Abstract
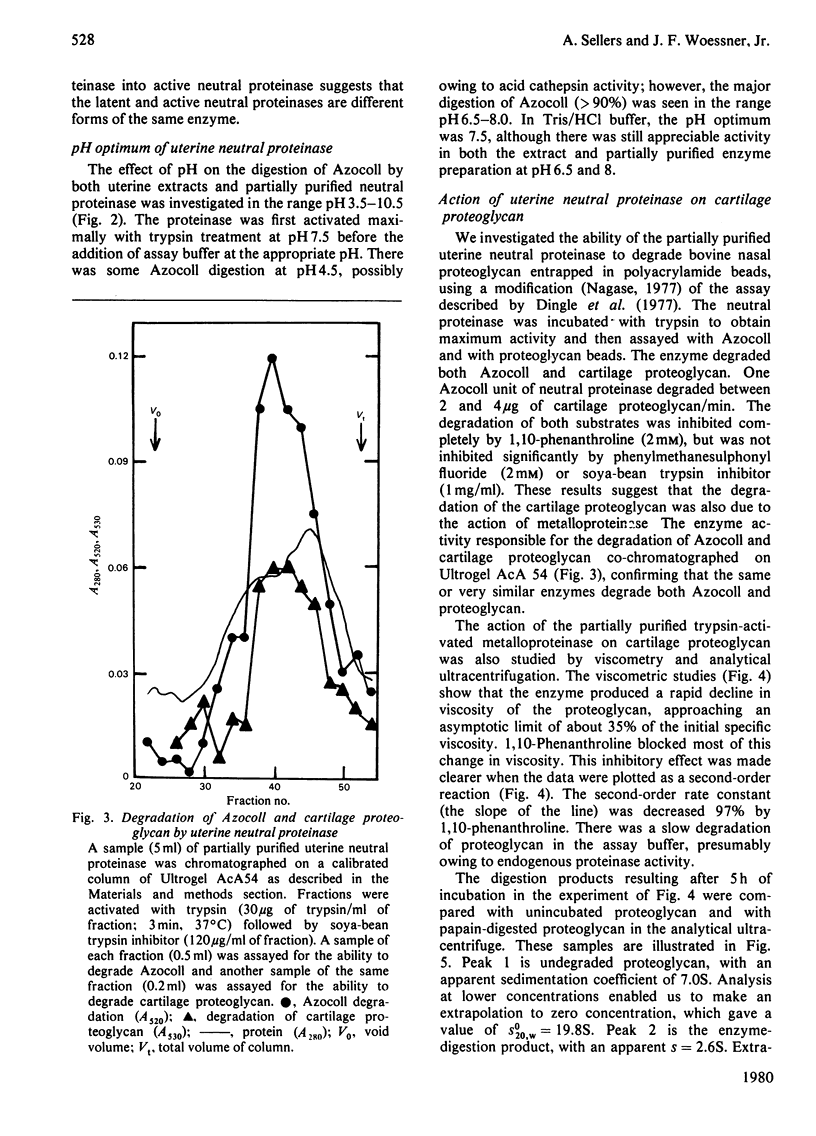
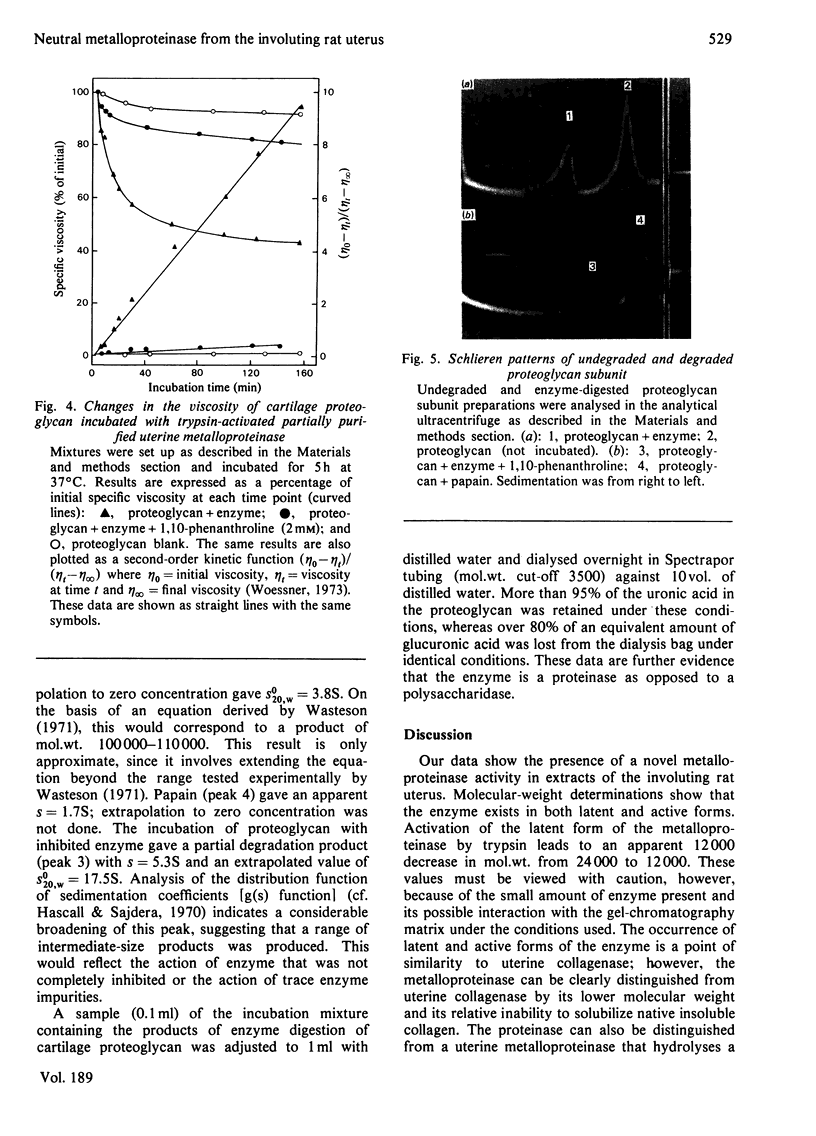
1. Homogenates of rat uteri removed 1 and 2 days post partum were centrifuged at 6000 g. Both pellets and supernatants degraded Azocoll, a general proteinase substrate, at pH 7.5. More than 80% of the total activity was in the pellet fraction. 2. Part of the pellet activity was in a latent form. Trypsin and 4-aminophenylmercuric acetate (a thiol-blocking agent) both activated this latent form, indicating that it is an enzyme--inhibitor complex. An endogenous serine proteinase activated part of the latent enzyme during the assay. 3. The enzyme activity was low before parturition and after involution; it was highest during the first 2 days post partum, when the largest losses of uterine wet weight and matrix macromolecules occur. 4. Up to 70% of the enzyme in the pellets was extracted by heating at 60 degrees C for 4 min in 0.1 M-CaCl2/0.05 M-Tris/HCl, pH 7.5. Approx. 30% of the extracted enzyme was still latent. 5. The extracted enzyme was a metalloproteinase, since it was inhibited completely by 1,10-phenanthroline, but not by inhibitors of thiol or serine proteinases. 6. The enzyme was further purified 15--30-fold by gel chromatography and precipitation with (NH4)2SO4. The apparent molecular weight, estimated by gel filtration, was 24000 for the latent form and 12000 for the active form. The pH optimum was 7--7.5. 7. The enzyme also degraded cartilage proteoglycan. This activity was studied by viscometry and the products were analysed by analytical ultracentrifugation. The major product had a mol.wt. of approx. 100000. The sites of cleavage were in the protein core, since no free oligosaccharides were detected. 8. This neutral metalloproteinase is distinct from uterine collagenase and from a uterine metal-dependent endopeptidase that hydrolyses a heptapeptide related to collagen.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Dingle J. T., Blow A. M., Barrett A. J., Martin P. E. Proteoglycan-degrading enzymes. A radiochemical assay method and the detection of a new enzyme cathepsin F. Biochem J. 1977 Dec 1;167(3):775–785. doi: 10.1042/bj1670775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS J., LAPIERE C. M. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1014–1022. doi: 10.1073/pnas.48.6.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARKNESS R. D., MORALEE B. E. The time-course and route of loss of collagen from the rat's uterus during post-partum involution. J Physiol. 1956 Jun 28;132(3):502–508. doi: 10.1113/jphysiol.1956.sp005543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr, Krane S. M. An endopeptidase from rheumatoid synovial tissue culture. Biochim Biophys Acta. 1972 Feb 28;258(2):566–576. doi: 10.1016/0005-2744(72)90249-5. