ABSTRACT
As an obligate aerobe, Mycobacterium tuberculosis relies on its branched electron transport chain (ETC) for energy production through oxidative phosphorylation. Regimens targeting ETC exhibit promising potential to enhance bactericidal activity against M. tuberculosis and hold the prospect of shortening treatment duration. Our previous research demonstrated that the bacteriostatic drug candidate TB47 (T) inhibited the growth of M. tuberculosis by targeting the cytochrome bc1 complex and exhibited synergistic activity with clofazimine (C). Here, we found synergistic activities between C and sudapyridine (S), a structural analog of bedaquiline (B). S has shown similar anti-tuberculosis efficacy and may share a mechanism of action with B, which inhibits ATP synthesis and the energy metabolism of bacteria. We evaluated the efficacy of SCT in combination with linezolid (L) or pyrazinamide (Z) using a well-established murine model of tuberculosis. Compared to the BPa(pretomanid)L regimen, SCT and SCTL demonstrated similar bactericidal and sterilizing activities. There was no significant difference in activity between SCT and SCTL. In contrast, SCZ and SCTZ showed much higher activities, with none of the 15 mice experiencing relapse after 2 months of treatment with either SCZ or SCTZ. However, T did not contribute to the activity of the SCZ. Our findings emphasize the efficacy and the potential clinical significance of combination therapy with ETC inhibitors. Additionally, cross-resistance exists not only between S and B but also between S/B and C. This is supported by our findings, as spontaneous S-resistant mutants exhibited mutations in Rv0678, which are associated with cross-resistance to B and C.
KEYWORDS: tuberculosis, electron transport chain, drug resistance, sudapyridine, TB47
INTRODUCTION
Tuberculosis (TB), caused by the bacillus Mycobacterium tuberculosis, ranks as the second leading cause of death worldwide from a single infectious agent after COVID-19 (1). The emergence of multidrug-resistant tuberculosis (MDR-TB) exacerbates the challenges associated with managing the disease. Current treatment options are hindered by prolonged duration, adverse effects, and poor adherence. A new short-course oral regimen BPaL, consisting of bedaquiline (B), pretomanid (Pa), and linezolid (L), has proven efficacy in treating MDR-TB and is known as the first regimen approved for this condition (2–4). However, the clinical use of BPaL raises concerns due to cardiotoxicity and hepatotoxicity of bedaquiline (5, 6), as well as hematologic and neurologic toxicity of linezolid (7, 8). Moreover, the emergence of resistance to second-line drugs further complicates individual treatment and TB control programs. To achieve the long-standing goals of shorter and safer treatment regimens with oral administration for both drug-susceptible and drug-resistant TB and to better restrain the emerging threats of drug resistance (9, 10), there is an urgent need for novel drugs and regimens with high efficacy and low toxicity to simplify and shorten TB treatment.
In our previous research, we identified TB47 (T) as a promising candidate drug belonging to the imidazopyridine amide class (11). TB47 exerts its action by inhibiting the cytochrome bc1 oxidase complex, specifically targeting the QcrB subunit (11). This mechanism leads to a reduction in intracellular ATP levels, ultimately hindering the growth of mycobacteria. Moreover, our studies demonstrated a synergistic effect between clofazimine (C) and TB47 against M. tuberculosis, both in vitro and in a murine model (12). This finding emphasizes the advantages of dual or multiple targeting of the electron transport chain for enhanced mycobacteria elimination (13–15). The pre-investigational new drug (pre-IND) application for TB47 (2024000634) was submitted to the Center for Drug Evaluation under the National Medical Products Administration of China on 31 January 2024.
Building upon these insights, we have developed various short-course oral regimens (16) and aim to combine CT with other drugs that target the electron transport chain. We opted to evaluate a novel diarylpyridinated antimycobacterial candidate called sudapyridine (S, previously known as WX-081). Sudapyridine was developed by substituting the bromoquinoline of bedaquiline with a 5-phenylpyridine (17). In vivo animal studies have demonstrated that sudapyridine exhibits a higher concentration in lung tissue, enhanced safety, and a reduced risk of QT interval prolongation compared to bedaquiline (18). Additionally, the drug has shown no adverse effects on heart rate, electrocardiograph morphology, or blood pressure in animal models (18). Following a successful phase II clinical trial (NCT04608955), a phase III clinical trial (NCT05824871) is currently underway.
In this study, we aimed to enhance the overall efficacy of regimens by incorporating linezolid or pyrazinamide (Z) into SCT. We initiated our investigation by employing an in vitro checkerboard method to assess potential synergistic or antagonistic interactions between the compounds sudapyridine, clofazimine, TB47, and linezolid. Moreover, we utilized a well-established BALB/c mouse model of TB to evaluate the anti-TB efficacy of SCT, SCTL, and SCTZ to identify alternative regimens by comparing their effectiveness to that of the BPaL regimen. At the same time, we also tested different drugs and drug combinations to investigate the interactions of sudapyridine, clofazimine, and TB47, as well as the contribution of each drug in different regimens in vivo.
RESULTS
Screening of sudapyridine-resistant mutant strains
To select mutants resistant to sudapyridine, approximately 1 × 108 M. tuberculosis H37Ra per plate was spread on 7H11 agar plates containing a 2 × dilution range of 0.125 to 8 mg/L of sudapyridine. The enumeration of colony-forming units (CFUs) for the titer was conducted in the absence of antibiotics. Seven and six resistant isolates were obtained on plates containing 0.5 and 1 mg/L sudapyridine, respectively. Seven mutants were randomly selected for the minimum inhibitory concentrations (MICs) determination and gene mutation analysis. Plates with other concentrations of sudapyridine either exhibited an excessive number of colonies or no colonies. The mutation frequency of sudapyridine-resistant mutants was approximately 1 × 10−7.
MICs determination and gene mutation analysis
It is well-known that three genes are associated with bedaquiline resistance (19). As shown in Table 1, out of the seven sudapyridine-resistant M. tuberculosis H37Ra isolates, one exhibited a twofold increase in MIC, with a point mutation detected solely in pepQ. The remaining six isolates showed a fourfold increase in MIC: five mutants bore mutations only in Rv0678, and one had codon shift mutations in both Rv0678 and pepQ. None of the tested isolates showed a mutation in atpE.
TABLE 1.
Mutation analysis of nine sudapyridine-resistant M. tuberculosis H37Ra mutants
| Strainsa | Gene/nucleotide change (Amino acid change) | MIC (mg/L) | |||
|---|---|---|---|---|---|
| Rv0678 | pepQ | atpE | Sudapyridine | Bedaquiline | |
| H37Ra | Wtb | Wt | Wt | 0.25 | 0.13 |
| S1-1 | G193 insertion (65 codon shift) | Wt | Wt | 1.00 | 0.50 |
| S1-2 | A117 insertion (39 codon shift) | G829 deletion (277 codon shift) | Wt | 1.00 | 0.50 |
| S1-3 | G193 insertion (65 codon shift) | Wt | Wt | 1.00 | 0.50 |
| S1-4 | C272T (T91I) | Wt | Wt | 1.00 | 0.50 |
| S0.5–1 | Wt | G774T (Q258H) | Wt | 0.50 | 0.25 |
| S0.5–2 | T354 deletion (118 codon shift) | Wt | Wt | 1.00 | 0.50 |
| S0.5–3 | G71T (G24V) | Wt | Wt | 1.00 | 0.50 |
S1-x indicates that strain x is isolated from a 7H11 agar plate containing sudapyridine at a concentration of 1 mg/L. Similarly, S0.5-y indicates that strain y is isolated from a 7H11 plate containing sudapyridine at a concentration of 0.5 mg/L, and so on.
