Abstract
Acinetobacter baumannii causes life-threatening infections that are becoming difficult to treat due to increasing rates of multi-drug resistance (MDR) among clinical isolates. This has led the World Health Organization and the CDC to categorize MDR A. baumannii as a top priority for the research and development of new antibiotics. Colistin is the last-resort antibiotic to treat carbapenem-resistant A. baumannii. Not surprisingly, reintroduction of colistin has resulted in the emergence of colistin-resistant strains. Diclofenac is a non-steroidal anti-inflammatory drug used to treat pain and inflammation associated with arthritis. In this work, we show that diclofenac sensitizes colistin-resistant A. baumannii clinical strains to colistin in vitro and in a murine model of pneumonia. Diclofenac also reduced the colistin minimal inhibitory concentration (MIC) of Klebsiella pneumoniae and Pseudomonas aeruginosa isolates. Transcriptomic and proteomic analyses revealed an upregulation of oxidative stress-related genes and downregulation of type IV pili induced by the combination treatment. Notably, the concentrations of colistin and diclofenac effective in the murine model were substantially lower than those determined in vitro, implying a stronger synergistic effect in vivo compared to in vitro. A pilA mutant strain, lacking the primary component of the type IV pili, became sensitive to colistin in the absence of diclofenac. This suggest that the downregulation of type IV pili is key for the synergistic activity of these drugs in vivo and indicates that colistin and diclofenac exert an anti-virulence effect. Together, these results suggest that diclofenac can be repurposed with colistin to treat MDR A. baumannii.
Author summary
Acinetobacter baumannii causes infections that are difficult to treat due to high rates of antibiotic resistance, leading the World Health Organization and CDC to classify this pathogen as a top priority for research and development of new antibiotics. Colistin is one of few drugs still able to treat A. baumannii infections, but, recently, resistant strains have emerged. In this work, we show that a combination of diclofenac, an FDA-approved anti-inflammatory drug, and colistin can kill colistin-resistant A. baumannii. Concentrations of colistin and diclofenac effective in the context of an animal model were substantially lower than in vitro, implying a stronger synergistic effect in the animal. Further studies revealed an increase in oxidative stress and decrease in type IV pili with the combination treatment. In the animal model, colistin-resistant A. baumannii lacking type IV pili became sensitive to colistin alone, which suggests that the decrease of type IV pili is key for the synergistic activity of these drugs. Diclofenac in combination with colistin was also efficient in killing other multidrug-resistant pathogens as well, including Klebsiella pneumoniae and Pseudomonas aeruginosa. Together, our results suggest that diclofenac can be used in combination with colistin to treat multidrug-resistant A. baumannii infections.
Introduction
Acinetobacter baumannii causes a wide range of life-threatening nosocomial infections, often in immunocompromised patients. Unfortunately, these infections are becoming more difficult to manage due to a sharp rise in multi-drug resistance rates among Acinetobacter species, which are now nearly four times higher than those observed in other Gram-negative pathogens [1]. One of the last resort antibiotics used to treat multi-drug resistant (MDR) isolates of A. baumannii is carbapenem. However, the rate of resistance to carbapenems in A. baumannii has now exceeded 40% in the U.S. [2]. Because of these increasing antibiotic resistance rates, the World Health Organization has ranked carbapenem-resistant A. baumannii as a top priority for research to develop new antimicrobial therapies [3]. Carbapenem-resistant strains are frequently resistant to all other agents except colistin [4].
Colistin (polymyxin E) is a nonribosomally synthesized cationic antimicrobial polypeptide that is part of the polymyxin class of antibiotics. Its bactericidal activity occurs when positively charged colistin interacts with the negatively charged lipid A moiety of lipopolysaccharide (LPS) in the outer membrane of Gram-negative bacteria [5]. This interaction leads to the displacement of Ca2+ and Mg2+ ions from the phosphate groups of LPS, causing destabilization of the membrane [5]. Although the direct mechanism of colistin killing is not well-understood, it is believed that disruption of both the outer and inner membranes leads to leaking of cytoplasmic contents and cell death [6]. Additional mechanisms of colistin-mediated killing have been described, such as formation of hydroxyl radicals as colistin molecules cross the membrane leading to production of reactive oxygen species (ROS) that damage the bacterial cell [7]. Colistin is considered the last-resort antibiotic for treating severe infections caused by carbapenem-resistant A. baumannii [8–13] due to its associated toxicity [14–22]. Not surprisingly, reintroduction of colistin to treat carbapenem-resistant infections has resulted in the emergence of colistin-resistant A. baumannii [2,23,24]. In A. baumannii, several mechanisms of colistin resistance have been identified including the alteration of the lipid A component of the LPS by incorporating a phosphoethanolamine moiety, that reduces the overall negative charge of the LPS. This is mediated by the chromosomal-encoded phosphoetanolamine transferases, PmrC or EptA, as well as plasmid-encoded mobile colistin resistant genes (mcr) [25]. Interestingly, some colistin-resistant A. baumannii strains have even evolved to completely lose LPS by inactivation of genes in its biosynthetic pathway, such as lpxA, lpxC, lpxD [26]. Another relevant mechanism involves the efflux of colistin from the cell mediated by the EmrAB system [27–29]. Thus, research into development of therapeutics or alternative mechanisms to treat carbapenem- and colistin-resistant A. baumannii is critical.
In a previous study, we described a novel stress response mechanism in A. baumannii, involving the phenylacetic acid (PAA) pathway. We found that in presence of subinhibitory concentrations of antibiotics and other stressors, Acinetobacter modulates the expression of the paa operon. This operon encodes enzymes responsible for the degradation of PAA [30]. Mutations in the paa operon results in increased susceptibility to antibiotics. These findings suggest that accumulation of PAA is detrimental for bacterial growth and that interfering with PAA levels disrupts the programmed antibiotic-mediated adaptations of A. baumannii. Therefore, we hypothesized that the exogenous addition of PAA derivatives may have therapeutic potential. Diclofenac is as non-steroidal anti-inflammatory drug (NSAID) that belongs to a family of PAA derivatives approved by the U.S. Food and Drug Administration (FDA) for use in humans. Diclofenac functions by inhibiting cyclooxygenases, COX-1 and COX-2 in the host [31]. It has been suggested that diclofenac may have antibiotic properties [32–35]. In this study, we explore the potential of repurposing the FDA approved PAA-derivative, diclofenac, to enhance antibiotic efficacy against MDR A. baumannii with a specific focus on the last-resort drug, colistin.
Results
Diclofenac increases colistin susceptibility in clinical A. baumannii isolates
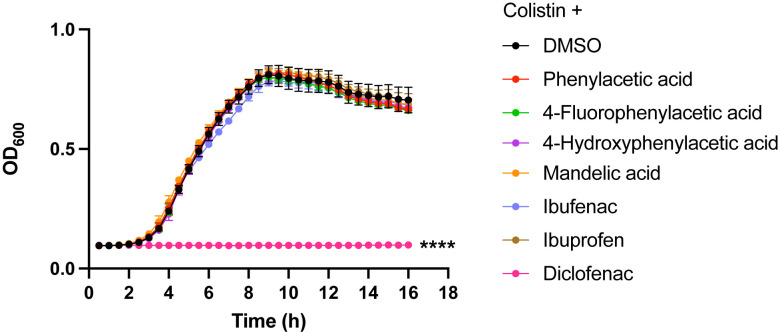
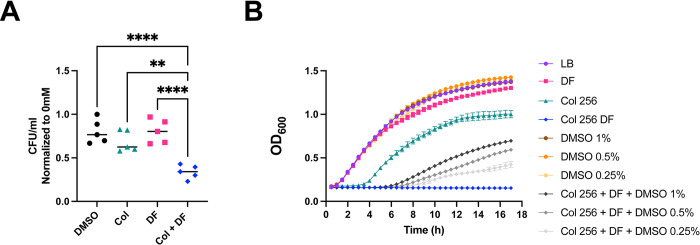
We previously observed that exogenous addition of PAA or inactivation of the paa operon increases the sensitivity of A. baumannii to multiple antibiotics [30]. Therefore, we hypothesized that FDA-approved PAA derivatives, such a diclofenac, could also interfere with the bacterial response to antibiotics. As MDR A. baumannii is rapidly losing susceptibility to colistin, we first investigated whether FDA-approved PAA derivatives could increase susceptibility to this last line of defense antibiotic. To test this, we used A. baumannii ARC6851 [36], a strain encoding several genes related to colistin resistance as pmrC (OB946_RS03455), pmrAB (OB946_RS03460-5) and the efflux system, emrAB (OB946_RS14565-70), and exhibiting high resistance to colistin with a minimum inhibitory concentration (MIC) >1024 μg/ml. We treated ARC6851 with a sub-MIC concentration of colistin (256 μg/ml) in combination with 100 μM phenylacetic acid, 4-fluorophenylacetic acid, 4-hydroxyphenylacetic acid, mandelic acid, ibufenac, ibuprofen, or diclofenac. We found that colistin, in conjunction with diclofenac but no other PAA derivatives, inhibits the growth of ARC6851 (Fig 1).
Fig 1. Diclofenac in combination with colistin inhibits A. baumannii growth.
Representative growth curves of ARC6851 in LB containing 256 μg/ml colistin and either the solvent control DMSO or 100 μM phenylacetic acid, 4-fluorophenylacitic acid, 4-hydroxyphenylacetic acid, mandelic acid, ibufenac, ibuprofen, or diclofenac. Data is representative of three biological replicates. ****P<0.0001 (One-way ANOVA with Tukey’s test for multiple comparisons, results were compared to the control group treated with DMSO).
To determine whether the enhancement of colistin susceptibility by diclofenac was specific to ARC6851, we assessed the MIC of several A. baumannii strains with varied susceptibilities to colistin. Additionally, we assessed other prominent Gram-negative pathogens, including Klebsiella pneumoniae, Pseudomonas aeruginosa, and Enterobacter cloacae. This analysis was conducted using a 2-fold broth dilution microtiter assay (S1 Table). We observed that diclofenac increased the susceptibility of all six A. baumannii strains tested. The most significant change in MIC, with an increase of ≥4-fold, occurred in the colistin-resistant strains AB347, AB431, AB774 and ARC6851. We also found that diclofenac renders K. pneumoniae, E. cloacae and P. aeruginosa more susceptible to colistin (S1 Table). Hence, the susceptibility to colistin mediated by diclofenac is not exclusive to A. baumannii. While colistin does not typically target Gram-positive bacteria, we assessed the MIC for Staphylococcus aureus. However, we did not observe any changes in the MIC in the presence of diclofenac.
