Abstract
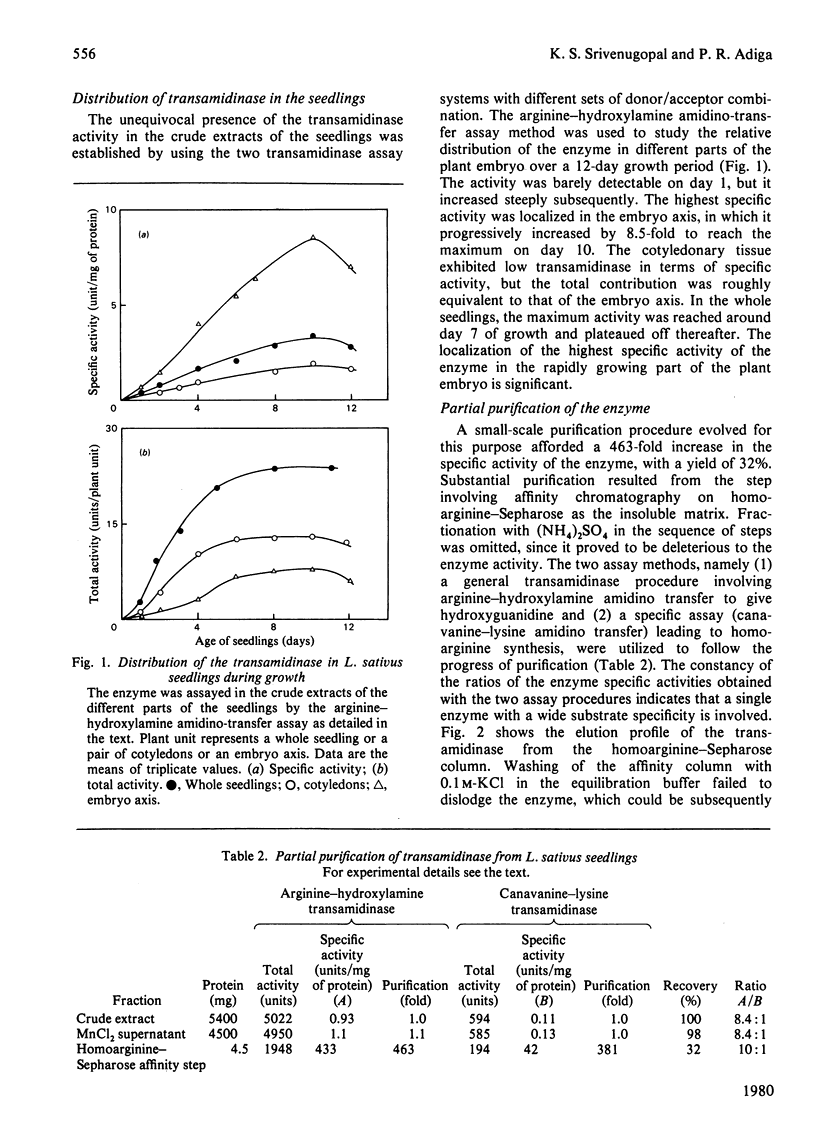
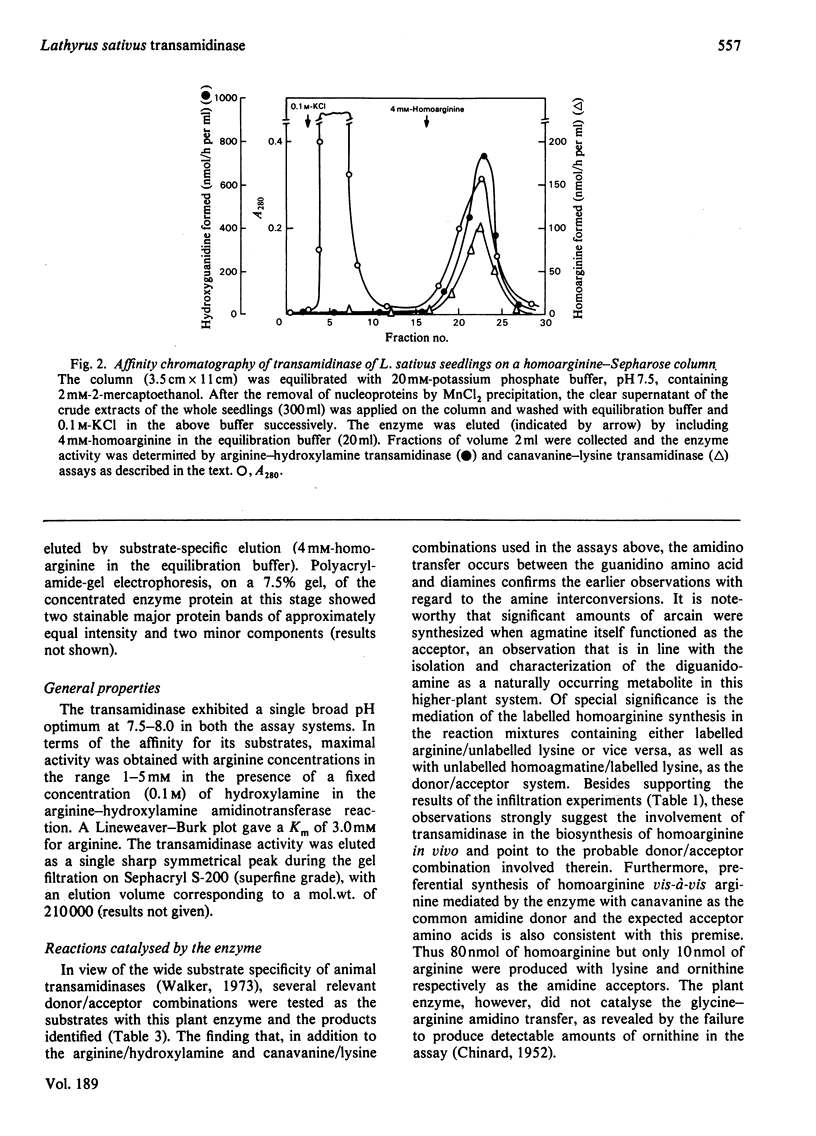
A transamidinase was purified 463-fold from Lathyrus sativus seedlings by affinity chromatography on homoarginine--Sepharose. The enzyme exhibited a wide substrate specificity, and catalysed the reversible transfer of the amidino groups from donors such as arginine, homoarginine and canavanine to acceptors such as lysine, putrescine, agmatine, cadaverine and hydroxylamine. The enzyme could not be detected in the seeds, and attained the highest specific activity in the embryo axis on day 10 after seed germination. Its thiol nature was established by strong inhibition by several thiol blockers and thiol compounds in the presence of ferricyanide. In the absence of an exogenous acceptor, it exhibited weak hydrolytic activity towards arginine. It had apparent mol.wt. 210000, and exhibited Michaelis--Menten kinetics with Km 3.0 mM for arginine. Ornithine competitively inhibited the enzyme, with Ki 1.0 mM in the arginine--hydroxylamine amidino-transfer reaction. Conversion experiments with labelled compounds suggest that the enzyme is involved in homoarginine catabolism during the development of plant embryo to give rise to important amino acids and amine metabolites. Presumptive evidence is also provided for its involvement in the biosynthesis of the guanidino amino acid during seed development. The natural occurrence of arcain in L. sativus and mediation of its synthesis in vitro from agmatine by the transamidinase are demonstrated.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AKAMATSU S., WATANABE T. The quantitative determination of arginine. J Biochem. 1961 Jun;49:566–569. doi: 10.1093/oxfordjournals.jbchem.a127345. [DOI] [PubMed] [Google Scholar]
- Audit C., Viala B., Robin Y. Biogénèse des dérivés diguanidiques chez la sangsue, Hirudo medicinalis L. I. Origine des groupements guanidiques et de la chîne carbonée. Comp Biochem Physiol. 1967 Sep;22(3):775–785. doi: 10.1016/0010-406x(67)90770-0. [DOI] [PubMed] [Google Scholar]