Wt, wild type.
The MICs (mg/L) on solid media to M. tuberculosis H37Rv used for infection were bedaquiline 0.125, sudapyridine 0.25, pretomanid 0.25, and linezolid 1.
Drug interaction profile in vitro
As shown in Table 2, the interactions of sudapyridine/linezolid, sudapyridine/TB47, linezolid/clofazimine, and linezolid/TB47 were considered indifferent, with 1.0 < FICIs (Fractional Inhibitory Concentration Index) ≤4.0. Additionally, the interaction of clofazimine/TB47 was synergistic with FICI = 0.375, as observed in our previous studies (12). It is worth noting that sudapyridine/clofazimine was also synergistic with FICI = 0.375. The checkerboard assay was independently performed three times using the selectable marker-free autoluminescent H37Rv strain (UAlRv) (20), and the results were consistent.
TABLE 2.
In vitro assessment of drug interaction profiles via checkerboard assaya
| Drug A Drug B |
MIC alone | MIC in combination | FICI | Classification |
|---|---|---|---|---|
| Clofazimine | 1 | 0.25 | 0.375 | Synergistic |
| TB47 | 0.00625 | 0.000781 | ||
| Sudapyridine | 0.25 | 0.015625 | 1.0625 | Indifferent |
| Linezolid | 0.5 | 0.5 | ||
| Sudapyridine | 0.25 | 0.03125 | 0.375 | Synergistic |
| Clofazimine | 1 | 0.25 | ||
| Sudapyridine | 0.25 | 0.25 | 1.5 | Indifferent |
| TB47 | 0.00625 | 0.003125 | ||
| Linezolid | 0.5 | 0.125 | 1.25 | Indifferent |
| Clofazimine | 1 | 1 | ||
| Linezolid | 0.5 | 0.5 | 1.125 | Indifferent |
| TB47 | 0.00625 | 0.000781 |
FICI = (MICA in combination)/(MICA alone) + (MICB in combination)/(MICB alone), where MICA alone and MICB alone were the MIC obtained when each drug was tested alone, while MICB in combination and MICB in combination were the concentrations of each compound at the lowest effective combination. The FICI was calculated to classify the interactions as one of five kinds: synergistic (FICI ≤ 0.5), partially synergistic (0.5 < FICI < 1.0), additive (FICI = 1.0), indifferent (1.0 < FICI ≤ 4.0), or antagonistic (FICI > 4.0).
Comparison of bactericidal activity in experiment 1
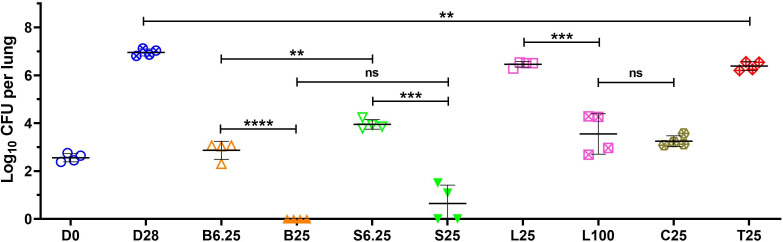
The activities of bedaquiline, sudapyridine, linezolid, clofazimine, and TB47, focusing on the difference between sudapyridine and bedaquiline, were tested in an acute TB mouse model. Treatment initiation commenced 2 days after infection. At the start of treatment (day 0), mean (±SD) lung CFU counts were 2.55 ± 0.17 log10. After 4 weeks of administration, the untreated mice exhibited a rise to 6.95 ± 0.15, indicating the successful establishment of the infection model (Fig. 1). Comparing the monotherapy groups with the untreated control group, CFU counts were lower in all monotherapy groups. The low-dose groups of bedaquiline, sudapyridine, and linezolid demonstrated significantly lower activity compared to the high-dose groups. Specifically, the bedaquiline (6.25 mg/kg) group showed higher activity than the sudapyridine (6.25 mg/kg) group (P < 0.01). In the bedaquiline (25 mg/kg) group, all four mice tested negative, while in the sudapyridine (25 mg/kg) group, two mice were negative, and the lung CFUs of the other two mice were at 1.08 and 1.51 log10, respectively (Fig. 1). This suggests that the in vivo bactericidal activity of bedaquiline and sudapyridine was similar, with bedaquiline exhibiting a little stronger activity. Lung CFU counts of the linezolid (100 mg/kg) group were 3.55 ± 0.85, whereas the linezolid (25 mg/kg) group had a significantly higher count at 6.46 ± 0.12 (P < 0.001). This suggests a potential dose-dependent effect for linezolid, indicating that a higher dose might be necessary in subsequent combination efficacy evaluations. The clofazimine (25 mg/kg) showed similar antitubercular activity to that of linezolid (100 mg/kg; P > 0.05). The activity of TB47, previously reported by us (12), was verified again in this study. TB47 inhibited the proliferation of M. tuberculosis because the lung CFUs in the TB47 group after a 4-week administration period were significantly lower compared to the untreated group (P < 0.01).
Fig 1.
Activities of different drugs with the indicated dose (in mg/kg) against M. tuberculosis in an acute mouse model after 1 month (4 weeks) of treatment in experiment 1. The drug administration began 2 days after infection (D0), and lung CFU counts were determined after continuous administration for 4 weeks (5 days per week). The drugs are abbreviated as follows: B for bedaquiline, S for sudapyridine, L for linezolid, C for clofazimine, and T for TB47. The dosage corresponds to the number behind the drug. Values are means ± SD; n = 4. ns, not significant; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Lung CFU counts during treatment in experiment 2
A high-dose aerosol infection of BALB/c mice was used to evaluate the bactericidal and sterilizing activity of sudapyridine, clofazimine, and TB47 pairwise combinations, as well as multi-drug combination regimens (Table 3). One day after infection of mice with M. tuberculosis H37Rv, mean (± SD) lung CFU counts were 4.09 ± 0.10 log10. CFU counts increased over the following 14 days to 7.58 ± 0.15 when treatment began. The untreated nine mice died of TB infection 21–23 days after infection indicating the strain used was virulent enough. The mice treated with clofazimine increased weakness at the beginning of the administration, characterized by disheveled fur, sluggish movement, and lethargy, resembling the untreated control group. Three of them died within 21–23 days after infection (i.e., within 7–9 days after treatment initiation), while the remaining two survived and progressively regained health. This could be explained by the delayed antimicrobial activity of clofazimine (21). All the other mice survived until the end of the experiment.
