Abstract
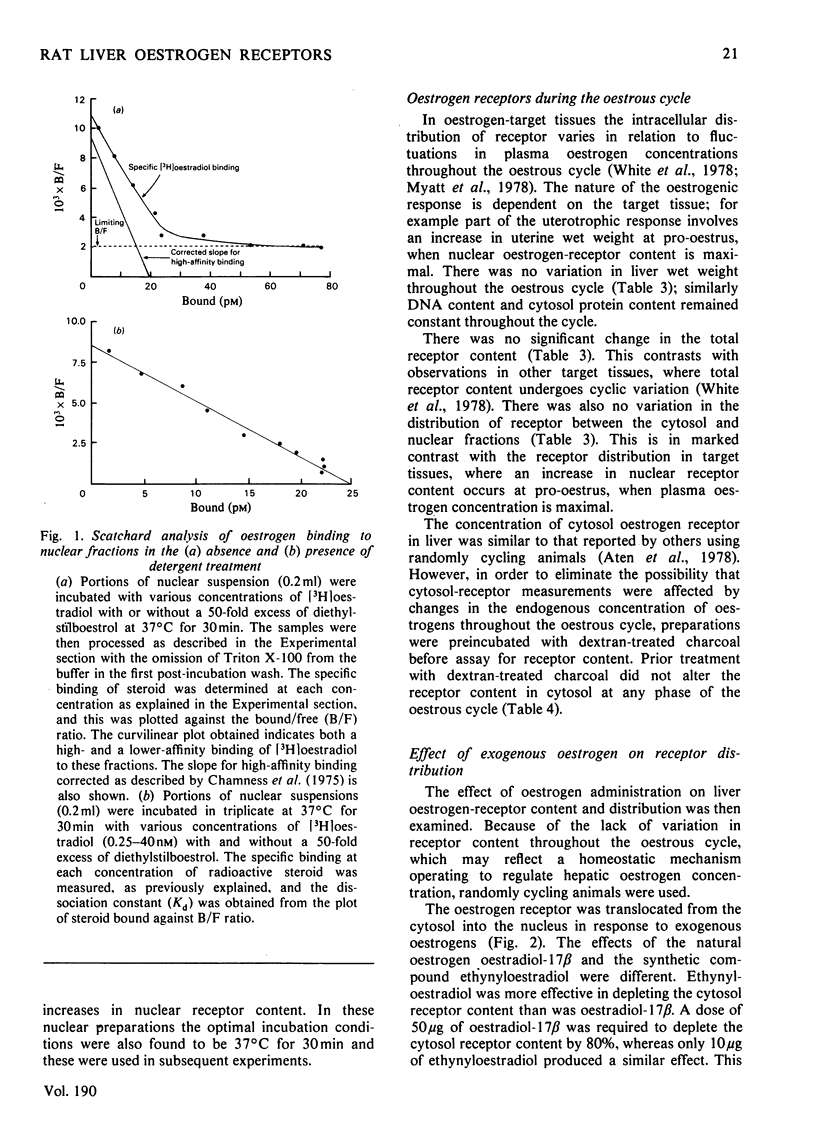
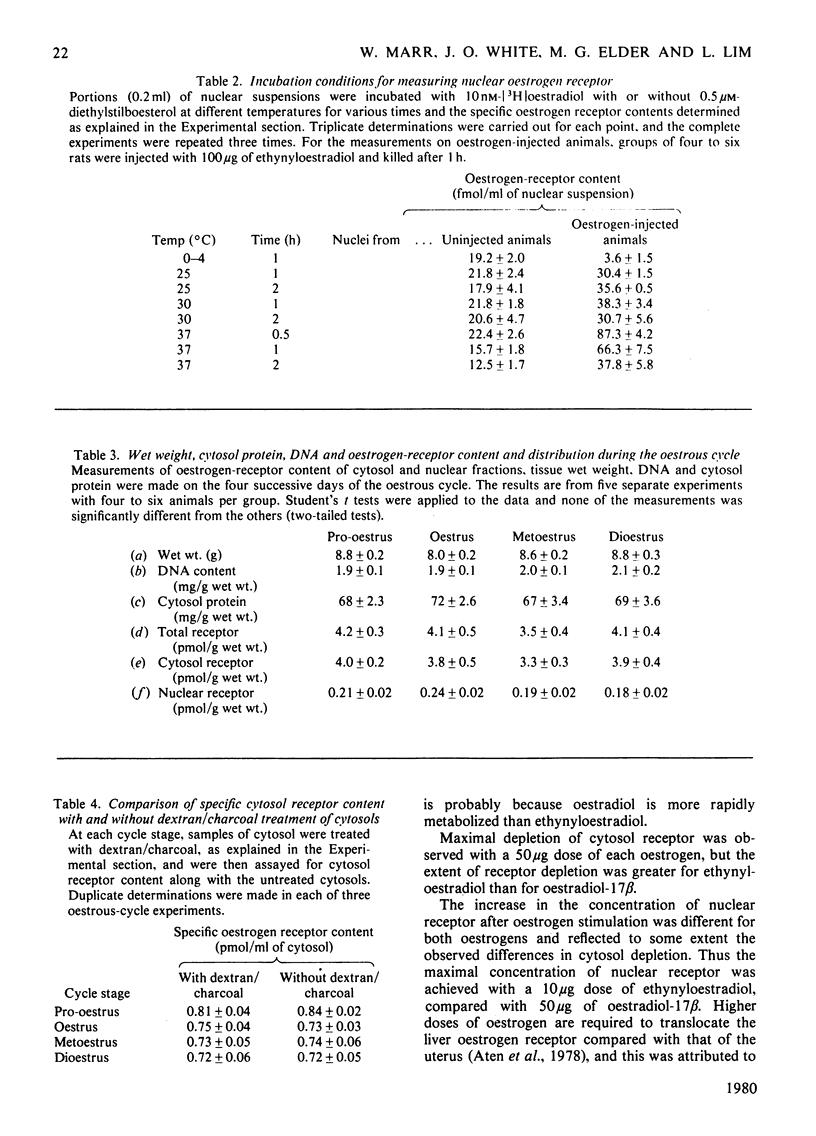
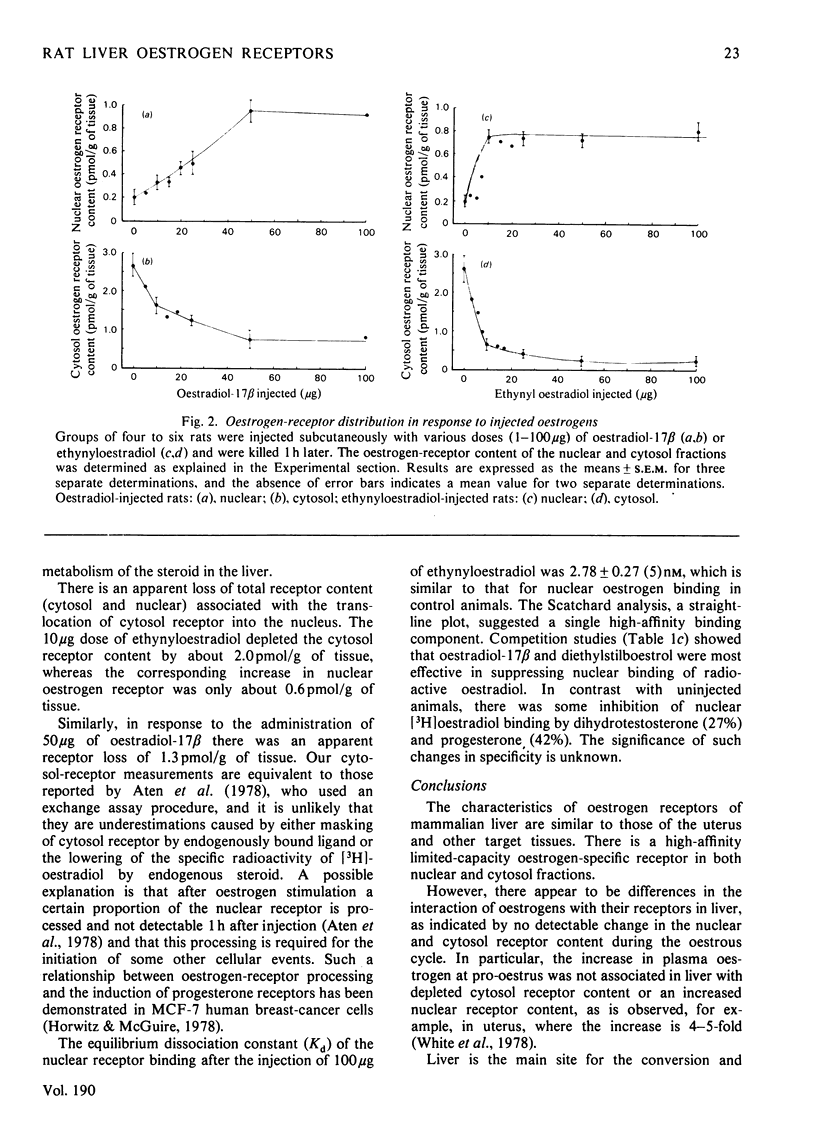
Oestrogen receptors were measured in the cytosolic and purified nuclear fractions of rat liver. Both cytosolic and nuclear receptors bind oestrogen with high affinity (Kd = 1.47 and 2.28 nM respectively) and specificity similar to that of receptors in order oestrogen-target tissues such as the uterus. During the 4-day oestrous cycle the receptor content and distribution between cytosol and nucleus did not vary; in particular, the content of nuclear receptor did not appear to fluctuate in concert with known cyclic changes in the concentration of plasma oestrogen. Injection of 50 micrograms of oestradiol-17 beta or 10 micrograms of ethynyloestradiol resulted in a 4--6-fold increase in the nuclear receptor content, with a concomitant decrease in the unoccupied-receptor content of cytosol 1 h after injection. The nuclear receptors present after injection bind oestrogens with similar affinity (Kd = 2.78 nM) and specificity to receptors present in uninjected animals. The administration of lower doses of either oestrogen was less effective in producing increases in nuclear receptor content. Hence there is apparently substantial translocation of receptor to the nucleus in response to hyperphysiological doses of oestrogen, but not to the physiological changes in plasma oestrogen concentrations during the oestrous cycle. The response to exogenous oestrogens is discussed in relation to the clinical use of synthetic oestrogens and progestogens.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J., Clark J. H., Peck E. J., Jr Oestrogen and nuclear binding sites. Determination of specific sites by ( 3 H)oestradiol exchange. Biochem J. 1972 Feb;126(3):561–567. doi: 10.1042/bj1260561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aten R. F., Weinberger M. J., Eisenfeld A. J. Estrogen receptor in rat liver: translocation to the nucleus in vivo. Endocrinology. 1978 Feb;102(2):433–442. doi: 10.1210/endo-102-2-433. [DOI] [PubMed] [Google Scholar]