- Brown E. G., Al-Baldowi N. F. Biosynthesis of the pyrimidinyl amino acid lathyrine by Lathyrus tingitanus L. Biochem J. 1977 Jun 15;164(3):589–594. doi: 10.1042/bj1640589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHINARD F. P. Photometric estimation of proline and ornithine. J Biol Chem. 1952 Nov;199(1):91–95. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Guarino L. A., Cohen S. S. Mechanism of toxicity of putrescine in Anacystis nidulans. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3660–3664. doi: 10.1073/pnas.76.8.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Mizutani A. A new method for isolation of polyamines from animal tissues. Anal Biochem. 1973 Dec;56(2):408–416. doi: 10.1016/0003-2697(73)90206-6. [DOI] [PubMed] [Google Scholar]
- Jasiorowska B., Kleczkowski K. Lysine as substrate for ornithine carbamoyltransferase. Bull Acad Pol Sci Biol. 1970;18(7):373–378. [PubMed] [Google Scholar]
- KALYANKAR G. D., IKAWA M., SNELL E. E. The enzymatic cleavage of canavanine to homoserine and hydroxyguanidine. J Biol Chem. 1958 Nov;233(5):1175–1178. [PubMed] [Google Scholar]
- Lawrence J. M., Grant D. R. Nitrogen Mobilization in Pea Seedlings. II. Free Amino Acids. Plant Physiol. 1963 Sep;38(5):561–566. doi: 10.1104/pp.38.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Prescott L. M., Jones M. E. Modified methods for the determination of carbamyl aspartate. Anal Biochem. 1969 Dec;32(3):408–419. doi: 10.1016/s0003-2697(69)80008-4. [DOI] [PubMed] [Google Scholar]
- RAO S. L., RAMACHANDRAN L. K., ADIGA P. R. The isolation and characterization of l-homoarginine from seeds of Lathyrus sativus. Biochemistry. 1963 Mar-Apr;2:298–300. doi: 10.1021/bi00902a019. [DOI] [PubMed] [Google Scholar]
- Rosenthal G. A. The preparation and colorimetric analysis of L-canaline. Anal Biochem. 1973 Feb;51(2):354–361. doi: 10.1016/0003-2697(73)90488-0. [DOI] [PubMed] [Google Scholar]
- Smith T. A. The occurrence, metabolism and functions of amines in plants. Biol Rev Camb Philos Soc. 1971 May;46(2):201–241. doi: 10.1111/j.1469-185x.1971.tb01182.x. [DOI] [PubMed] [Google Scholar]
- Suresh M. R., Adiga P. R. Putrescine-sensitive (artifactual) and insensitive (biosynthetic) S-adenosyl-L-methionine decarboxylase activities of Lathyrus sativus seedlings. Eur J Biochem. 1977 Oct 3;79(2):511–518. doi: 10.1111/j.1432-1033.1977.tb11835.x. [DOI] [PubMed] [Google Scholar]
- WALKER J. B. Further studies on the mechanism of transamidinase action: transamidination in Streptomyces griseus. J Biol Chem. 1958 Mar;231(1):1–9. [PubMed] [Google Scholar]