TABLE 3.
Lung CFU counts and proportions of mice relapsed after treatment completion in experiment 2e
| Regimena | Mean (±SD) lung log10 CFUb | Proportion of mice with culture-positive lungs after treatmentc | ||||||
|---|---|---|---|---|---|---|---|---|
| D-14 | D0 | M1 | M1.5 | M2 | M1.5 | M2 | M2.5 | |
| Untreated | 4.09 ± 0.10 | 7.58 ± 0.15 | ||||||
| BPaL | 3.43 ± 0.26 | 1.55 ± 0.39 | 0.98 ± 0.61e1 | 10/15 | 4/15 | |||
| S | 4.84 ± 0.34 | |||||||
| C | 5.55 ± 0.04d | |||||||
| T | 7.66 ± 0.39 | |||||||
| SC | 3.62 ± 0.57 | |||||||
| ST | 4.65 ± 0.31 | |||||||
| CT | 4.06 ± 0.43 | |||||||
| SCT | 3.20 ± 0.29 | 1.97 ± 0.15 | 0.71 ± 0.69e2 | 5/15 | 2/15 | |||
| SCTL | 2.78 ± 0.49 | 2.07 ± 0.18 | 0.46 ± 0.65e3 | 15/15 | 7/15 | 0/15 | ||
| SCL | 3.47 ± 0.51 | 2.84 ± 0.35 | 1.83 ± 0.50 | 14/15 | ||||
| STL | 4.55 ± 0.36 | 3.59 ± 0.40 | 2.88 ± 0.47 | 15/15 | 15/15 | |||
| SCZ | 1.97 ± 0.15 | 1.12 ± 0.71e1 | 0.24 ± 0.54e4 | 0/15 | ||||
| SCTZ | 1.99 ± 0.27 | 1.43 ± 0.29 | 0.34 ± 0.49e3 | 1/15 | 0/15 | 0/15 | ||
Abbreviations and doses (mg/kg): B, bedaquiline, 25; Pa, pretomanid, 100; L, linezolid, 100; S, sudapyridine, 25; C, clofazimine, 25; T, TB47, 25; and Z, pyrazinamide, 150. All the drugs were administered orally once a day, 5 days per week.
Time points are shown as days (D-14 or D0) or months (M) of treatment. D-14, 1 day after aerosol infection with M. tuberculosis H37Rv; D0, day of treatment initiation; M1, 1 month (4 weeks) after treatment initiation, and so on.
Upon completing the prescribed course of treatment, 15 mice were designated for relapse assessment at each time point, as indicated by the proportions in the table. The mice were held for 4 additional months after treatment completion.
Only two out of five mice had survived until this time point.
en, N out of 5 mice showed 0 lung CFU. For example, e3 means three out of five mice were culture negative.
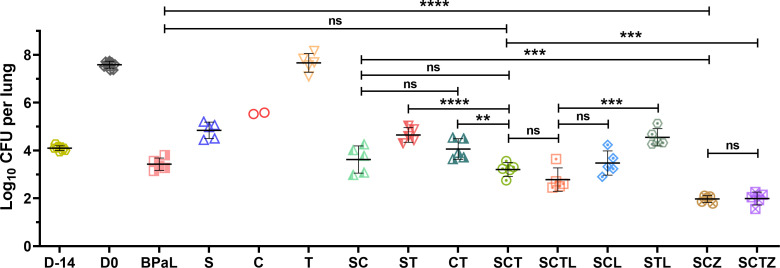
After 1 month of treatment, the single-drug sudapyridine exhibited significant bactericidal activity (P < 0.0001, Table 3; Fig. 2), resulting in a decrease of lung CFU counts from 7.58 ± 0.15 (D0 lung CFU) to 4.84 ± 0.34. Although clofazimine initially demonstrated almost no antibacterial activity, it exhibited bactericidal activity in the two mice that survived after 1 month of treatment. The lung CFU counts for the remaining two mice decreased to 5.55 ± 0.04. Although the TB47 single drug could not effectively lower the CFUs, it did show bacteriostatic activity. All five mice in this group survived well until the end of the experiment, and the CFU counts of M1 were 7.66 ± 0.39, close to the count at the beginning of treatment (Table 3; Fig. 2). Among the two-drug combinations, the SC and CT exhibited combined activity, and the bactericidal effects of these combinations were significantly more potent than those of any single drug. However, the activity of the ST was comparable to that of sudapyridine alone, with no statistical significance (P > 0.05). These results were consistent with the in vitro drug interaction study. Each trial group demonstrated effective bactericidal activity in vivo in combinations involving three or four drugs (Table 3). The bactericidal activity of the SCT was significantly superior to that of the CT (P < 0.01), similar to the BPaL positive control group (P > 0.05). The addition of linezolid slightly enhanced the SCT activity but without statistical significance (P > 0.05). However, upon the removal of clofazimine from SCTL, the activity was significantly reduced (P < 0.001). It is noteworthy that the SCZ and SCTZ exhibited remarkable bactericidal activity in the presence of pyrazinamide, reducing CFU counts to about two logs after only 1 month of administration, surpassing the performance of all other experimental groups.
Fig 2.
Efficacy of different drugs or regimens against M. tuberculosis in an active TB disease model after 1 month (4 weeks) of treatment in experiment 2. Nine mice (three from each infection run) were humanely sacrificed one day after infection (D-14) and on the day of treatment initiation (D0) to assess lung CFU counts, respectively. The experimental group of mice received either monotherapy, dual medication, or a regimen for 1 month before CFU detection at sacrifice. Abbreviations and doses (mg/kg): B, bedaquiline, 25; Pa, pretomanid, 100; L, linezolid, 100; S, sudapyridine, 25; C, clofazimine, 25; T, TB47, 25; and Z, pyrazinamide, 150. All the drugs were administered orally once a day, 5 days per week. Values are means ± SD; n = 5. ns, not significant; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
After 1.5 months of treatment, lung CFU counts in BPaL decreased to 1.55 ± 0.39. The CFU counts of SCT and SCTL were similar, both around two logs (Table 3). Conversely, the CFU counts in the SCL and STL were significantly higher than those in the SCT (P < 0.001). Notably, the SCZ and SCTZ groups exhibited continuously robust bactericidal activity, with lung CFU decreasing further. Remarkably, after just 1.5 months of treatment, one mouse lung in the SCZ group even showed 0 CFU.