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Physical properties and polydispersity of proteoglycan from bovine nasal cartilage. J Biol Chem. 1970 Oct 10;245(19):4920–4930. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Hauser P., Vaes G. Degradation of cartilage proteoglycans by a neutral proteinase secreted by rabbit bone-marrow macrophages in culture. Biochem J. 1978 May 15;172(2):275–284. doi: 10.1042/bj1720275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz A. L., Kelman J. A., Crystal R. G. Activation of alveolar macrophage collagenase by a neutral protease secreted by the same cell. Nature. 1976 Dec 23;264(5588):772–774. doi: 10.1038/264772a0. [DOI] [PubMed] [Google Scholar]
- MONTFORT I., PEREZ-TAMAYO R. Studies on uterine collagen during pregnancy and puerperium. Lab Invest. 1961 Nov-Dec;10:1240–1258. [PubMed] [Google Scholar]
- Reynolds J. J., Murphy G., Sellers A., Cartwright E. A new factor that may control collagen resorption. Lancet. 1977 Aug 13;2(8033):333–335. doi: 10.1016/s0140-6736(77)91490-8. [DOI] [PubMed] [Google Scholar]
- Rosenberg L., Pal S., Beale R., Schubert M. A comparison of proteinpolysaccharides of bovine nasal cartilage isolated and fractionated by different methods. J Biol Chem. 1970 Aug 25;245(16):4112–4122. [PubMed] [Google Scholar]
- Sapolsky A. I., Keiser H., Howell D. S., Woessner J. F., Jr Metalloproteases of human articular cartilage that digest cartilage proteoglycan at neutral and acid pH. J Clin Invest. 1976 Oct;58(4):1030–1041. doi: 10.1172/JCI108526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers A., Reynolds J. J., Meikle M. C. Neutral metallo-proteinases of rabbit bone. Separation in latent forms of distinct enzymes that when activated degrade collagen, gelatin and proteoglycans. Biochem J. 1978 May 1;171(2):493–496. doi: 10.1042/bj1710493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sopata I., Dancewicz A. M. Presence of a gelatin-specific proteinase and its latent form in human leucocytes. Biochim Biophys Acta. 1974 Dec 29;370(2):510–523. doi: 10.1016/0005-2744(74)90112-0. [DOI] [PubMed] [Google Scholar]
- Steven F. S., Torre-Blanco A., Hunter J. A. A neutral protease in rheumatoid synovial fluid capable of attacking the telopeptide regions of polymeric collagen fibrils. Biochim Biophys Acta. 1975 Sep 9;405(1):188–200. doi: 10.1016/0005-2795(75)90329-3. [DOI] [PubMed] [Google Scholar]
- Tansey T. R., Padykula H. A. Cellular responses to experimental inhibition of collagen degradation in the postpartum rat uterus. Anat Rec. 1978 Jul;191(3):287–309. doi: 10.1002/ar.1091910303. [DOI] [PubMed] [Google Scholar]
