CH, Kim HD, Chang SH, Kim S, Jeon YK, Park YJ, Choi G, Kim J, et al. Type 17 immunity promotes the exhaustion of CD8+ T cells in cancer. J ImmunoTher Cancer. 2021;9:e002603. doi: 10.1136/jitc-2021-002603 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the Major Resources Table in the Supplemental Material.