After 2 months of treatment, the lung homogenate culture of some mice turned negative. This included one mouse in the BPaL, two mice in the SCT, three mice in the SCTL, four mice in the SCZ, and three mice in the SCTZ. The combined bactericidal activity of the SCZ and SCTZ surpassed that of the positive control, and their activities were consistently close at each time point. Interestingly, the contribution of TB47 to the SCTZ combination was not evident. While the bactericidal activity of the three drugs in the SCT was slightly lower than that of SCZ, no statistical significance was reached (P > 0.05), and it remained similar to BPaL (P > 0.05). However, linezolid, despite its potent single-drug activity, did not exhibit a contribution to the SCTL combination as SCTL showed no difference or only a little better than SCT, and the bactericidal activities of either SCL or STL were much lower than that of SCT. In other words, if removing either clofazimine or TB47 from SCTL, the regimens could become much worse (P < 0.001 and P < 0.01). However, if removing linezolid, the regimen became only a little weaker (P > 0.05). There was no significant difference in activity between the SCL and the SCT after 1 month of administration. However, with the progression of the administration period, a significant disparity in activity emerged between the two groups after 2 months (P < 0.05). Notably, all mice in the SCL tested positive. This observation once again confirmed that TB47 can substantially enhance the bactericidal activity of clofazimine.
Relapse rates after treatment completion in experiment 2
Significant differences in sterilizing activities were observed among the different regimens. As shown in Table 3 and Table S4, only 1/15 mice relapsed in the SCTZ only after 1.5 months of treatment, which is even much fewer than that (10/15) in the BPaL (P < 0.01) treated for 2 months. After 2 months of treatment, no mice relapsed in the SCTZ and SCZ groups, which indicated that both of them are more sterilizing than BPaL (P < 0.01). It was a missed opportunity that we previously underestimated the efficacy of SCZ and did not set other time points for relapse detection. There was no significant difference among relapse rates of the following regimens BPaL (10/15), SCTL (5/15), and SCT (7/15; P > 0.05 for any pairwise comparison). The sterilization activity of STL was obviously poorer, with no mice exhibiting negative culture (P < 0.001, P < 0.01, P < 0.0001, and P < 0.0001 for STL vs SCT, STL vs SCTL, STL vs SCZ, and STL vs SCTZ, respectively). After 2.5 months of treatment, no relapses were observed once more in the most powerful SCTZ group. The SCTL and SCT groups showed 0/15 and 2/15 mice relapsed respectively, which showed slightly better sterilizing activities than that 4/15 of BPaL against (P > 0.05 and P > 0.05, for BPaL vs SCTL and BPaL vs SCT), although the differences were not statistically significant (Table 3). The relapse rates of BPaL found in this study were comparable with those found in a recent study published by the Nuermberger group (22). In that study, 1 out of 15 mice still relapsed even after 3 months of BPaL treatment.
DISCUSSION
The BPaL regimen, approved by the US Food and Drug Administration (FDA) and recommended by the World Health Organization (WHO) for the clinical treatment of MDR and extensively drug-resistant TB (XDR-TB) (2, 3), derives from the bactericidal and sterilization activity observed in a well-established mouse model of TB (23). Despite this, the emergence of resistance and prevalence of adverse drug reactions emphasizes the value of ongoing optimization of BPaL.
Drugs that target the electron transport chain complexes exhibit promising potential (13, 24). Sudapyridine, a structural analog of bedaquiline, demonstrates similar efficacy and improved safety profiles (18, 25). The mechanisms of action and resistance for sudapyridine have not been previously reported. This study revealed that sudapyridine-resistant M. tuberculosis mutants containing the same mutations in Rv0678 or pepQ as those reported in bedaquiline-resistant M. tuberculosis mutants exhibit cross-resistance, indicating a shared resistance mechanism in M. tuberculosis. Spontaneous-resistant mutants of M. smegmatis have been reported to display cross-resistance to sudapyridine and bedaquiline (17). However, we did not identify any sudapyridine-resistant M. tuberculosis mutants with atpE mutations or other significant mutations, leaving the question of whether sudapyridine and bedaquiline share the same mechanism of action unresolved. Additionally, there are currently no reports on the evaluation of sudapyridine in combination with other drugs, particularly those targeting the electron transport chain.
Previously, we identified a synergistic effect against actively growing M. tuberculosis between clofazimine and TB47, creating a potent combination. Furthermore, CT- and CTZ-containing regimens demonstrated excellent bactericidal and sterilizing activities (12, 16). Our conjecture revolves around delineating the conceivable mechanism underpinning this synergistic effect. TB47 expeditiously reroutes electron flux from the mycobacterial cytochrome bc1:aa3 complex to cytochrome bd, thereby diminishing the efficiency of proton motive force and hence ATP production (11). This process may increase carbon catabolism and tricarboxylic acid cycle activity. Consequently, this may enhance the ability of clofazimine to transfer electrons from NDH-2 to oxygen, increasing the production of reactive oxygen and oxidative stress (13, 26). It is interesting that the synergistic effects against nonreplicating M. tuberculosis in both glycerol-enriched and cholesterol-enriched media under low oxygen conditions were detected in a recent study (27). However, additional experiments are required to clarify the precise mechanism. In the current study, we uncovered a synergistic interaction between sudapyridine and clofazimine in vitro using a checkerboard assay (Table 2). We propose that incorporating sudapyridine to CT may augment the efficacy of the CT-containing regimens.
To enhance the precision of evaluating drugs and regimens activities, we employed low- and high-dose infection models to assess the activities of single drugs and combinations, respectively. The primary distinction between the two infection models was the control of bacterial fluid concentration at the time of infection and the regulation of the time interval between infection and treatment. This was crucial to ensure that the mouse lung CFU fell within the anticipated range when treatment commenced. Here, we demonstrated that sudapyridine, clofazimine, and TB47 in all two-drug combination regimens exhibited greater bacterial kill than any of the drugs alone, with the exception of ST, which showed comparable efficacy to sudapyridine alone (P > 0.05, Table 3; Fig. 2). According to the results, the contribution of sudapyridine and clofazimine in the SCT regimen was significant, compared with CT and ST (P < 0.01 and P < 0.0001). The difference between SC and SCT was not statistically significant. Despite the limitations imposed by the toxicity of linezolid (7, 8), its antituberculosis activity remains acknowledged. Our study also demonstrated the efficacy of linezolid as a single treatment in our study (Fig. 1). However, the addition of linezolid to the SCT regimen did not show an enhanced efficacy in combination activity at each time point and in relapse outcomes. Interestingly, although the monotherapy efficacy of TB47 was notably lower than that of linezolid (Fig. 1), SCL showed weaker bactericidal activities at each time point (P < 0.0001) and weaker sterilization activity at 2 months compared to SCT (P < 0.01, Table 3). The addition of pyrazinamide to SC has an incredible effect, with no relapses in 2 months. Pyrazinamide, as a first-line drug with an unclear mechanism, also plays a crucial role in multiple regimens for the treatment of TB based on its potent anti-TB activity (28). Based on our current findings, the contribution of TB47 to SCZ cannot be proved. Further investigation is required to determine whether the effect of TB47 is counteracted or masked by other factors. Interestingly, there were still some mice that showed positive lung culture after completion of 2 months of treatment of SCZ or SCTZ (Table 3); however, the relapse rates of these two groups at this time point were both 0%. This could be because the half-lives of clofazimine and sudapyridine are long, and they have bactericidal activities especially in combination, as they could continue to eradicate bacteria even after treatment stopped. A similar phenomenon was observed in a study testing the potent and long half-life rifapentine in a latent TB chemotherapy mouse model (29).