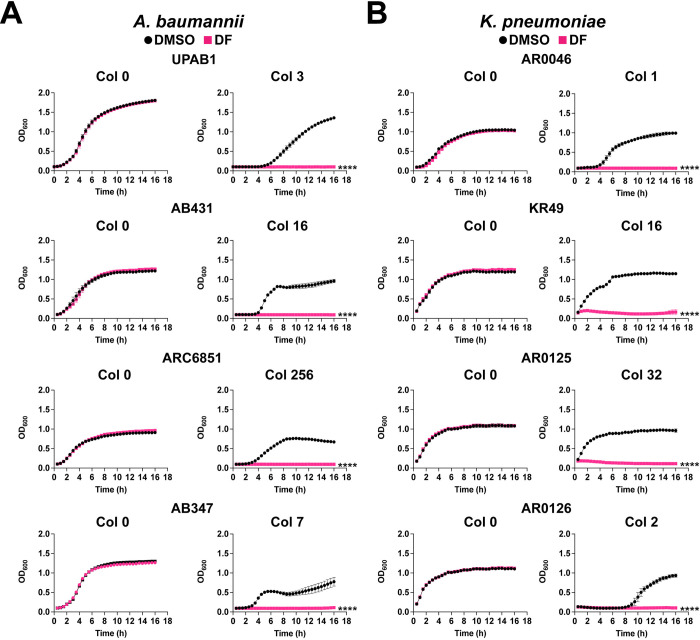
Treatment of A. baumannii UPAB1, AB431, ARC6851 and AB347 with either 100 μM diclofenac or the solvent control DMSO, did not affect Acinetobacter growth in the absence of colistin (Fig 2A, left panel). However, when adding sub-MIC concentrations of colistin, diclofenac inhibited the growth of the four strains tested (Fig 2A, right panel). Similar results were obtained for a panel of K. pneumoniae (Fig 2B) and P. aeruginosa (S1 Fig) strains.
Fig 2. Diclofenac inhibits A. baumannii and K. pneumoniae growth in the presence of colistin.
(A) Representative growth curves of A. baumannii UPAB1, AB431, ARC6851 and AB347 strains in LB containing DMSO or 100 μM diclofenac (DF) (left panel) and 3 μg/ml, 16 μg/ml, 256 μg/ml, or 7 μg/ml colistin respectively (right panel). (B) Representative growth curves of K. pneumoniae AR0046, KR49, AR0125, AR0126 strains in LB containing DMSO or 100 μM diclofenac (DF) (left panel) and 1 μg/ml, 16 μg/ml, 32 μg/ml, or 2 μg/ml colistin respectively (right panel). Data is representative of three biological replicates. ****P<0.0001, unpaired t tests at 16 h for Col + DF 100 compared to DMSO control. Col (colistin), DF (diclofenac).
Colistin, but not diclofenac, increases the permeability of ARC6851
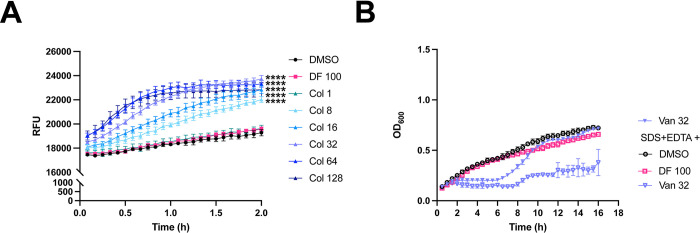
Colistin targets LPS in the outer membrane [5,7]. On the other hand, it has been reported that large concentrations of diclofenac inhibits bacterial DNA synthesis [37,38]. Therefore, we hypothesized that the growth inhibition caused by colistin plus diclofenac may result from the membrane-permeabilizing activity of colistin. To test this hypothesis, we measured the membrane permeability of our most colistin-resistant isolate, ARC6851, under treatment with colistin alone, diclofenac alone, and in combination. Permeability was analyzed by measuring the accumulation of the fluorescent dye Hoechst H33342, which binds to DNA, in treated cells [39,40]. As anticipated, colistin increased bacterial permeability in a dose-dependent manner (Fig 3A). Notably, we found that 100 μM diclofenac (the concentration employed in our MIC assays) did not alter the permeability of ARC6851 when used alone (Fig 3A) or in combination with different concentrations of colistin (S2A Fig). Moreover, similar results were obtained when measuring cell permeability with N-phenyl-1-naphthylamine (NPN). The addition of the efflux pump inhibitor Carbonyl cyanide-m-chlorophenylhydrazone (CCCP) did not modify cell permeability (S2B Fig). These results indicate that colistin, but not diclofenac, affects membrane permeability in ARC6851. This result is in agreement with previous work in which colistin increased the permeability of bacteria to other molecules [41].
Fig 3. Colistin, but not diclofenac, increases membrane permeability of ARC6851.
(A) Membrane permeability was assessed by measuring uptake of Hoescht 33342 (H33342) fluorescent dye. Bacterial cells were suspended in PBS supplemented with DMSO, 100 μM diclofenac (DF) or increasing concentrations of colistin (Col 1 μg/ml, 8 μg/ml, 16 μg/ml, 32 μg/ml, 64 μg/ml, or 128 μg/ml). Relative fluorescent units (RFU) were monitored over the course of 2 hours. (B) Representative growth curves of ARC6851 in LB containing 32 μg/ml Vancomycin or 0.01% SDS + 0.075 mM EDTA in combination with the solvent control DMSO, 100 μM Diclofenac or 32 μg/ml Vancomycin. Data is representative of three biological replicates, ****P<0.0001, (One-way ANOVA with Tukey’s test for multiple comparisons, results were compared to the control group treated with DMSO). Col (colistin), DF (diclofenac), Van (Vancomycin), SDS (sodium dodecyl sulfate), EDTA (ethylenediaminetetraacetic acid).
Bacterial cell permeability is not sufficient for diclofenac-mediated killing
The effect of colistin on permeability prompted us to question whether colistin merely acts as a permeabilizing agent to enable the diclofenac-mediated elimination of A. baumannii. To test this possibility, we performed growth curves of ARC6851 treated with 100 μM diclofenac with the combination of a different permeabilizing agent, consisting of 0.01% sodium dodecyl sulfate (SDS) and 0.075 mM ethylenediaminetetraacetic acid (EDTA) (Fig 3B) [42]. As a control, we included ARC6851 treated with vancomycin, a large compound targeting the cell wall that typically is only used to treat Gram-positive bacterial infections [43, 44]. However, previous reports have shown that increasing the cell permeability of Gram-negative bacteria can lead to susceptibility to vancomycin [43,44]. Interestingly, although vancomycin in combination with SDS/EDTA increased killing of ARC6851, 100 μM diclofenac in combination with SDS/EDTA treatment did not affect ARC6851 growth (Fig 3B). Polymyxin B Nonapeptide (PBNP) is a derivative that lacks a fatty acid tail and is significantly less active than colistin and polymixin B against species such as E. coli and K. pneumonia, but still possesses outer membrane-permeabilizing activity. PBNP did not show any activity against A. baumannii (S3A Fig). Together, our data demonstrate that indeed, both colistin and diclofenac are required to kill A. baumannii.
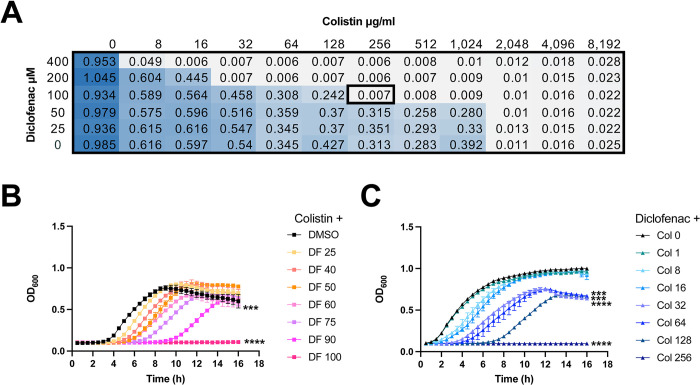
We then investigated the combinatorial potential of diclofenac with colistin in a checkerboard assay of ARC6851 (Fig 4A). Colistin alone only killed ARC6851 at a concentration higher than 1024 μg/ml. Diclofenac alone did not have any effect on growth of ARC6851, even at 4,000 μM concentration (Figs 4A and S3B). In contrast, when we evaluated the combination of colistin plus diclofenac, we found growth inhibition at 256 μg/ml colistin plus 100 μM diclofenac (Fig 4A). Furthermore, the effect on Acinetobacter growth was dependent on both colistin and diclofenac concentrations (Fig 4B and 4C). These results indicate that diclofenac and colistin exert synergistic effects against A. baumanni.
Fig 4. Colistin and diclofenac inhibit growth of ARC6851 in a synergistic manner.
(A) Checkerboard microdilution assay for ARC6851 with colistin and diclofenac. Blue color represents cell density. (B) Growth curves of ARC6851 in LB containing 256 μg/ml colistin with either DMSO or increasing concentrations of diclofenac (25 μM, 40 μM, 50 μM, 60 μM, 75 μM, 90 μM, or 100 μM) (C) Growth curves of ARC6851 in LB containing 100 μM diclofenac with either DMSO or increasing concentrations of colistin (1 μg/ml, 8 μg/ml, 16 μg/ml, 32 μg/ml, 64 μg/ml, 128 μg/ml, or 256 μg/ml). Data is representative of three biological replicates ***P<0.001, ****P<0.0001 (One-way ANOVA with Tukey’s test for multiple comparisons, results were compared to the control group treated with DMSO). Col (colistin), DF (diclofenac).
It was previously reported that combinations of colistin and fatty acid synthesis (FAS), inhibitors such as triclosan, a FabI inhibitor, synergized with colistin against mcr-1-expressing E. coli [41] and that the phenotype was rescued by adding fatty acids [41]. Indeed, ARC6851 colistin MIC was reduced in the presence of triclosan and addition of oleic acid or linoleic acid abolished the synergy (S2 Table). However, the addition of palmitic acid, araquidonic acid, linoleic acid, oleic acid and stearic acid had no effect on Acinetobacter colistin MIC in the presence of diclofenac (S3 Table). These results indicate that the synergistic effects mediated by diclofenac and FAS inhibitors with colistin occur through different molecular mechanisms. Additionally, we found that the addition of diclofenac did not significantly alter the MICs of either ARC6851 or UPAB1 to other antibiotics such as, ciprofloxacin, erythromycin, tetracycline, vancomycin, imipenem and sulfomethoxazole/trimethoprim (S4 Table).