- Bieri-Bonniot F., Joss U., Dierks-Ventling C. Stimulation of RNA polymerase i activity by 17beta-estradiol-receptor complex on chick liver nucleolar chromatin. FEBS Lett. 1977 Sep 1;81(1):91–96. doi: 10.1016/0014-5793(77)80935-6. [DOI] [PubMed] [Google Scholar]
- Booth B. A., Dyer R. D., Colás A. E. Separation of progesterone-specific from CBG-like binding sites by chromatography on columns of spheroidal hydroxylapatite. J Steroid Biochem. 1977 Nov;8(11):1179–1182. doi: 10.1016/0022-4731(77)90070-x. [DOI] [PubMed] [Google Scholar]
- Buller R. E., O'Malley B. W. The biology and mechanism of steroid hormone receptor interaction with the eukaryotic nucleus. Biochem Pharmacol. 1976 Jan;25(1):1–12. doi: 10.1016/0006-2952(76)90164-7. [DOI] [PubMed] [Google Scholar]
- CHAUVEAU J., MOULE Y., ROUILLER C. Isolation of pure and unaltered liver nuclei morphology and biochemical composition. Exp Cell Res. 1956 Aug;11(2):317–321. doi: 10.1016/0014-4827(56)90107-0. [DOI] [PubMed] [Google Scholar]
- Chamness G. C., Huff K., McGuire W. L. Protamine-precipitated estrogen receptor: a solid-phase ligand exchange assay. Steroids. 1975 May;25(5):627–635. doi: 10.1016/0039-128x(75)90017-3. [DOI] [PubMed] [Google Scholar]
- Clark J. H., Peck E. J. Nuclear retention of receptor-oestrogen complex and nuclear acceptor sites. Nature. 1976 Apr 15;260(5552):635–637. doi: 10.1038/260635a0. [DOI] [PubMed] [Google Scholar]
- Conard J., Samama M., Salomon Y. Antithrombin 3 and the estrogen content of combined estro-progestagen contraceptives. Lancet. 1972 Nov 25;2(7787):1148–1149. doi: 10.1016/s0140-6736(72)92757-2. [DOI] [PubMed] [Google Scholar]
- Eisenfeld A. J., Aten R. F., Haselbacher G. K., Halpern K. Specific macromolecular binding of estradiol in the mammalian liver supernatant. Biochem Pharmacol. 1977 May 15;26(10):919–922. doi: 10.1016/0006-2952(77)90466-x. [DOI] [PubMed] [Google Scholar]
- Eisenfeld A. J., Aten R., Weinberger M., Haselbacher G., Halpern K., Krakoff L. Estrogen receptor in the mammalian liver. Science. 1976 Feb 27;191(4229):862–865. doi: 10.1126/science.175442. [DOI] [PubMed] [Google Scholar]