This study has shown that the efficacy of SCZ is superior to that of BPaL, while the SCT exhibits a comparable effectiveness. These two therapeutic strategies present viable options for mitigating the toxicity issues associated with bedaquiline and linezolid. Nevertheless, certain limitations persist. First, sudapyridine remains in the phase III clinical trials (NCT05824871) which needs to be proved further. Second, TB47 is currently in the pre-IND stage (2024000634), and its bacteriostatic activity necessitates its use in conjunction with other bactericidal drugs. Third, despite its efficacy as a second-line drug, clofazimine’s propensity to cause skin pigmentation continues to limit its application (30). Fourth, the mutants screened in vitro in our study, which harbor gene mutations especially in Rv0678 (six out of seven) causing cross resistant to bedaquiline and sudapyridine, are likely to be resistant to clofazimine as well (30). We acknowledge that the emergence of mutations conferring cross-resistance to clofazimine and sudapyridine during treatment could significantly diminish the efficacy, or even lead to the failure, of regimens based on the combination of sudapyridine and clofazimine. Drugs that could prevent the emergence of such mutants and the rapid diagnosis of such preexisting mutants are highly needed. Finally, vigilance toward pyrazinamide is warranted due to the increasing resistance rates among MDR-TB cases in recent years (31, 32). For example, a recent study from southern China reported a resistance rate of up to 60.04% in MDR/XDR-TB cases (33). It is noteworthy that the increase in alanine aminotransferase enzyme levels is primarily associated with the toxic effects of pyrazinamide during the treatment of MDR-TB patients (34). The WHO endorsed operational research to assess the efficacy of modified short-treatment regimens for rifampin-resistant TB, aiming to guide national programmatic implementation. Notably, a clinical trial in Vietnam, which employed a five-drug combination including bedaquiline, clofazimine, and pyrazinamide, demonstrated that 85% of TB patients achieved culture conversion within 2 months of treatment (34). It is compelling to investigate whether patients infected with M. tuberculosis susceptible to all three drugs exhibit a higher cure rate in such studies.
This study has several limitations. First, only M. tuberculosis H37Rv and BALB/c mice were used in the in vivo activity evaluation experiments. For future investigations, if conditions permit, it is advisable to include drug-resistant strains, particularly rifampin-resistant M. tuberculosis, alongside the C3HeB/FeJ mouse model, in which the strain capable of developing caseous necrotic lesions. Second, M. tuberculosis colonies isolated from the lungs of relapsed mice were not collected for drug susceptibility testing. Third, we did not assess the role of sudapyridine in different regimens nor compare its efficacy with bedaquiline. Finally, due to the complexity involved in multi-drug combinations, we have yet to unravel the mechanism behind the synergistic bactericidal effect that necessitates further research.
In conclusion, our findings ranked the tested regimens according to their sterilizing activities as SCTZ = SCZ > SCTL = SCT ≥ BPaL and demonstrated that the potential of novel drug combinations containing SCT with linezolid or pyrazinamide, providing a new foundation for universally effective short-course drug-resistant TB treatment regimens. The inclusion of pyrazinamide is anticipated to further shorten the treatment if isolates remain susceptible to pyrazinamide. Although the treatment-shortening potential observed in mice cannot be directly extrapolated to human TB cases, but the results of this study provide support and a basis for further clinical trials.
MATERIALS AND METHODS
Mycobacterial strains and growth conditions
The mouse-passaged M. tuberculosis H37Rv strain (ATCC 27294) preserved at −80°C was subcultured in Middlebrook 7H9 broth (Difco, Detroit, MI, USA) supplemented with 10% oleic-acid-albumin-dextrose-catalase (OADC; BD, Sparks, MD, USA), 0.2% glycerol, and 0.05% Tween 80 at 37°C. Broth cultures of H37Rv at the logarithmic phase with OD600 0.8–1.0 were used. MICs (mg/L) on solid media to M. tuberculosis H37Rv used were rifampin 0.25, isoniazid 0.1, clofazimine 0.25, and TB47 0.003 on 7H11 agar (Difco) and pyrazinamide 10 on Löwenstein-Jensen medium (pH 5.5), which were previously described (12). The attenuated M. tuberculosis H37Ra strain (ATCC 25177) was used for the screening of sudapyridine-resistant spontaneous mutant strains and the subsequent determination of MICs. The selectable marker-free autoluminescent H37Rv strain (UAlRv), previously developed and characterized in our laboratory (20), was utilized in a checkerboard assay. The preservation and growth conditions for H37Ra and UAlRv were consistent with those outlined for H37Rv, as mentioned above.
Antimicrobials
Bedaquiline, pretomanid, and linezolid were purchased from Biochempartner (Shanghai, China). Clofazimine and pyrazinamide were purchased from Meilun Bio (Dalian, China). Sudapyridine was provided by Shanghai Jiatan Pharmatech Co., Ltd (Shanghai, China) with a purity of ≥98%. TB47 was synthesized and supplied by Boji Ltd (Guangzhou, China) with a purity of ≥98%. Dexamethasone was purchased from Sigma-Aldrich (St. Louis, MO, USA). For the in vitro study, water-insoluble drugs, including clofazimine, sudapyridine, TB47, and linezolid, were dissolved in dimethylsulfoxide. The drug configuration for the mouse model is elaborated in detail in the chemotherapy section below.
Sudapyridine-resistant mutant selection
In brief, M. tuberculosis H37Ra was cultured to the logarithmic growth stage with a concentration adjusted to approximately 1 × 108 CFU/mL. Subsequently, 0.5 mL of the culture was spread on 7H11 agar plates containing various concentrations of sudapyridine (0.125, 0.25, 0.5, 1, 2, 4, and 8 mg/L), followed by incubation at 37°C for 4–5 weeks. The mutant strains were isolated from individual colonies on each petri dish and then expanded for MICs determination, as well as for DNA extraction.
MICs determination
MICs of bedaquiline and sudapyridine to M. tuberculosis H37Ra and the derived sudapyridine-resistant mutants were determined with the microplate alamar blue assay as described in our recent study (35). Briefly, a 0.1 mL bacterial suspension with an inoculum of 2 × 105 CFU/mL was added to microplates, along with varying concentrations of the test compounds. After a 6-day incubation at 37°C, 0.02 mL alamar blue and 0.0125 mL 20% Tween 80 were introduced into the wells, and the plates were further incubated for 24 hours. The MICs were defined as the lowest concentration of the compounds that prevented a color change from blue to pink, indicating inhibition of microbial growth.
The MICs to M. tuberculosis H37Rv used for animal infection in this study were determined using 7H11 agar similarly described in the previous study (36). Serial dilutions of drugs were prepared and added to Middlebrook 7H11 agar with 10% OADC. M. tuberculosis H37Rv was grown to an optical density at 600 nm (OD600) of 0.3–0.7, and a series of 10-fold dilutions were plated. Plates were inoculated with 0.5 mL aliquots and incubated at 37°C for 4 weeks before colony counting. The MIC was defined as the lowest drug concentration that inhibited at least 99% of the bacterial growth observed for drug-free control plates. Each assay was performed in duplicate.