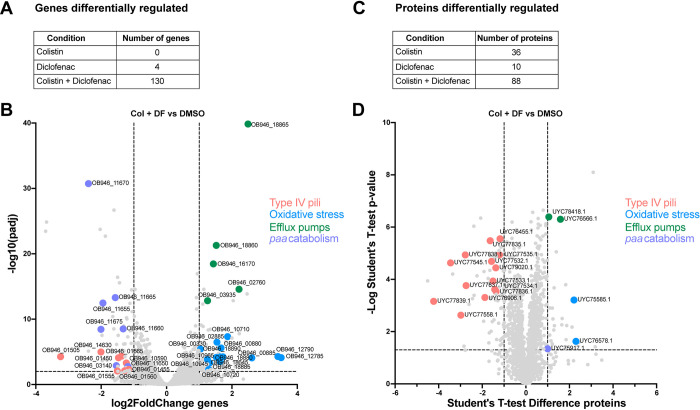
Colistin plus diclofenac significantly alter ARC6851’s gene and protein expression
To dissect the molecular mechanism underlying the synergistic effect of colistin plus diclofenac treatment, we performed whole-cell transcriptomic of ARC6851 grown in LB, LB plus colistin (1 μg/ml), LB plus diclofenac (100 μM), or LB plus colistin (1 μg/ml) and diclofenac (100 μM). We employed sub-MIC colistin concentrations that did not induce significant growth defects in vitro (S4 Fig). Only 4 genes involved in lipid metabolism and homeostasis were upregulated in response to diclofenac alone, while no genes were downregulated in this condition (Figs 5A and S5 and S5 Table). No statistically significant changes in gene expression were detected in response to colistin alone (Fig 5A). However, the combination treatment of colistin plus diclofenac led to differential expression of 130 genes, with 69 genes upregulated and 61 genes downregulated (Fig 5A and 5B and S6 Table). Remarkably, genes involved in the synthesis of type IV pili and catabolism of PAA were the most significantly repressed in presence of both drugs. The genes most upregulated by diclofenac and colistin are involved in oxidative stress response. Several efflux pumps were also induced. The complete functional analysis of the transcriptomic data using eggNOG Mapper [45,46] is shown in S6 Fig. As controls, we performed the comparisons between treatment groups colistin plus diclofenac against colistin (S7 Table) and colistin plus diclofenac against diclofenac (S8 Table) and found similar results than those of the colistin plus diclofenac against DMSO (Fig 5A and 5B and S6 Table). We further performed whole-cell differential proteomics of ARC6851 grown in the same experimental conditions (Fig 5C and 5D and S9, S10 and S11 Tables). In agreement with the transcriptomic data, the most dramatic change detected by proteomics is the repression of type IV pili synthesis.
Fig 5. Oxidative stress response and type IV pili are differentially regulated in ARC6851 under colistin and diclofenac combination treatment.
(A) Number of differentially regulated genes in ARC6851 when treated with 1 μg/ml colistin, 100 μM diclofenac, or the combination. (B) Volcano plot showing differentially expressed genes in ARC6851 with colistin and diclofenac combination treatment vs DMSO control. (C) Number of differentially regulated proteins in ARC6851 when treated with 1 μg/ml colistin, 100 μM diclofenac, or the combination. (D) Volcano plot showing differentially expressed proteins in ARC6851 in colistin plus diclofenac combination treatment vs DMSO control. Col (colistin), DF (diclofenac). Five biological replicates were prepared for each condition.
The transcriptomics and proteomics suggest that induction of oxidative stress and repression of pili expression are the two most important processes affected by diclofenac and colistin. Indeed, significantly fewer ARC6851 CFUs were recovered when exposed to 5mM H2O2 in the presence of colistin plus diclofenac compared to the untreated cells. (Fig 6A). Furthermore, the presence of up to 1% DMSO, a ROS scavenging agent shown to reduce ROS-mediated killing in E. coli [47], improved ARC6851 growth in the presence of DF and colistin (Fig 6B). These results indicated that treatment of ARC6851 with both drugs renders bacteria susceptible to oxidative stress. Subsequently, we performed qRT-PCR to assess the expression of pilA, a crucial component of type IV pili, in ARC6851 and in the A. baumannii clinical isolate AB347 (S7A Fig). We observed a ~4 and ~3-fold downregulation of pilA respectively, when comparing the combination treatment of colistin plus diclofenac against DMSO, colistin, or diclofenac alone, consistent with the transcriptomic data (Fig 5B). Furthermore, a significant reduction in the quantity of type IV pili under the colistin plus diclofenac treatment was observed by transmission electron microscopy (S7B and S7C Fig).
Fig 6. Diclofenac and colistin–dependent bacterial killing is mediated by ROS.
(A) ARC6851 was grown to mid-exponential phase in LB + DMSO, LB+ colistin (1 μg/ml) + DMSO, LB + diclofenac (100 μM), or LB + colistin (1 μg/ml) + diclofenac (100 μM), and treated with 0 mM or 5 mM H2O2 for 2 h. Survival was measured by serial dilution and quantification of recoverable CFU/mL. Symbols represent five biological replicates; the horizontal line represents the Median. (B) Representative growth curves of ARC6851 in LB containing 256 μg/ml colistin, 100 μM diclofenac and increasing concentrations of DMSO (0.25%, 0.5% and 1%). Data is representative of three biological replicates **P<0.01, ****P< 0.0001; One-way ANOVA, Tukey’s test for multiple comparisons. Col (colistin), DF (diclofenac).
Colistin plus diclofenac is effective against colistin-resistant A. baumannii in vivo
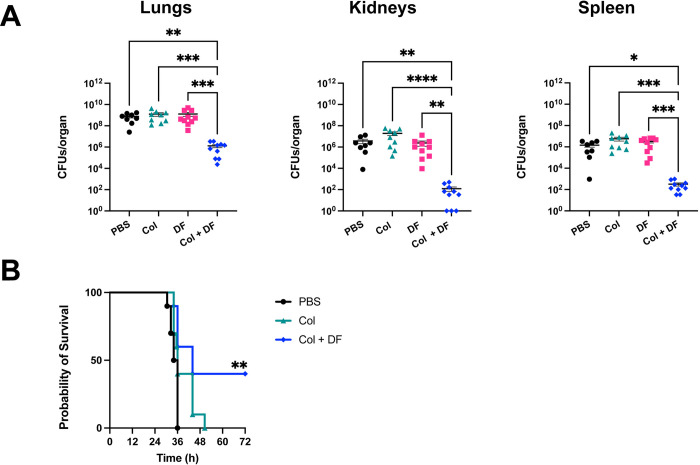
Building on the encouraging in vitro findings, we conducted experiments to assess the efficacy of combining colistin and diclofenac for the treatment of A. baumannii pneumonia in an acute murine model of infection [36,48]. Briefly, mice were intranasally inoculated with ARC6851 and, at 4 h post-infection (hpi), mice were injected intraperitoneally (IP) with PBS, 2.5 mg/kg colistin, 1.25 mg/kg diclofenac, or 2.5 mg/kg colistin plus 1.25 mg/kg diclofenac. Colistin treatment was administered every 8 hours, starting at 4 hours post-infection (4, 12, and 20 hpi), in both the colistin-only and colistin plus diclofenac treatment groups. Diclofenac treatment was performed once at 4 hpi in the diclofenac-only and colistin plus diclofenac groups based on previously established murine treatment protocols [49–51]. At 24 hpi, the lungs, spleens and kidneys were harvested and processed to determine the bacterial burden. Individual treatments of colistin or diclofenac alone did not produce any significant changes in bacterial burden. However, the combination treatment of colistin plus diclofenac produced a significant reduction in recovered CFUs in lungs, kidneys, and spleens compared to all other groups (Fig 7A). The concentrations of diclofenac and colistin found to be effective in this model are 2.5 and 20 times lower, respectively, than those determined in MIC experiments. Moreover, no inhibition of cyclooxygenase COX-2 activity was observed in vivo under our model conditions (S8 Fig). This indicates that the synergistic effect of colistin plus diclofenac is significantly greater in vivo than in vitro. Furthermore, all mice treated with PBS or colistin alone succumbed to the infection within 36 hpi and 50.5 hpi, respectively (Fig 7B). In contrast, 40% of mice treated with the combination of colistin plus diclofenac survived up to 72 hpi. (end-point of the experiment). Notably, mice in the dual treatment group that survived the entirety of the survival study exhibited no symptoms associated with pneumonia (e.g., ruffled fur, hunching, dyspnea, reduced activity) by 72 hpi, consistent with resolution of disease.
Fig 7. ARC6851 is attenuated in an acute murine pneumonia model under colistin and diclofenac combination treatment.
(A) C57BL/6 mice were infected with ~5 × 107 CFU of mid-exponential ARC6851. At 4 hpi, mice were IP injected with 1.25 mg/kg diclofenac. At 4, 12, and 20 hpi, mice were IP injected with 2.5 mg/kg colistin. At 24 h post-infection, lungs, kidneys, and spleens were harvested, and bacterial load was determined. Each symbol represents an individual mouse, and the horizontal bar represents the Mean with SEM. Data collected from two independent experiments. (B) Survival rate of C57BL/6 mice (n = 10 per group) following infection with ARC6851 for the different therapies shown in A. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001, Kruskal-Wallis test. Col (colistin), DF (diclofenac).