- Ginsburg M., Greenstein B. D., MacLusky N. J., Morris I. D., Thomas P. J. An improved method for the study of high-affinity steroid binding:-oestradiol binding in brain and pituitary. Steroids. 1974 Jun;23(6):773–792. doi: 10.1016/0039-128x(74)90053-1. [DOI] [PubMed] [Google Scholar]
- Gorski J., Gannon F. Current models of steroid hormone action: a critique. Annu Rev Physiol. 1976;38:425–450. doi: 10.1146/annurev.ph.38.030176.002233. [DOI] [PubMed] [Google Scholar]
- Helton E. D., Goldzieher J. W. The pharmacokinetics of ethynyl estrogens. A review. Contraception. 1977 Mar;15(3):255–284. doi: 10.1016/0010-7824(77)90112-3. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., McGuire W. L. Estrogen control of progesterone receptor in human breast cancer. Correlation with nuclear processing of estrogen receptor. J Biol Chem. 1978 Apr 10;253(7):2223–2228. [PubMed] [Google Scholar]
- Jost J. P., Ohno T., Panyim S., Schuerch A. R. Appearance of vitellogenin mRNA sequences and rate of vitellogenin synthesis in chicken liver following primary and secondary stimulation by 17 beta-estradiol. Eur J Biochem. 1978 Mar 15;84(2):355–361. doi: 10.1111/j.1432-1033.1978.tb12175.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Munro H. N. The determination of nucleic acids. Methods Biochem Anal. 1966;14:113–176. doi: 10.1002/9780470110324.ch5. [DOI] [PubMed] [Google Scholar]
- Myatt L., Chaudhuri G., Elder M. G., Lim L. The oestrogen receptor in the rat uterus in relation to intra-uterine devices and the oestrous cycle. Biochem J. 1978 Nov 15;176(2):523–529. doi: 10.1042/bj1760523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménard J., Corvol P., Foliot A., Raynaud J. P. Effects of estrogens on renin substrate and uterine weights in rats. Endocrinology. 1973 Sep;93(3):747–751. doi: 10.1210/endo-93-3-747. [DOI] [PubMed] [Google Scholar]
- Powell-Jones W., Davies P., Griffiths K. Specific binding of (3H) oestradiol by cytoplasmic protein components of female rat liver. J Endocrinol. 1976 Apr;69(1):167–168. doi: 10.1677/joe.0.0690167. [DOI] [PubMed] [Google Scholar]