MICs determined by different methods to different strains (autoluminescent or not) even to the same M. tuberculosis can be different.
PCR and DNA sequencing
M. tuberculosis genomic DNA was extracted using the genomic DNA purification kit (Mei5 bio, Beijing, China) according to the manufacturer’s protocol. Afterward, the bedaquiline resistance-associated genes (Rv0678, pepQ, and atpE) were subjected to PCR amplification using wild-type H37Ra and spontaneously isolated sudapyridine-resistant strains as templates. The primers used in this study are listed in Table S1. The PCR products were purified and sequenced at Sangon Biotech Co., Ltd (Shanghai, China).
Checkerboard assay
A checkerboard assay was used to assess the interaction profiles of drugs in vitro, following a previously outlined method (12). FICI was calculated using the formula: (MICA in combination)/(MICA alone) + (MICB in combination)/(MICB alone). Here, MICA alone and MICB alone represent the MIC of each drug tested individually, while MICA in combination and MICB in combination denoted the concentrations of each compound at the lowest effective combination. The FICI values were categorized as follows for interaction classification: synergistic (FICI ≤ 0.5), partially synergistic (0.5 < FICI < 1.0), additive (FICI = 1.0), indifferent (1.0 < FICI ≤ 4.0), and antagonistic (FICI > 4.0) (12).
Aerosol infection mouse model
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Guangzhou Institutes of Biomedicine and Health (N2022081). Five to six weeks old female BALB/c mice were purchased from Zhejiang Vital River Laboratory Animal Technology Co., Ltd (Jiaxing, China). Following several days of acclimatization, mice were infected with M. tuberculosis H37Rv by an inhalation exposure system (Glas-Col, Terre Haute, IN).
For experiment 1, 40 mice were infected with a low dose of M. tuberculosis and randomly grouped, with four mice per group (Table S2). Four mice were sacrificed 2 days after infection (D0) to determine the CFU counts of bacteria implanted in the lungs at the onset of treatment. Following this, mice from each group were sacrificed to assess the bactericidal activity of each drug.
For experiment 2, 372 mice were infected with a high dose of M. tuberculosis, divided into three runs. Before the commencement of treatment, mice were block-randomized into treatment groups and timed sacrifice cohorts (Table S3). Nine mice (three mice from each run) were humanely sacrificed 1 day after infection (D-14) and on the day of treatment initiation (D0) to determine CFU counts implanted in the lungs at both time points, respectively. The in vivo bactericidal activities of the regimens were measured by lung CFU counts at various time points as indicated (Table S3).
Chemotherapy
Bedaquiline and sudapyridine were formulated in 20% hydroxypropyl-β-cyclodextrin solution acidified with 1.5% 1 mol/L HCl. Pretomanid was suspended in a cyclodextrin micelle (CM-2) formulation containing 10% hydroxypropyl—cyclodextrin (Sigma, St.Louis, MO, USA) and 10% lecithin (ICN Pharmaceuticals Inc., OH, USA) (37). Linezolid, clofazimine, and TB47 were suspended in 0.5% sodium carboxymethyl cellulose (Solarbio, Beijing, China). Pyrazinamide was dissolved in distilled water. Bedaquiline and pretomanid were prepared separately and administered together after mixing just before administration. Sudapyridine and pyrazinamide were individually prepared and administered. Clofazimine and TB47 were prepared collectively and administered at least 1 hour subsequent to the administration of other drugs. In regimens containing linezolid, it was administered last, with a 4-hour interval to minimize potential interference with the absorption of accompanying agents. All drugs were prepared to deliver the specified doses in a total volume of 0.2 mL per gavage, based on the average mouse body mass of 20 g. The drug solutions and suspensions were prepared weekly and stored at 4°C. Treatment was initiated either 2 days (experiment 1) or 14 days (experiment 2) after infection. In experiment 1, bedaquiline and sudapyridine were administered at doses of 6.25 or 25 mg/kg. Linezolid was administered at doses of either 25 or 100 mg/kg. Both clofazimine and TB47 were administered at 25 mg/kg. The loading dose of sudapyridine is 450 mg per day, which is equivalent to approximately 25 mg/kg in mice. In experiment 2, the daily doses (in mg/kg) were as follows: bedaquiline, 25; pretomanid, 100; linezolid, 100; sudapyridine, 25; clofazimine, 25; TB47, 25; and pyrazinamide, 150. Treatment was orally administered by gavage 5 days per week.
Assessment of treatment efficacy
Assessed the efficacy by CFU counts at specific treatment intervals, providing insights into bactericidal activity. Additionally, the proportion of mice experiencing culture-positive relapse post-treatment served as an indicator of sterilizing activity. Mouse lungs were dissected, stored in 2.0 mL sterile phosphate buffered saline (PBS), and homogenized in glass tissue grinders. Both undiluted lung homogenate and 10-fold serial dilutions were cultured and spread with 0.5 mL per 7H11 agar plate. To mitigate the potential impact of clofazimine carryover on M. tuberculosis growth, lung homogenates from clofazimine-containing groups underwent plating on 7H11 agar supplemented with 0.4% (weight/volume) activated charcoal to absorb residual clofazimine (38). CFU counting was performed following a 4–5 week incubation at 37°C. Relapses were evaluated 4 months post-treatment cessation, involving two immunodepression phases (administering 10 mg/kg dexamethasone dissolved in PBS subcutaneously once a week during the final 2 weeks) (16). The entire lung homogenate was evenly distributed across four plates for culturing. If ≥1 colony appeared, the mouse was considered as relapse.
Statistical analysis
CFU counts (x) were log-transformed as log10 (x + 1) before performing the statistical analysis. Comparisons of CFU means between different groups were carried out by one-way analysis of variance with Dunnett’s post-test to correct for multiple comparisons. Differences in relapse proportions were assessed by Fisher’s Exact test using the Holm-Bonferroni correction for multiple comparisons. Differences were considered to be statistically significant for a P value of <0.05. All statistical analyses and visual representations were performed using GraphPad Prism version 8.0.2 (GraphPad Software, La Jolla, CA, USA).
ACKNOWLEDGMENTS
This study was supported by the National Key R&D Program of China (2021YFA1300900), the National Natural Science Foundation of China (21920102003, 82304575, and 32300152), Guangdong Provincial Basic and Applied Basic Research Fund (2022A1515110883 and 2022 A1515110505), China Postdoctoral Science Foundation (2022M723164), Guangzhou Science and Technology Plan-Youth Doctoral "Sail" Project (2024A04J4273), Chinese Academy of Sciences (CAS), President’s International Fellowship Initiative (2023VBC0015), National Foreign Expert Project - Foreign Young Talents Program (QN2022031002L), and the State Key Lab of Respiratory Disease, Guangzhou Institute of Respiratory Diseases, First Affiliated Hospital of Guangzhou Medical University (grants SKLRD-Z-202301, SKLRD-OP-202324, SKLRD-Z-202414, and SKLRD-Z-202412).