In vivo, type IV pili synergistic inhibition yields anti-virulence effects
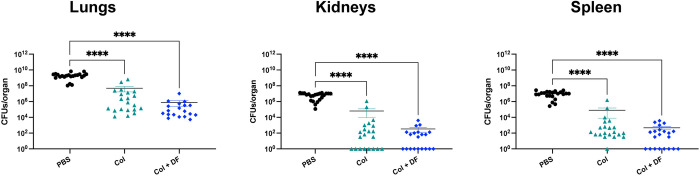
Type IV pili is crucial for virulence in various pathogens such as P. aeruginosa, V. cholerae, and Neisseria spp., among others [52–56]. Transcriptomic and proteomic analyses revealed a drastic reduction in the expression of type IV pili-related genes in ARC6851 when exposed to the combined treatment of colistin and diclofenac. We hypothesized that the reduction of type IV pili by treatment with colistin and diclofenac enhances the bacterial clearance through an anti-virulence mechanism. To test this hypothesis, we constructed a ΔpilA strain. TEM analysis confirmed the absence of type IV pili in ΔpilA (S9A Fig). In vitro, ΔpilA MIC values and growth in the presence of colistin, diclofenac or the combination treatment was similar to the parental wild-type strain (S9B, S9C and S9D Fig). Unlike in other bacterial species, deletion of the pilA gene did not affect bacterial burden in the respiratory model (S10 Fig). However, treatment with colistin alone was sufficient to reduce the burden of the ΔpilA strain in lung, kidney and spleen to a similar extent as observed with colistin plus diclofenac treatment (Fig 8). These results indicate that the downregulation of type IV pili is key for the synergistic activity of these drugs in vivo.
Fig 8. ARC6851 ΔpilA is sensitive to colistin treatment in an acute murine pneumonia model.
C57BL/6 mice were infected with ~5 × 107 CFU of mid-exponential ARC6851 ΔpilA. At 4 hpi, mice were IP injected with 1.25 mg/kg diclofenac or PBS. At 4, 12, and 20 hpi, mice were IP injected with 2.5 mg/kg colistin or PBS. At 24 h post-infection, lungs, kidneys, and spleens were harvested, and bacterial load was determined. Each symbol represents an individual mouse, and the horizontal bar represents the Mean with SEM. Data collected from two independent experiments. ****P<0.0001, Kruskal-Wallis test. Col (colistin), DF (diclofenac).
Discussion
Colistin, despite its nephrotoxicity and neurotoxicity, has been used as a last resort antibiotic to treat carbapenem-resistant A. baumannii infections. The increasing use of colistin has resulted in the emergence of colistin-resistant strains, and therefore, alternative therapies to address carbapenem- and colistin-resistant A. baumannii infections are needed. Combining and repurposing existing drugs as potential therapies offers numerous advantages over traditional de novo drug discovery approaches, such as reduced development costs and expedited treatment implementation. In this study, we uncovered the synergistic effect between colistin and diclofenac, exhibiting antibacterial activity against carbapenem- and colistin-resistant A. baumannii.
A. baumannii responds to antibiotics by differentially regulating the phenylacetic acid catabolism. Diclofenac is a non-metabolizable PAA-derivative classified as a non-steroidal anti-inflammatory drug (NSAID) approved by the U.S. FDA. Previous studies have shown that diclofenac has antibacterial activity against both Gram-negative and Gram-positive pathogens. Specifically, it has been reported that diclofenac has anti-listerial activity in vivo [57], is effective against Escherichia coli in an in vivo urinary model [33,34], and eliminates Mycobacterium tuberculosis in an in vivo model when used as monotherapy or in combination with streptomycin [32]. Moreover, diclofenac displayed antibacterial activity against the Gram-positive methicillin-resistant Staphylococcus aureus in combination with β-lactams [35]. However, we found that diclofenac did not have an effect against A. baumannii, E. faecalis, P. aeruginosa or K. pneumoniae by itself. In our study, we found that diclofenac enhanced the bactericidal activity of colistin against MDR-clinical A. baumannii isolates, leading to a decrease in the MIC values. Moreover, the combination treatment of colistin plus diclofenac decreased the MICs in K. pneumoniae, E. faecalis and P. aeruginosa, therefore expanding the potential of diclofenac to target a broader spectrum of Gram-negative pathogens.
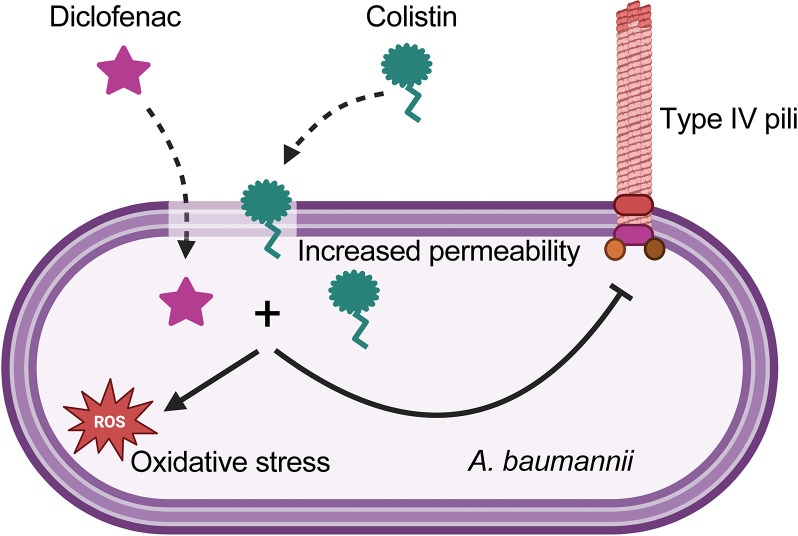
The results of this work are summarized in Fig 9. To investigate the synergistic mechanism of colistin in combination with diclofenac, we performed transcriptomics and proteomics analyses. Our data showed no transcriptional changes in response to treatment with 1 μg/ml colistin. Under treatment with 100 μM diclofenac alone, we observed the upregulation of just 4 genes involved in lipid transport and metabolism, including fadB (acyl-CoA dehydrogenase) and fahA (involved in breakdown of aromatic amino acids). Remarkably, the combination of both drugs significantly changed the expression of ~130 genes. These include the upregulation of genes primarily involved in oxidative stress such as oxidoreductases, monooxygenases, and poroxiredoxin [58]. Additionally, we observed significant induction of sulfate transport and taurine metabolism pathways. This is also indicative of oxidative stress as these pathways are induced by hydrogen peroxide treatment in A. baumannii [58]. In fact, Acinetobacter was more suceptible to H2O2 treatment in the presence of colistin plus diclofenac and addition of the ROS scavenger DMSO rescued bacterial growth in the presence of colistin plus diclofenac, suggesting that oxidative stress is one of the ways in which both drugs synergistically kill A. baumannii. Moreover, during the revision of this manuscript and in agreement with our results, Qingxia Fu et al. [59], reported that ROS production under colistin and diclofenac treatment was found to be significantly increased compared to both the monotherapy and blank treatment groups.
Fig 9. Current model for the mechanism of colistin and diclofenac.
Created in Biorender.
Type IV pili is crucial for virulence in various pathogens [52–56]. Type IV pili modulates twitching motility [60–63] and is also involved in bacterial self-organization in microcolonies and biofilms [64,65]. Prior research documented the inhibition of type IV pili-mediated biofilm in A. baumannii by phenothiazine compounds, a class of heterocyclic compound [66]. Remarkably, the combination treatment of colistin plus diclofenac leads to the drastic downregulation of type IV pili. Despite being as virulent as the wild-type strain in an acute murine model, the pilA deletion mutant strain became sensitive to colistin in the absence of diclofenac in vivo, indicating that the downregulation of type IV pili is key for the synergistic activity of these drugs during infection. It is tempting to speculate that repression of type IV pili by the combined therapy disrupts the formation of microcolonies or biofilm-like structures that protect the bacteria from the action of colistin. In A. baumannii type IV pili is generally glycosylated with negatively charged sugars [67]. It is also possible that these negatively charged sugars sequester the positively charged colistin, thereby reducing the actual concentration of colistin in the bacterial microenvironment. Further research will be necessary to fully elucidate the anti-virulence mechanism through which colistin and diclofenac are active at much lower concentrations in vivo than in vitro. The combination of colistin and diclofenac also reduced the growth of K. pneumoniae, E. faecalis, and P. aeruginosa in vitro. Further testing is needed to determine if the antivirulence effect observed in A. baumannii in mice also applies to these pathogens. This is particularly intriguing given that K. pneumoniae does not encode a type IV pilus. If colistin and diclofenac demonstrate an antivirulence effect in K. pneumoniae, it would suggest that these drugs target a different virulence factor in this bacterium. For instance, they might affect the type II secretion system (T2SS), which is structurally related to the type IV pilus.
Both colisitin and diclofenac cause nephrotoxicity in both, human and mice [68–76]. The maximum concentration (Cmax) of colistin in serum during treatment in humans ranges from 3.5 to 33.33 mg/L [77]. In mice, similar Cmax values are observed with administration of ~20 mg/kg every 8 h [50]. Regarding diclofenac, human therapeutic treatment results in a serum Cmax ranging from 2.6 to 21.5 mg/L in [78]. In mice, a single 60 mg/kg dose resulted Cmax of 264 mg/L [79]. In our experiments we achieved a drastic reduction in bacterial burden and 40% increase in survival employing doses of colistin (2.5 mg/kg) and diclofenac (1.25 mg/kg) much lower than what is typically required for human treatment. If these lower doses were equally efficient in humans, colistin and diclofenac could be employed against A. baumannii with minimal toxicity. Although the immediate use of diclofenac together with colistin is not precluded, it may be possible to modify the molecule to improve its antibacterial activity and reduce its side effects. Leveraging the well-understood interaction between diclofenac and COX-2, there appears to be a significant opportunity to synthesize a series of diclofenac-derivatives and select for compounds retaining their synergistic effect with colistin specifically tailored for its bacterial target but no longer able to inhibit COX-2 in the host.
Materials and methods
Ethics statement
All animal experiments were approved by the Washington University Animal Care and Use Committee, and we have complied with all relevant ethical regulations (Approval number 23–0071).
Study design
The aim of this study was to assess the efficacy of combining colistin with diclofenac against multi-drug resistant (MDR) Acinetobacter baumannii. We proposed that FDA-approved phenylacetic acid (PAA) derivatives, like diclofenac, in combination with colistin might disrupt the bacterial response to antibiotics. We treated a highly colistin-resistant A. baumannii strain, ARC6851, with colistin in combination with various PAA derivatives in vitro. Our results indicate that colistin combined with diclofenac effectively eradicates ARC6851 and other A. baumannii isolates. Additionally, Klebsiella pneumoniae and Enterobacter cloacae isolates were susceptible to this combination treatment. To elucidate the molecular mechanism underlying the synergistic effect of colistin and diclofenac, we conducted permeability assays. Our findings revealed that colistin induces permeability in ARC6851, whereas diclofenac does not impact permeability. Furthermore, whole-cell transcriptomics and proteomics showed that the combination of colistin and diclofenac downregulates type IV pili and upregulates oxidative stress genes. Additionally, efflux pumps were induced by the combination treatment. Encouraged by the promising in vitro results, we evaluated the combination treatment for A. baumannii pneumonia using an acute murine infection model. This assessment demonstrated a reduction in colony-forming units (CFU) in the lungs, kidneys, and spleens. Sample sizes were determined based on prior studies [36,48], with the specific number of biological and technical replicates outlined in the figure legend for each experiment. Mice within the same litter were randomly assigned to either the control or treatment group. No animals were excluded from the experiments. A limitation of the study is that it only included female mice. It was previously observed that female mice were more susceptible to the acute pneumonia infection with A. baumannii that male mice [80].
