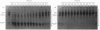Abstract
The denaturation of transferrin by urea has been studied by (a) electrophoresis in polyacrylamide gels incorporating a urea gradient, (b) measurements of the loss of iron-binding capacity and (c) u.v. difference spectrometry. In human serum transferrin and hen ovotransferrin the N-terminal and C-terminal domains of the iron-free protein were found to denature at different urea concentrations.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AZARI P. R., FEENEY R. E. Resistance of metal complexes of conalbumin and transferrin to proteolysis and to thermal denaturation. J Biol Chem. 1958 May;232(1):293–302. [PubMed] [Google Scholar]
- Aisen P., Leibman A., Reich H. A. Studies on the binding of iron to transferrin and conalbumin. J Biol Chem. 1966 Apr 25;241(8):1666–1671. [PubMed] [Google Scholar]
- Aisen P., Leibman A., Zweier J. Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem. 1978 Mar 25;253(6):1930–1937. [PubMed] [Google Scholar]
- Creighton T. E. Electrophoretic analysis of the unfolding of proteins by urea. J Mol Biol. 1979 Apr 5;129(2):235–264. doi: 10.1016/0022-2836(79)90279-1. [DOI] [PubMed] [Google Scholar]
- Donovan J. W., Ross K. D. Iron binding to conalbumin. Calorimetric evidence for two distinct species with one bound iron atom. J Biol Chem. 1975 Aug 10;250(15):6026–6031. [PubMed] [Google Scholar]
- Evans R. W., Donovan J. W., Williams J. Calorimetric studies on the binding of iron and aluminium to the amino- and carboxyl-terminal fragments of hen ovotransferrin. FEBS Lett. 1977 Nov 1;83(1):19–22. doi: 10.1016/0014-5793(77)80632-7. [DOI] [PubMed] [Google Scholar]
- Evans R. W., Williams J. Studies of the binding of different iron donors to human serum transferrin and isolation of iron-binding fragments from the N- and C-terminal regions of the protein. Biochem J. 1978 Aug 1;173(2):543–552. doi: 10.1042/bj1730543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAZER A. N., McKENZIE H. A. The denaturation of proteins. IV. Conalbumin and iron(III)-conalbumin in urea solution. Biochim Biophys Acta. 1963 Apr 2;71:109–123. doi: 10.1016/0006-3002(63)90990-9. [DOI] [PubMed] [Google Scholar]
- Graham I., Williams J. A comparsion of glycopeptides from the transferrins of several species. Biochem J. 1975 Feb;145(2):263–279. doi: 10.1042/bj1450263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienenberger J. M., Simon D. Structure and biosynthesis of the ribosomal ribonucleic acids from the oncogenic bacterium Agrobacterium tumefaciens. Biochem J. 1975 Jul;149(1):23–30. doi: 10.1042/bj1490023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibman A., Aisen P. Distribution of iron between the binding sites of transferrin in serum: methods and results in normal human subjects. Blood. 1979 Jun;53(6):1058–1065. [PubMed] [Google Scholar]
- Makey D. G., Seal U. S. The detection of four molecular forms of human transferrin during the iron binding process. Biochim Biophys Acta. 1976 Nov 26;453(1):250–256. doi: 10.1016/0005-2795(76)90270-1. [DOI] [PubMed] [Google Scholar]
- Williams J., Moreton K. The distribution of iron between the metal-binding sites of transferrin human serum. Biochem J. 1980 Feb 1;185(2):483–488. doi: 10.1042/bj1850483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh Y., Iwai S., Feeney R. E. Conformations of denatured and renatured ovotransferrin. Biochemistry. 1979 Mar 6;18(5):882–889. doi: 10.1021/bi00572a023. [DOI] [PubMed] [Google Scholar]