Contributor Information
Tianyu Zhang, Email: zhang_tianyu@gibh.ac.cn.
Sean Wasserman, St. George's, University of London, London, United Kingdom.
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aac.00124-24.
Tables S1 to S4.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. World Health Organization . 2023. Global tuberculosis report 2023. World Health Organization. Geneva, Switzerland. [Google Scholar]
- 2. Conradie F, Bagdasaryan TR, Borisov S, Howell P, Mikiashvili L, Ngubane N, Samoilova A, Skornykova S, Tudor E, Variava E, et al. 2022. Bedaquiline-pretomanid-linezolid regimens for drug-resistant tuberculosis. N Engl J Med 387:810–823. doi: 10.1056/NEJMoa2119430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burki T. 2019. BPaL approved for multidrug-resistant tuberculosis. Lancet Infect Dis 19:1063–1064. doi: 10.1016/S1473-3099(19)30489-X [DOI] [PubMed] [Google Scholar]
- 4. Collaborative Group for the Meta-Analysis of Individual Patient Data in MDR-TB treatment–2017, Ahmad N, Ahuja SD, Akkerman OW, Alffenaar J-WC, Anderson LF, Baghaei P, Bang D, Barry PM, Bastos ML, et al. 2018. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet 392:821–834. doi: 10.1016/S0140-6736(18)31644-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gao M, Gao J, Xie L, Wu G, Chen W, Chen Y, Pei Y, Li G, Liu Y, Shu W, Fan L, Wu Q, Du J, Chen X, Tang P, Xiong Y, Li M, Cai Q, Jin L, Mei Z, Pang Y, Li L. 2021. Early outcome and safety of bedaquiline-containing regimens for treatment of MDR- and XDR-TB in China: a multicentre study. Clin Microbiol Infect 27:597–602. doi: 10.1016/j.cmi.2020.06.004 [DOI] [PubMed] [Google Scholar]
- 6. Jones J, Mudaly V, Voget J, Naledi T, Maartens G, Cohen K. 2019. Adverse drug reactions in South African patients receiving bedaquiline-containing tuberculosis treatment: an evaluation of spontaneously reported cases. BMC Infect Dis 19:544. doi: 10.1186/s12879-019-4197-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hashemian SMR, Farhadi T, Ganjparvar M. 2018. Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther 12:1759–1767. doi: 10.2147/DDDT.S164515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thirot H, Briquet C, Frippiat F, Jacobs F, Holemans X, Henrard S, Tulkens PM, Spinewine A, Van Bambeke F. 2021. Clinical use and adverse drug reactions of linezolid: a retrospective study in four Belgian hospital centers. Antibiotics (Basel) 10:530. doi: 10.3390/antibiotics10050530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chesov E, Chesov D, Maurer FP, Andres S, Utpatel C, Barilar I, Donica A, Reimann M, Niemann S, Lange C, Crudu V, Heyckendorf J, Merker M. 2022. Emergence of bedaquiline resistance in a high tuberculosis burden country. Eur Respir J 59:2100621. doi: 10.1183/13993003.00621-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen TVA, Anthony RM, Bañuls A-L, Nguyen TVA, Vu DH, Alffenaar J-WC. 2018. Bedaquiline resistance: its emergence, mechanism, and prevention. Clin Infect Dis 66:1625–1630. doi: 10.1093/cid/cix992 [DOI] [PubMed] [Google Scholar]
- 11. Liu Y, Gao Y, Liu J, Tan Y, Liu Z, Chhotaray C, Jiang H, Lu Z, Chiwala G, Wang S, Makafe G, Islam MM, Hameed HMA, Cai X, Wang C, Li X, Tan S, Zhang T. 2019. The compound TB47 is highly bactericidal against Mycobacterium ulcerans in a buruli ulcer mouse model. Nat Commun 10:524. doi: 10.1038/s41467-019-08464-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu W, Chiwala G, Gao Y, Liu Z, Sapkota S, Lu Z, Guo L, Khan SA, Zhong N, Zhang T. 2020. TB47 and clofazimine form a highly synergistic sterilizing block in a second-line regimen for tuberculosis in mice. Biomed Pharmacother 131:110782. doi: 10.1016/j.biopha.2020.110782 [DOI] [PubMed] [Google Scholar]
- 13. Lamprecht DA, Finin PM, Rahman MdA, Cumming BM, Russell SL, Jonnala SR, Adamson JH, Steyn AJC. 2016. Turning the respiratory flexibility of Mycobacterium tuberculosis against itself. Nat Commun 7:12393. doi: 10.1038/ncomms12393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee BS, Hards K, Engelhart CA, Hasenoehrl EJ, Kalia NP, Mackenzie JS, Sviriaeva E, Chong SMS, Manimekalai MSS, Koh VH, Chan J, Xu J, Alonso S, Miller MJ, Steyn AJC, Grüber G, Schnappinger D, Berney M, Cook GM, Moraski GC, Pethe K. 2021. Dual inhibition of the terminal oxidases eradicates antibiotic-tolerant Mycobacterium tuberculosis. EMBO Mol Med 13:e13207. doi: 10.15252/emmm.202013207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berube BJ, Parish T. 2018. Combinations of respiratory chain inhibitors have enhanced bactericidal activity against Mycobacterium tuberculosis. Antimicrob Agents Chemother 62:e01677-17. doi: 10.1128/AAC.01677-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu W, Yusuf B, Wang S, Tian X, Hameed HMA, Lu Z, Chiwala G, Alam MS, Cook GM, Maslov DA, Zhong N, Zhang T. 2021. Sterilizing effects of novel regimens containing TB47, clofazimine, and linezolid in a murine model of tuberculosis. Antimicrob Agents Chemother 65:e0070621. doi: 10.1128/AAC.00706-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang Z, Luo W, Xu D, Guo F, Yang M, Zhu Y, Shen L, Chen S, Tang D, Li L, Li Y, Wang B, Franzblau SG, Ding CZ. 2022. Discovery and preclinical profile of sudapyridine (WX-081), a novel anti-tuberculosis agent. Bioorg Med Chem Lett 71:128824. doi: 10.1016/j.bmcl.2022.128824 [DOI] [PubMed] [Google Scholar]
- 18. Yao R, Wang B, Fu L, Li L, You K, Li Y-G, Lu Y. 2022. Sudapyridine (WX-081), a novel compound against Mycobacterium tuberculosis. Microbiol Spectr 10:e0247721. doi: 10.1128/spectrum.02477-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hameed HMA, Islam MM, Chhotaray C, Wang C, Liu Y, Tan Y, Li X, Tan S, Delorme V, Yew WW, Liu J, Zhang T. 2018. Molecular targets related drug resistance mechanisms in MDR-, XDR-, and TDR-Mycobacterium tuberculosis strains. Front Cell Infect Microbiol 8:114. doi: 10.3389/fcimb.2018.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang F, Njire MM, Liu J, Wu T, Wang B, Liu T, Cao Y, Liu Z, Wan J, Tu Z, Tan Y, Tan S, Zhang T. 2015. Engineering more stable, selectable marker-free autoluminescent mycobacteria by one step. PLoS One 10:e0119341. doi: 10.1371/journal.pone.0119341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ammerman NC, Swanson RV, Tapley A, Moodley C, Ngcobo B, Adamson J, Dorasamy A, Moodley S, Mgaga Z, Bester LA, Singh SD, Almeida DV, Grosset JH. 2017. Clofazimine has delayed antimicrobial activity against Mycobacterium tuberculosis both in vitro and in vivo . J Antimicrob Chemother 72:455–461. doi: 10.1093/jac/dkw417 [DOI] [PubMed] [Google Scholar]