Bacterial strains and growth conditions
Bacterial strains and plasmids used in this study are listed in S12 and S13 Tables. Unless otherwise noted, strains were grown in lysogeny broth (LB) liquid medium at 37°C with shaking (200 rpm). For cultures used for phenotypic assays, strains were struck out on solid LB-agar plates, and overnight cultures were always grown without antibiotics.
Hoechst H33342 permeability assays
Permeability assays were performed as previously described [39, 40]. Briefly, wild-type ARC6851 cultures were grown overnight in LB. Bacterial cells were washed twice with PBS and normalized to an OD600 of 1.0. Bacteria were then mixed with MgSO4 (1 mM final concentration), colistin (128 μg/ml, 64 μg/ml, 32 μg/ml, 16 μg/ml, 8 μg/ml, 8 μg/ml, 1 μg/ml), diclofenac (100 μM) and Hoescht 33342 dye (1.25 μM final concentration). Samples were prepared in 200 μL final volume in technical sextuples and were added to a 96-well black, clear-bottomed plate. Fluorescence was monitored with excitation and emission wavelengths of 361 and 497, respectively, over the course of 2 h.
N-phenyl-1-naphthylamine (NPN) Uptake Assay
Wild-type ARC6851 cultures were grown overnight in LB. Bacterial cells were washed twice with PBS and normalized to an OD600 of 1.0 in 5mM Sodium HEPES, pH 7.2. Bacteria were then mixed with 128 μg/ml colistin, 100 μM diclofenac and NPN (10 μM final concentration) with or without the efflux pump inhibitor Carbonyl cyanide-m-chlorophenylhydrazone (CCCP). Samples were prepared in 200 μL final volume in technical sextuples and were added to a 96-well black, clear-bottom plate. Fluorescence was monitored with excitation and emission wavelengths of 350 and 420, respectively.
MIC determinations
Antimicrobial susceptibility was determined using the 2-fold broth dilution microtiter assay as previously described [81,82]. Briefly, overnight cultures grown in LB were subcultured for 3 hours and inoculated at OD600 of 0.01 in a 96-well microtiter plate containing 2-fold dilutions of the appropriate antibiotics. MICs were determined using OD600 after plates were incubated at 37°C under shaking conditions for 16 h. MICs were defined as <10% growth compared to the non-treated controls. Experiments were performed at least 3 independent times.
Growth curve assays
Growth curves were performed in sterile, round-bottom, polystyrene, 96-well plates (Corning 3788). Overnight cultures were subcultured and incubated at 37°C and 200 rpm for 3 h to mid-exponential phase. Cultures were diluted in fresh medium and inoculated at a final OD600 of 0.01 and a final volume of 150 μL. Colistin, diclofenac, DMSO, Polimixin B Nonapeptide (PBNP) and other compounds were added as indicated. Plates were incubated at 37°C in shaking conditions for 16 h in a BioTek microplate reader, with OD600 values measured at 30 min intervals.
Checkerboard broth microdilution assays
An overnight culture of ARC6851 grown in LB was subcultured for 3 hours. Checkerboard analyses were conducted with a clear, flat-bottom 96-well assay plate containing twofold dilutions of colistin and diclofenac in LB in an 8 × 12 dose-point matrix and ARC6851 culture was inoculated at OD600 of 0.01 in the 96-well microtiter plate. The plates were incubated at 37°C with shaking overnight for 16h then the absorbance at OD600 was measured. MIC of the combination colistin plus diclofenac was defined as <10% growth compared to the non-treated controls. Experiments were performed at least 3 independent times.
H2O2 killing assays
H2O2 killing assays were performed as previously described [58]. Briefly, ARC6851overnight cultures were subcultured at OD600 0.05 in 10 mL LB plus DMSO, LB plus colistin (1 μg/ml) plus DMSO, LB plus diclofenac (100 μM), or LB plus colistin (1 μg/ml) plus diclofenac (100 μM) and incubated at 37°C and 200 rpm for 3 h to mid-exponential phase. Cultures were normalized to 0.3 OD600, and 1 mL was aliquoted into 14 mL polypropylene round-bottom tubes. Cultures were treated with 0 or 5 mM H2O2 (Supelco HX0635-3) plus treatments of DMSO, colistin (1 μg/ml) plus DMSO, diclofenac (100 μM), colistin (1 μg/ml) plus diclofenac (100 μM) for 2 h at 37°C and 200 rpm. Survival was measured by serial dilution and quantification of recoverable CFUs. Experiments were performed at least five independent times.
Culture preparation for RNA sequencing
ARC6851 was grown overnight in LB before being diluted into 10mL of LB, LB plus diclofenac (100 μM), LB plus colistin (1 μg/ml), or LB plus diclofenac and colistin and grown for 2 h at 37°C with shaking. Five individual overnight and 10mL culture biological replicates were prepared for each condition. Cultures were normalized, and the equivalent of 1 mL OD600 1.0 was pelleted quickly at 6.2 x g, treated with RNAprotect (Qiagen) for 5 minutes at room temperature, and pelleted again. Pellets were flash frozen and stored at -80°C until extraction. For RNA extractions, pellets were thawed on ice, resuspended in 600μL Trizol (Invitrogen) with 4 μl of glycogen at 5 mg/ml and lysed via bead-beating. Samples were pelleted, and supernatants were treated with chloroform. RNA was extracted from the aqueous phase using the RNeasy Mini Kit (Qiagen), and RNA quality was checked by agarose gel electrophoresis and A260/A280 measurements. RNA was stored at -80°C with SUPERase-IN RNase inhibitor (Life Technologies) until library preparation.
RNA sequencing and analysis
RNA sequencing (RNA-Seq) was performed as previously described [83]. Briefly, 400 ng of total RNA from each sample was used for generating cDNA libraries following the RNAtag-Seq protocol. PCR amplified cDNA libraries were sequenced on an Illumina NextSeq500, generating a high sequencing depth of ~7.5 million reads per sample. Raw reads were demultiplexed by 5’ and 3’ indices, trimmed to 59 base pairs, and quality filtered (96% sequence quality>Q14). Filtered reads were mapped to the corresponding reference genome using bowtie2 with the-very-sensitive option (-D 20 –R 3 –N 0 –L 20 –i S, 1, 0.50). Mapped reads were aggregated by feature Count, and differential expression was calculated with DESeq2 [84, 85]. In each pair-wise differential expression comparison, significant differential expression is filtered based on two criteria: |log2foldchange| > 1 and adjusted p-value (padj) <0.05. All differential expression comparisons were made between Arc6851 in LB and either ARC6851 plus Colistin, ARC6851 plus Diclofenac, or ARC6851 plus colistin plus diclofenac. The reproducibility of the transcriptomic data was confirmed by an overall high Spearman correlation across biological replicates (R > 0.95).
Sample preparation for Proteomic analysis
Proteomic cultures were prepared under the same conditions as RNAseq. Briefly, ARC6851 was grown overnight in LB before being diluted into 10mL of LB, LB + diclofenac (0.1 mM), LB + colistin 1 μg/ml) or LB + diclofenac + colistin and grown for 2 h at 37°C with shaking. Five individual overnight and 10mL culture biological replicates were prepared for each condition. Whole cells were harvested by pelleting the 10 mL cultures at 4°C, washing with ice-cold phosphate-buffered saline (PBS), and pelleting again before resuspending them in 4% SDS, 100mM Tris pH 8.5. Resuspended protein samples were solubilized by boiling them for 10 min at 95°C. The protein concentrations were then assessed by bicinchoninic acid protein assays (Thermo Fisher Scientific) and 200μg of each biological replicate prepared for digestion using Micro S-traps (Protifi, USA) according to the manufacturer’s instructions. Briefly, samples were reduced with 10mM DTT for 20 minutes at 95°C and then alkylated with 50mM IAA in the dark for 1 hour. Samples were acidified to 1.2% phosphoric acid and diluted with seven volumes of S-trap wash buffer (90% methanol, 100mM Tetraethylammonium bromide pH 7.1) before being loaded onto S-traps and washed 3 times with S-trap wash buffer. Samples were then digested with 4 μg of Trypsin/lys-C (Promega) overnight at 37°C before being collected by centrifugation with washes of 100mM Tetraethylammonium bromide, followed by 0.2% formic acid followed by 0.2% formic acid / 50% acetonitrile. Samples were dried down and further cleaned up using C18 Stage [86, 87] tips to ensure the removal of any particulate matter.
Reverse phase Liquid chromatography–mass spectrometry
C18 enriched proteome samples were re-suspended in Buffer A* (2% acetonitrile, 0.01% trifluoroacetic acid) and separated using a two-column chromatography setup composed of a PepMap100 C18 20-mm by 75-μm trap (Thermo Fisher Scientific) and a PepMap C18 500-mm by 75-μm analytical column (Thermo Fisher Scientific) using a Dionex Ultimate 3000 UPLC (Thermo Fisher Scientific). Samples were concentrated onto the trap column at 5 μl/minutes for 6 minutes with Buffer A (0.1% formic acid, 2% DMSO) and then infused into an Orbitrap 480 (Thermo Fisher Scientific) at 300 nl/minute via the analytical columns. Peptides were separated by altering the buffer composition from 3% Buffer B (0.1% formic acid, 77.9% acetonitrile, 2% DMSO) to 28% B over 70 minutes, then from 23% B to 40% B over 4 minutes and then from 40% B to 80% B over 3 minutes. The composition was held at 80% B for 2 minutes before being returned to 3% B for 10 minutes. The Orbitrap 480 Mass Spectrometer was operated in a data-dependent mode automatically switching between the acquisition of a single Orbitrap MS scan (300–160 m/z, maximal injection time of 25 ms, an Automated Gain Control (AGC) set to a maximum of 300% and a resolution of 120k) and 3 seconds of Orbitrap MS/MS HCD scans of precursors (Stepped NCE of 28;32;40%, a maximal injection time of 55 ms, a AGC of 300% and a resolution of 30k).