- Vaes G., Eeckhout Y., Lenaers-Claeys G., François-Gillet C., Druetz J. E. The simultaneous release by bone explants in culture and the parallel activation of procollagenase and of a latent neutral proteinase that degrades cartilage proteoglycans and denatured collagen. Biochem J. 1978 May 15;172(2):261–274. doi: 10.1042/bj1720261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vater C. A., Mainardi C. L., Harris E. D., Jr Inhibitor of human collagenase from cultures of human tendon. J Biol Chem. 1979 Apr 25;254(8):3045–3053. [PubMed] [Google Scholar]
- WOESSNER J. F., Jr Catabolism of collagen and non-collagen protein in the rat uterus during post-partum involution. Biochem J. 1962 May;83:304–314. doi: 10.1042/bj0830304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasteson A. Properties of fractionated chondroitin sulphate from ox nasal septa. Biochem J. 1971 May;122(4):477–485. doi: 10.1042/bj1220477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks J. G., Halme J., Woessner J. F., Jr Extraction of collagenase from the involuting rat uterus. Biochim Biophys Acta. 1976 Aug 12;445(1):205–214. doi: 10.1016/0005-2744(76)90173-x. [DOI] [PubMed] [Google Scholar]
- Werb Z., Dingle J. T., Reynolds J. J., Barrett A. J. Proteoglycan-degrading enzymes of rabbit fibroblasts and granulocytes. Biochem J. 1978 Sep 1;173(3):949–958. doi: 10.1042/bj1730949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Gordon S. Elastase secretion by stimulated macrophages. Characterization and regulation. J Exp Med. 1975 Aug 1;142(2):361–377. doi: 10.1084/jem.142.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb Z., Reynolds J. J. Stimulation by endocytosis of the secretion of collagenase and neutral proteinase from rabbit synovial fibroblasts. J Exp Med. 1974 Dec 1;140(6):1482–1497. doi: 10.1084/jem.140.6.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr A latent form of collagenase in the involuting rat uterus and its activation by a serine proteinase. Biochem J. 1977 Mar 1;161(3):535–542. doi: 10.1042/bj1610535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Inhibition by oestrogen of collagen breakdown in the involuting rat uterus. Biochem J. 1969 May;112(5):637–645. doi: 10.1042/bj1120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woessner J. F., Jr Purification of cathepsin D from cartilage and uterus and its action on the protein-polysaccharide complex of cartilage. J Biol Chem. 1973 Mar 10;248(5):1634–1642. [PubMed] [Google Scholar]
- Woessner J. F., Jr Separation of collagenase and a metal-dependent endopeptidase of rat uterus that hydrolyzes a heptapeptide related to collagen. Biochim Biophys Acta. 1979 Dec 7;571(2):313–320. doi: 10.1016/0005-2744(79)90101-3. [DOI] [PubMed] [Google Scholar]
- Woessner J. F., Jr Total, latent and active collagenase during the course of post-partum involution of the rat uterus. Effect of oestradiol. Biochem J. 1979 Apr 15;180(1):95–102. doi: 10.1042/bj1800095. [DOI] [PMC free article] [PubMed] [Google Scholar]