- 22. Nuermberger EL, Martínez-Martínez MS, Sanz O, Urones B, Esquivias J, Soni H, Tasneen R, Tyagi S, Li S-Y, Converse PJ, et al. 2022. GSK2556286 is a novel antitubercular drug candidate effective in vivo with the potential to shorten tuberculosis treatment. Antimicrob Agents Chemother 66:e0013222. doi: 10.1128/aac.00132-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tasneen R, Betoudji F, Tyagi S, Li SY, Williams K, Converse PJ, Dartois V, Yang T, Mendel CM, Mdluli KE, Nuermberger EL. 2016. Contribution of oxazolidinones to the efficacy of novel regimens containing bedaquiline and pretomanid in a mouse model of tuberculosis. Antimicrob Agents Chemother 60:270–277. doi: 10.1128/AAC.01691-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harikishore A, Mathiyazakan V, Pethe K, Grüber G. 2023. Novel targets and inhibitors of the Mycobacterium tuberculosis cytochrome bd oxidase to foster anti-tuberculosis drug discovery. Expert Opin Drug Discov 18:917–927. doi: 10.1080/17460441.2023.2224553 [DOI] [PubMed] [Google Scholar]
- 25. Xiao H, Yu X, Shang Y, Ren R, Xue Y, Dong L, Zhao L, Jiang G, Huang H. 2023. In vitro and intracellular antibacterial activity of sudapyridine (WX-081) against tuberculosis. Infect Drug Resist 16:217–224. doi: 10.2147/IDR.S390187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yano T, Kassovska-Bratinova S, Teh JS, Winkler J, Sullivan K, Isaacs A, Schechter NM, Rubin H. 2011. Reduction of clofazimine by mycobacterial type 2 NADH: quinone oxidoreductase: a pathway for the generation of bactericidal levels of reactive oxygen species. J Biol Chem 286:10276–10287. doi: 10.1074/jbc.M110.200501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tian X, Ma W, Yusuf B, Li C, Hameed HMA, Wang X, Zhong N, Hu J, Zhang T. 2024. High-throughput screening of compounds against autoluminescent nonreplicating Mycobacterium tuberculosis under diverse conditions. bioRxiv. doi: 10.1101/2024.03.10.584296 [DOI]
- 28. Murray JF, Schraufnagel DE, Hopewell PC. 2015. Treatment of tuberculosis. a historical perspective. Ann Am Thorac Soc 12:1749–1759. doi: 10.1513/AnnalsATS.201509-632PS [DOI] [PubMed] [Google Scholar]
- 29. Zhang T, Zhang M, Rosenthal IM, Grosset JH, Nuermberger EL. 2009. Short-course therapy with daily rifapentine in a murine model of latent tuberculosis infection. Am J Respir Crit Care Med 180:1151–1157. doi: 10.1164/rccm.200905-0795OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stadler JAM, Maartens G, Meintjes G, Wasserman S. 2023. Clofazimine for the treatment of tuberculosis. Front Pharmacol 14:1100488. doi: 10.3389/fphar.2023.1100488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Juma SP, Maro A, Pholwat S, Mpagama SG, Gratz J, Liyoyo A, Houpt ER, Kibiki GS, Mmbaga BT, Heysell SK. 2019. Underestimated pyrazinamide resistance may compromise outcomes of pyrazinamide containing regimens for treatment of drug susceptible and multi-drug-resistant tuberculosis in Tanzania. BMC Infect Dis 19:129. doi: 10.1186/s12879-019-3757-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang Z, Tang Z, Heidari H, Molaeipour L, Ghanavati R, Kazemian H, Koohsar F, Kouhsari E. 2023. Global status of phenotypic pyrazinamide resistance in Mycobacterium tuberculosis clinical isolates: an updated systematic review and meta-analysis. J Chemother 35:583–595. doi: 10.1080/1120009X.2023.2214473 [DOI] [PubMed] [Google Scholar]
- 33. Hameed HA, Tan Y, Islam MM, Lu Z, Chhotaray C, Wang S, Liu Z, Fang C, Tan S, Yew WW, Zhong N, Liu J, Zhang T. 2020. Detection of novel gene mutations associated with pyrazinamide resistance in multidrug-resistant Mycobacterium tuberculosis clinical isolates in Southern China. Infect Drug Resist 13:217–227. doi: 10.2147/IDR.S230774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maia MV, Cunha M da GS, Cunha CS. 2013. Adverse effects of alternative therapy (minocycline, ofloxacin, and clofazimine) in multibacillary leprosy patients in a recognized health care unit in Manaus, Amazonas, Brazil. An Bras Dermatol 88:205–210. doi: 10.1590/S0365-05962013000200003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li C, Tian X, Huang Z, Gou X, Yusuf B, Li C, Gao Y, Liu S, Wang Y, Yang T, Liu Z, Sun Q, Zhang T, Luo Y. 2023. Structure-activity relationship of novel pyrimidine derivatives with potent inhibitory activities against Mycobacterium tuberculosis. J Med Chem 66:2699–2716. doi: 10.1021/acs.jmedchem.2c01647 [DOI] [PubMed] [Google Scholar]
- 36. Zhang T, Bishai WR, Grosset JH, Nuermberger EL. 2010. Rapid assessment of antibacterial activity against Mycobacterium ulcerans by using recombinant luminescent strains. Antimicrob Agents Chemother 54:2806–2813. doi: 10.1128/AAC.00400-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tyagi S, Nuermberger E, Yoshimatsu T, Williams K, Rosenthal I, Lounis N, Bishai W, Grosset J. 2005. Bactericidal activity of the nitroimidazopyran PA-824 in a murine model of tuberculosis. Antimicrob Agents Chemother 49:2289–2293. doi: 10.1128/AAC.49.6.2289-2293.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tyagi S, Ammerman NC, Li SY, Adamson J, Converse PJ, Swanson RV, Almeida DV, Grosset JH. 2015. Clofazimine shortens the duration of the first-line treatment regimen for experimental chemotherapy of tuberculosis. Proc Natl Acad Sci U S A 112:869–874. doi: 10.1073/pnas.1416951112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S4.