Proteomic data analysis
Identification and LFQ analysis were accomplished using MaxQuant (v2.2.0.0) [88] using the ARC6851 Proteome (NCBI accession: GCA_025677625.1 / ASM2567762v1) with Carbamidomethyl (C) allowed as a fixed modification and Acetyl (Protein N-term) as well as Oxidation (M) allowed as variable modifications with the LFQ and “Match Between Run” options enabled. The resulting data files were processed using Perseus (version 1.6.0.7) [89] with missing values imputed based on the total observed protein intensities with a range of 0.3 σ and a downshift of 1.8 σ. Statistical analysis was undertaken in Perseus using two-tailed unpaired T-tests and ANOVAs. Matching of protein homologs between the strain ATCC17978 (Uniprot accession: UP000072389) and UPAB1 Proteome (NCBI GCF_006843645.1 / ASM684364v1) was undertaken using the proteome comparison tool within PATRIC, the bacterial bioinformatics database and analysis resource [90].
Murine pneumonia model of infection
The murine pneumonia infections were performed as previously described for A. baumannii [36,48]. Briefly, overnight cultures of bacteria were subcultured into 100 mL at OD600 0.05 and grown at 37°C and shaking for 3 h to mid-exponential growth. Cultures were washed two times and resuspended in PBS. 6- to 8-week-old female C57BL/6 mice (Charles River Laboratories, Wilmington, MA, USA) were anesthetized by inhalation of 4% isoflurane, and then intranasally inoculated with 5 × 107 CFU of resuspended bacteria. At 4, 12, and 20 h post-infection, mice in the colistin and colistin plus diclofenac treatment groups were intraperitoneally (IP) injected with 2.5 mg/kg colistin sulfate salt in 100 μL PBS. Colistin treatment every 8 h was based on previous studies and was required due to the limited half-life of the compound in mouse serum [49–51]. At 4 h post-infection, mice in the diclofenac and colistin plus diclofenac treatment groups were IP injected with 1.25 mg/kg diclofenac sodium salt in 100 μL PBS, with the one timepoint in 24 h based on previous studies [91,92]. Groups not receiving a treatment(s) at 4, 12, and 20 h timepoints, were IP injected with a mock treatment of 100 μL PBS. At 24 h post-infection, the mice were sacrificed, and the lungs, kidneys, and spleens were aseptically removed. The bacterial load present in each tissue was determined by homogenizing each organ in PBS and plating serial dilutions on LB agar supplemented with chloramphenicol. Alternatively, survival rates were determined over a 72-hour period post infection.
Statistical analyses
All statistical analyses were performed using GraphPad Prism version 10.
Supporting information
Representative growth curves of P. aeruginosa 358800, 369569, and 409957 strains in LB containing either the solvent control DMSO or 100 μM diclofenac (DF) (left panel) and LB containing 16 μg/ml, 2 μg/ml, or 1 μg/ml colistin respectively in the presence of DMSO or 100 μM diclofenac (right panel). ****P<0.0001, unpaired t tests at 16 h for Col + DF 100 compared to DMSO control. Col (colistin), DF (diclofenac).
(TIF)
(A) Representative membrane permeability assay measuring uptake of Hoescht 33342 fluorescent dye. Bacterial cells were suspended in PBS supplemented with increasing concentrations of colistin (Col 1 μg/ml, 8 μg/ml, 16 μg/ml, 32 μg/ml, 64 μg/ml, or 128 μg/ml) in combination with the solvent control DMSO or 100 μM DF. Relative fluorescent units (RFU) were monitored over the course of 2 hours. (B) Cell membrane permeabilization assay measuring NPN uptake with or without efflux pump inhibitor CCCP. ****P<0.0001 (One-way ANOVA with Tukey’s test for multiple comparisons, in A results were compared to the control group treated with DMSO). Col (colistin), DF (diclofenac).
(TIF)
(A) Polymyxin B nanopetide and diclofenac do not affect ARC6851 growth. Representative growth curves of ARC6851 in LB containing increasing concentrations of Polimixin B nanopeptide (PBNP) with or without 100 μM diclofenac. (B) Diclofenac does not affect ARC6851 growth at concentrations up to 4,000 μM. Representative growth curves of ARC6851 in LB containing increasing concentrations of diclofenac (100 μM, 200 μM, 300 μM, 400 μM, 500 μM, 1,000 μM, 2,000 μM, 3,000 μM, or 4000 μM). DF (diclofenac).
(TIF)
Representative growth curves of ARC6851 in LB containing either the solvent control DMSO or 1 μg/ml colistin. Col (colistin).
(TIF)
Volcano plot of Supplemental data set showing differentially expressed genes in ARC6851 in diclofenac treatment vs DMSO control. DF (diclofenac).
(TIF)
(A) Number of differentially regulated genes in ARC6851 when treated with 1μg/ml colistin, 100 μM diclofenac, or in combination. (B) Donut charts representing the functional classifications of differentially regulated genes of the combination treatment against DMSO. Classifications were determined based on the designated clusters of orthologous groups (COGs) using eggNOG Mapper. Col (colistin), DF (diclofenac).
(TIF)
(A) Relative gene expression of pilA in ARC6851 grown in LB + DMSO, LB+ colistin (1 μg/ml) + DMSO, LB + diclofenac (100 μM), or LB + colistin (1 μg/ml) + diclofenac (100 μM) as determined by qRT-PCR. Dotted lines represent 2-fold change. (B) Transmission electron microscopy of ARC6851 WT in LB plus DMSO and LB plus colistin (1 μg/ml) plus diclofenac (100 μM). Scale bar of 500 nm is shown. (C) Quantification of type IV pili in ARC6851 WT was performed in 20 bacterial cells. Col (colistin), DF (diclofenac). Unpaired t tests *P < 0.05.
(TIF)
Lung lysates containing protease inhibitors were used to determine COX-2 activities using a COX fluorescent activity assay kit. Resorufin fluorescence can be analyzed with an excitation wavelength between 530–540 nm and an emission wavelength between 585–595 nm. DF (diclofenac).
(TIF)
(A) Transmission electron microscopy of ARC6851 ΔpilA in LB plus DMSO and LB plus colistin (1 μg/ml) plus diclofenac (100 μM). Scale bar of 500 nm is shown. (B) ΔpilA was screened for changes in MICs to colistin in combination with the solvent control DMSO and 100 μM diclofenac using a 2-fold broth dilution method. MIC was determined as <10% growth compared to a non-treated culture. (C) Representative growth curves of ARC6851 ΔpilA in LB containing 256 μg/ml colistin with increasing concentrations of diclofenac (25 μM, 40 μM, 50 μM, 60 μM, 75 μM, 90 μM, or 100 μM). (D) Representative growth curves of ARC6851 ΔpilA in LB containing 100 μM diclofenac with increasing concentrations of colistin (1 μg/ml, 8 μg/ml, 16 μg/ml, 32 μg/ml, 64 μg/ml, 128 μg/ml, or 256 μg/ml). *P<0.05, ***P<0.001, ****P<0.0001.
(TIF)
C57BL/6 mice were infected with ~5 × 107 CFU of mid-exponential ARC6851 WT or ΔpilA. At 24 h post-infection, the lungs, kidneys, and spleens were harvested, and the bacterial load present in each tissue was determined with serial dilutions. Each symbol represents an individual mouse, and the horizontal bar represents the Mean with SEM. Data collected from two independent experiments. *P < 0.05, **P < 0.01, Kruskal-Wallis test.
(TIF)
Gram-negative and Gram-positive bacteria were screened for changes in MICs to colistin in combination with the solvent control DMSO or 100 μM diclofenac using a 2-fold broth dilution method. MIC was determined as <10% growth compared to a non-treated culture. DF (diclofenac).
(TIF)
ARC6851 was screened for changes in MICs to triclosan in combination with either the solvent control DMSO or 20 μg/ml of oleic acid and linoleic acid, using a 2-fold broth dilution method. MIC was determined as <10% growth compared to a non-treated culture.
(TIF)
ARC6851 was screened for changes in MICs to colistin in combination with either the solvent control DMSO or 20 μg/ml of palmitic acid, araquidonic acid, linoleic acid, oleic acid, and stearic acid using a 2-fold broth dilution method. MIC was determined as <10% growth compared to a non-treated culture. Col (colistin), DF (diclofenac).
(TIF)
ARC6851 and UPAB1 were screened for changes in MICs to ciprofloxacin, erythromycin, tetracycline, vancomycin, imipenem, or sulfomethoxazole/trimethoprim in combination with the solvent control DMSO or 100 μM diclofenac using a 2-fold broth dilution method. MIC was determined as <10% growth compared to a non-treated culture. DF (diclofenac).
(TIF)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank Entasis Therapeutics for the ARC6851 isolate. We thank the Melbourne Mass Spectrometry and Proteomics Facility of The Bio21 Molecular Science and Biotechnology Institute for access to MS instrumentation. We thank David Rosen for the Klebsiella pneumoniae strains used in this study. We thank Michele LeRoux for the Pseudomonas strains used in this study. We thank Dakota Hall for technical support and the members of the Feldman lab for critical reading of the manuscript. We thank the imaging laboratory of the Molecular Microbiology Department at Washington University in St. Louis. We want to thank Wandy Beatty for her collaboration in obtaining the electron microscopy images shown in this work. We thank Lance Bottini for his diligent care of our instruments.
Data Availability
The transcriptomics data has been deposited in SRA database with the data set identifier: PRJNA1109398 https://www.ncbi.nlm.nih.gov/sra/PRJNA1109398. The mass spectrometry proteomics data including the full search results have been deposited in the Proteome Xchange Consortium via the PRIDE partner repository with the data set identifier: PXD047586.