- Rössner S., Larsson-Cohn U., Carlson L. A., Boberg J. Effects of an oral contraceptive agent on plasma lipids, plasma lipoproteins, the intravenous fat tolerance and the post-heparin lipoprotein lipase activity. Acta Med Scand. 1971 Oct;190(4):301–305. doi: 10.1111/j.0954-6820.1971.tb07435.x. [DOI] [PubMed] [Google Scholar]
- Song C. S., Rifkind A. B., Gillette P. N., Kappas A. Hormones and the liver. The effect of estrogens, progestins, and pregnancy on hepatic function. Am J Obstet Gynecol. 1969 Nov 1;105(5):813–847. [PubMed] [Google Scholar]
- Stokes T., Wynn V. Serum-lipids in women on oral contraceptives. Lancet. 1971 Sep 25;2(7726):677–680. doi: 10.1016/s0140-6736(71)92247-1. [DOI] [PubMed] [Google Scholar]
- Thrower S., Hall C., Lim L., Davison A. N. The selective isolation of the uterine oestradiol-receptor complex by binding to oligo(dT)-cellulose. The mediation of an essential activator in the transformation of cytosol receptor. Biochem J. 1976 Nov 15;160(2):271–280. doi: 10.1042/bj1600271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrower S., Lim L. Characterization of rat hypothalamic progestin binding by spheroidal hydroxylapatite chromatography. Biochem J. 1980 Jan 15;186(1):295–300. doi: 10.1042/bj1860295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. O., Lim L. The oestrogen receptor in the rat hypothalamus: nuclear responses to oestrogen administration [proceedings]. Biochem Soc Trans. 1978;6(6):1310–1312. doi: 10.1042/bst0061310. [DOI] [PubMed] [Google Scholar]
- White J. O., Thrower S., Lim L. Intracellular relationships of the oestrogen receptor in the rat uterus and hypothalamus during the oestrous cycle. Biochem J. 1978 Apr 15;172(1):37–47. doi: 10.1042/bj1720037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn V., Adams P. W., Godsland I., Melrose J., Niththyananthan R., Oakley N. W., Seed M. Comparison of effects of different combined oral-contraceptive formulations on carbohydrate and lipid metabolism. Lancet. 1979 May 19;1(8125):1045–1049. doi: 10.1016/s0140-6736(79)92949-0. [DOI] [PubMed] [Google Scholar]
- Zava D. T., McGuire W. L. Estrogen receptor. Unoccupied sites in nuclei of a breast tumor cell line. J Biol Chem. 1977 Jun 10;252(11):3703–3708. [PubMed] [Google Scholar]