Funding Statement
This work was supported by the National Institutes of Health grants R01AI166359 to MFF and R01AI148470 to TVO and JOM; the Australian Research Council Future Fellowship FT200100270 to NES; the Discovery Project Grant DP210100362 to NES, and the Australian National Health and Medical Research Council Ideas grant 2018980 to NES. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Giammanco A, Cala C, Fasciana T, Dowzicky MJ. Global Assessment of the Activity of Tigecycline against Multidrug-Resistant Gram-Negative Pathogens between 2004 and 2014 as Part of the Tigecycline Evaluation and Surveillance Trial. mSphere. 2017;2(1). doi: 10.1128/mSphere.00310-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia Casallas JC, Robayo-Amortegui H, Corredor-Rozo Z, Carrasco-Marquez AM, Escobar-Perez J. Bacteremia by colistin-resistant Acinetobacter baumannii isolate: a case report. J Med Case Rep. 2019;13(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–27. doi: 10.1016/S1473-3099(17)30753-3 [DOI] [PubMed] [Google Scholar]
- 4.Gurjar M. Colistin for lung infection: an update. J Intensive Care. 2015;3(1):3. doi: 10.1186/s40560-015-0072-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Velkov T, Thompson PE, Nation RL, Li J. Structure—activity relationships of polymyxin antibiotics. J Med Chem. 2010;53(5):1898–916. doi: 10.1021/jm900999h [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gogry FA, Siddiqui MT, Sultan I, Haq QMR. Current Update on Intrinsic and Acquired Colistin Resistance Mechanisms in Bacteria. Front Med (Lausanne). 2021;8:677720. doi: 10.3389/fmed.2021.677720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sampson TR, Liu X, Schroeder MR, Kraft CS, Burd EM, Weiss DS. Rapid killing of Acinetobacter baumannii by polymyxins is mediated by a hydroxyl radical death pathway. Antimicrob Agents Chemother. 2012;56(11):5642–9. doi: 10.1128/AAC.00756-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Falagas ME, Kasiakou SK. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin Infect Dis. 2005;40(9):1333–41. doi: 10.1086/429323 [DOI] [PubMed] [Google Scholar]
- 9.Gounden R, Bamford C, van Zyl-Smit R, Cohen K, Maartens G. Safety and effectiveness of colistin compared with tobramycin for multi-drug resistant Acinetobacter baumannii infections. BMC Infect Dis. 2009;9:26. doi: 10.1186/1471-2334-9-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linden PK, Paterson DL. Parenteral and inhaled colistin for treatment of ventilator-associated pneumonia. Clin Infect Dis. 2006;43 Suppl 2:S89–94. doi: 10.1086/504485 [DOI] [PubMed] [Google Scholar]
- 11.Garnacho-Montero J, Ortiz-Leyba C, Jimenez-Jimenez FJ, Barrero-Almodovar AE, Garcia-Garmendia JL, Bernabeu-Wittel IM, et al. Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis. 2003;36(9):1111–8. doi: 10.1086/374337 [DOI] [PubMed] [Google Scholar]
- 12.Kwa AL, Loh C, Low JG, Kurup A, Tam VH. Nebulized colistin in the treatment of pneumonia due to multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Clin Infect Dis. 2005;41(5):754–7. doi: 10.1086/432583 [DOI] [PubMed] [Google Scholar]
- 13.Michalopoulos A, Fotakis D, Virtzili S, Vletsas C, Raftopoulou S, Mastora Z, et al. Aerosolized colistin as adjunctive treatment of ventilator-associated pneumonia due to multidrug-resistant Gram-negative bacteria: a prospective study. Respir Med. 2008;102(3):407–12. doi: 10.1016/j.rmed.2007.10.011 [DOI] [PubMed] [Google Scholar]
- 14.Hartzell JD, Neff R, Ake J, Howard R, Olson S, Paolino K, et al. Nephrotoxicity associated with intravenous colistin (colistimethate sodium) treatment at a tertiary care medical center. Clin Infect Dis. 2009;48(12):1724–8. doi: 10.1086/599225 [DOI] [PubMed] [Google Scholar]
- 15.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, et al. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis. 2006;6(9):589–601. doi: 10.1016/S1473-3099(06)70580-1 [DOI] [PubMed] [Google Scholar]
- 16.Falagas ME, Kasiakou SK, Kofteridis DP, Roditakis G, Samonis G. Effectiveness and nephrotoxicity of intravenous colistin for treatment of patients with infections due to polymyxin-only-susceptible (POS) gram-negative bacteria. Eur J Clin Microbiol Infect Dis. 2006;25(9):596–9. doi: 10.1007/s10096-006-0191-2 [DOI] [PubMed] [Google Scholar]
- 17.Falagas ME, Rizos M, Bliziotis IA, Rellos K, Kasiakou SK, Michalopoulos A. Toxicity after prolonged (more than four weeks) administration of intravenous colistin. BMC Infect Dis. 2005;5:1. doi: 10.1186/1471-2334-5-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deryke CA, Crawford AJ, Uddin N, Wallace MR. Colistin dosing and nephrotoxicity in a large community teaching hospital. Antimicrob Agents Chemother. 2010;54(10):4503–5. doi: 10.1128/AAC.01707-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilic I, Ayar Y, Ceylan I, Kaya PK, Caliskan G. Nephrotoxicity caused by colistin use in ICU: a single centre experience. BMC Nephrol. 2023;24(1):302. doi: 10.1186/s12882-023-03334-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim LM, Ly N, Anderson D, Yang JC, Macander L, Jarkowski A, 3rd, et al. Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics, and dosing. Pharmacotherapy. 2010;30(12):1279–91. doi: 10.1592/phco.30.12.1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozkarakas H, Kose I, Zincircioglu C, Ersan S, Ersan G, Senoglu N, et al. Risk factors for colistin-associated nephrotoxicity and mortality in critically ill patients. Turk J Med Sci. 2017;47(4):1165–72. doi: 10.3906/sag-1604-60 [DOI] [PubMed] [Google Scholar]
- 22.Prasannan BK, Mukthar FC, Unni VN, Mohan S, Vinodkumar K. Colistin Nephrotoxicity-Age and Baseline kidney Functions Hold the Key. Indian J Nephrol. 2021;31(5):449–53. doi: 10.4103/ijn.IJN_130_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henry R, Vithanage N, Harrison P, Seemann T, Coutts S, Moffatt JH, et al. Colistin-resistant, lipopolysaccharide-deficient Acinetobacter baumannii responds to lipopolysaccharide loss through increased expression of genes involved in the synthesis and transport of lipoproteins, phospholipids, and poly-beta-1,6-N-acetylglucosamine. Antimicrob Agents Chemother. 2012;56(1):59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soudeiha MAH, Dahdouh EA, Azar E, Sarkis DK, Daoud Z. In vitro Evaluation of the Colistin-Carbapenem Combination in Clinical Isolates of A. baumannii Using the Checkerboard, Etest, and Time-Kill Curve Techniques. Front Cell Infect Microbiol. 2017;7:209. doi: 10.3389/fcimb.2017.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beceiro A, Llobet E, Aranda J, Bengoechea JA, Doumith M, Hornsey M, et al. Phosphoethanolamine modification of lipid A in colistin-resistant variants of Acinetobacter baumannii mediated by the pmrAB two-component regulatory system. Antimicrob Agents Chemother. 2011;55(7):3370–9. doi: 10.1128/AAC.00079-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moffatt JH, Harper M, Harrison P, Hale JD, Vinogradov E, Seemann T, et al. Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother. 2010;54(12):4971–7. doi: 10.1128/AAC.00834-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boinett CJ, Cain AK, Hawkey J, Do Hoang NT, Khanh NNT, Thanh DP, et al. Clinical and laboratory-induced colistin-resistance mechanisms in Acinetobacter baumannii. Microb Genom. 2019;5(2). doi: 10.1099/mgen.0.000246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin MF, Lin YY, Lan CY. Contribution of EmrAB efflux pumps to colistin resistance in Acinetobacter baumannii. J Microbiol. 2017;55(2):130–6. doi: 10.1007/s12275-017-6408-5 [DOI] [PubMed] [Google Scholar]
- 29.Ni W, Li Y, Guan J, Zhao J, Cui J, Wang R, et al. Effects of Efflux Pump Inhibitors on Colistin Resistance in Multidrug-Resistant Gram-Negative Bacteria. Antimicrob Agents Chemother. 2016;60(5):3215–8. doi: 10.1128/AAC.00248-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hooppaw AJ, McGuffey JC, Di Venanzio G, Ortiz-Marquez JC, Weber BS, Lightly TJ, et al. The Phenylacetic Acid Catabolic Pathway Regulates Antibiotic and Oxidative Stress Responses in Acinetobacter. mBio. 2022;13(3):e0186321. doi: 10.1128/mbio.01863-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rowlinson SW, Kiefer JR, Prusakiewicz JJ, Pawlitz JL, Kozak KR, Kalgutkar AS, et al. A novel mechanism of cyclooxygenase-2 inhibition involving interactions with Ser-530 and Tyr-385. J Biol Chem. 2003;278(46):45763–9. doi: 10.1074/jbc.M305481200 [DOI] [PubMed] [Google Scholar]
- 32.Dutta NK, Mazumdar K, Dastidar SG, Park JH. Activity of diclofenac used alone and in combination with streptomycin against Mycobacterium tuberculosis in mice. Int J Antimicrob Agents. 2007;30(4):336–40. doi: 10.1016/j.ijantimicag.2007.04.016 [DOI] [PubMed] [Google Scholar]
- 33.Eman FA RM-B, Abo BFA, Nancy GF, Neveen AA, Gamal FMG. Evaluation of antibacterial activity of some non-steroidal anti-inflammatory drugs against Escherichia coli causing urinary tract infection. African J Microbiol Res. 2016;10:1408–1416. [Google Scholar]
- 34.Mazumdar K, Dutta NK, Dastidar SG, Motohashi N, Shirataki Y. Diclofenac in the management of E. coli urinary tract infections. In Vivo. 2006;20(5):613–9. [PubMed] [Google Scholar]
- 35.Zhang S, Qu X, Tang H, Wang Y, Yang H, Yuan W, et al. Diclofenac Resensitizes Methicillin-Resistant Staphylococcus aureus to beta-Lactams and Prevents Implant Infections. Adv Sci (Weinh). 2021;8(13):2100681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGuffey JC, Jackson-Litteken CD, Di Venanzio G, Zimmer AA, Lewis JM, Distel JS, et al. The tRNA methyltransferase TrmB is critical for Acinetobacter baumannii stress responses and pulmonary infection. mBio. 2023:e0141623. doi: 10.1128/mbio.01416-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dastidar SG, Ganguly K, Chaudhuri K, Chakrabarty AN. The anti-bacterial action of diclofenac shown by inhibition of DNA synthesis. Int J Antimicrob Agents. 2000;14(3):249–51. doi: 10.1016/s0924-8579(99)00159-4 [DOI] [PubMed] [Google Scholar]
- 38.Dutta NK, Annadurai S, Mazumdar K, Dastidar SG, Kristiansen JE, Molnar J, et al. Potential management of resistant microbial infections with a novel non-antibiotic: the anti-inflammatory drug diclofenac sodium. Int J Antimicrob Agents. 2007;30(3):242–9. doi: 10.1016/j.ijantimicag.2007.04.018 [DOI] [PubMed] [Google Scholar]
- 39.Coldham NG, Webber M, Woodward MJ, Piddock LJ. A 96-well plate fluorescence assay for assessment of cellular permeability and active efflux in Salmonella enterica serovar Typhimurium and Escherichia coli. J Antimicrob Chemother. 2010;65(8):1655–63. doi: 10.1093/jac/dkq169 [DOI] [PubMed] [Google Scholar]
- 40.Richmond GE, Chua KL, Piddock LJ. Efflux in Acinetobacter baumannii can be determined by measuring accumulation of H33342 (bis-benzamide). J Antimicrob Chemother. 2013;68(7):1594–600. doi: 10.1093/jac/dkt052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carfrae LA, Rachwalski K, French S, Gordzevich R, Seidel L, Tsai CN, et al. Inhibiting fatty acid synthesis overcomes colistin resistance. Nat Microbiol. 2023;8(6):1026–38. doi: 10.1038/s41564-023-01369-z [DOI] [PubMed] [Google Scholar]
- 42.Palmer LD, Minor KE, Mettlach JA, Rivera ES, Boyd KL, Caprioli RM, et al. Modulating Isoprenoid Biosynthesis Increases Lipooligosaccharides and Restores Acinetobacter baumannii Resistance to Host and Antibiotic Stress. Cell Rep. 2020;32(10):108129. doi: 10.1016/j.celrep.2020.108129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heesterbeek DAC, Martin NI, Velthuizen A, Duijst M, Ruyken M, Wubbolts R, et al. Complement-dependent outer membrane perturbation sensitizes Gram-negative bacteria to Gram-positive specific antibiotics. Sci Rep. 2019;9(1):3074. doi: 10.1038/s41598-019-38577-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Muheim C, Gotzke H, Eriksson AU, Lindberg S, Lauritsen I, Norholm MHH, et al. Increasing the permeability of Escherichia coli using MAC13243. Sci Rep. 2017;7(1):17629. doi: 10.1038/s41598-017-17772-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cantalapiedra CP, Hernandez-Plaza A, Letunic I, Bork P, Huerta-Cepas J. eggNOG-mapper v2: Functional Annotation, Orthology Assignments, and Domain Prediction at the Metagenomic Scale. Mol Biol Evol. 2021;38(12):5825–9. doi: 10.1093/molbev/msab293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huerta-Cepas J, Szklarczyk D, Heller D, Hernandez-Plaza A, Forslund SK, Cook H, et al. eggNOG 5.0: a hierarchical, functionally and phylogenetically annotated orthology resource based on 5090 organisms and 2502 viruses. Nucleic Acids Res. 2019;47(D1):D309–D14. doi: 10.1093/nar/gky1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mi H WD, Xue Y, Zhang Z, Niu J, Hong Y, Drlica K, Zhao X. Dimethyl sulfoxide protects Escherichia coli from rapid antimicrobial-mediated killing. Antimicrob Agents Chemother. 2016;60:5054–5058. doi: 10.1128/AAC.03003-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmer LD, Green ER, Sheldon JR, Skaar EP. Assessing Acinetobacter baumannii Virulence and Persistence in a Murine Model of Lung Infection. Methods Mol Biol. 2019;1946:289–305. doi: 10.1007/978-1-4939-9118-1_26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ku NS, Lee SH, Lim YS, Choi H, Ahn JY, Jeong SJ, et al. In vivo efficacy of combination of colistin with fosfomycin or minocycline in a mouse model of multidrug-resistant Acinetobacter baumannii pneumonia. Sci Rep. 2019;9(1):17127. doi: 10.1038/s41598-019-53714-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pachon-Ibanez ME, Docobo-Perez F, Lopez-Rojas R, Dominguez-Herrera J, Jimenez-Mejias ME, Garcia-Curiel A, et al. Efficacy of rifampin and its combinations with imipenem, sulbactam, and colistin in experimental models of infection caused by imipenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother. 2010;54(3):1165–72. doi: 10.1128/AAC.00367-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sanderink D, Cassisa V, Chenouard R, Mahieu R, Kempf M, Dubee V, et al. Colistin-glycopeptide combinations against multidrug-resistant Acinetobacter baumannii in a mouse model of pneumonia. Future Microbiol. 2019;14:581–6. doi: 10.2217/fmb-2019-0022 [DOI] [PubMed] [Google Scholar]
- 52.Attridge SR, Voss E, Manning PA. The role of toxin-coregulated pili in the pathogenesis of Vibrio cholerae O1 El Tor. Microb Pathog. 1993;15(6):421–31. doi: 10.1006/mpat.1993.1091 [DOI] [PubMed] [Google Scholar]
- 53.Comolli JC, Hauser AR, Waite L, Whitchurch CB, Mattick JS, Engel JN. Pseudomonas aeruginosa gene products PilT and PilU are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect Immun. 1999;67(7):3625–30. doi: 10.1128/IAI.67.7.3625-3630.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eugene E, Hoffmann I, Pujol C, Couraud PO, Bourdoulous S, Nassif X. Microvilli-like structures are associated with the internalization of virulent capsulated Neisseria meningitidis into vascular endothelial cells. J Cell Sci. 2002;115(Pt 6):1231–41. doi: 10.1242/jcs.115.6.1231 [DOI] [PubMed] [Google Scholar]
- 55.Melican K, Michea Veloso P, Martin T, Bruneval P, Dumenil G. Adhesion of Neisseria meningitidis to dermal vessels leads to local vascular damage and purpura in a humanized mouse model. PLoS Pathog. 2013;9(1):e1003139. doi: 10.1371/journal.ppat.1003139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor RK, Miller VL, Furlong DB, Mekalanos JJ. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci U S A. 1987;84(9):2833–7. doi: 10.1073/pnas.84.9.2833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dutta NK, Mazumdar K, Seok SH, Park JH. The anti-inflammatory drug Diclofenac retains anti-listerial activity in vivo. Lett Appl Microbiol. 2008;47(2):106–11. doi: 10.1111/j.1472-765X.2008.02391.x [DOI] [PubMed] [Google Scholar]
- 58.Juttukonda LJ, Green ER, Lonergan ZR, Heffern MC, Chang CJ, Skaar EP. Acinetobacter baumannii OxyR Regulates the Transcriptional Response to Hydrogen Peroxide. Infect Immun. 2019;87(1). doi: 10.1128/IAI.00413-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fu Q, Liu S, Hu P, Chen H, Zheng J, Shi S, et al. Diclofenac Sodium Restores the Sensitivity of Colistin-Resistant Gram-Negative Bacteria to Colistin. ACS Infectious Diseases. 2024;10(8):2860–9. doi: 10.1021/acsinfecdis.4c00207 [DOI] [PubMed] [Google Scholar]
- 60.Harshey RM. Bacterial motility on a surface: many ways to a common goal. Annu Rev Microbiol. 2003;57:249–73. doi: 10.1146/annurev.micro.57.030502.091014 [DOI] [PubMed] [Google Scholar]
- 61.Merz AJ, So M, Sheetz MP. Pilus retraction powers bacterial twitching motility. Nature. 2000;407(6800):98–102. doi: 10.1038/35024105 [DOI] [PubMed] [Google Scholar]
- 62.Skerker JM, Berg HC. Direct observation of extension and retraction of type IV pili. Proc Natl Acad Sci U S A. 2001;98(12):6901–4. doi: 10.1073/pnas.121171698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wall D, Kaiser D. Type IV pili and cell motility. Mol Microbiol. 1999;32(1):1–10. doi: 10.1046/j.1365-2958.1999.01339.x [DOI] [PubMed] [Google Scholar]
- 64.Hockenberry AM, Hutchens DM, Agellon A, So M. Attenuation of the Type IV Pilus Retraction Motor Influences Neisseria gonorrhoeae Social and Infection Behavior. mBio. 2016;7(6). doi: 10.1128/mBio.01994-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ponisch W, Weber CA, Juckeland G, Biais N, Zaburdaev V. Multiscale modeling of bacterial colonies: how pili mediate the dynamics of single cells and cellular aggregates. New J Phys. 2017;19(1). doi: 10.1088/1367-2630/aa5483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vo N, Sidner BS, Yu Y, Piepenbrink KH. Type IV Pilus-Mediated Inhibition of Acinetobacter baumannii Biofilm Formation by Phenothiazine Compounds. Microbiol Spectr. 2023;11(4):e0102323. doi: 10.1128/spectrum.01023-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ronish LA, Lillehoj E, Fields JK, Sundberg EJ, Piepenbrink KH. The structure of PilA from Acinetobacter baumannii AB5075 suggests a mechanism for functional specialization in Acinetobacter type IV pili. J Biol Chem. 2019;294(1):218–30. doi: 10.1074/jbc.RA118.005814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grattagliano I, Bonfrate L, Diogo CV, Wang HH, Wang DQ, Portincasa P. Biochemical mechanisms in drug-induced liver injury: certainties and doubts. World J Gastroenterol. 2009;15(39):4865–76. doi: 10.3748/wjg.15.4865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Purcell P, Henry D, Melville G. Diclofenac hepatitis. Gut. 1991;32(11):1381–5. doi: 10.1136/gut.32.11.1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Breen EG, McNicholl J, Cosgrove E, McCabe J, Stevens FM. Fatal hepatitis associated with diclofenac. Gut. 1986;27(11):1390–3. doi: 10.1136/gut.27.11.1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Helfgott SM, Sandberg-Cook J, Zakim D, Nestler J. Diclofenac-associated hepatotoxicity. JAMA. 1990;264(20):2660–2. [PubMed] [Google Scholar]
- 72.Banks AT, Zimmerman HJ, Ishak KG, Harter JG. Diclofenac-associated hepatotoxicity: analysis of 180 cases reported to the Food and Drug Administration as adverse reactions. Hepatology. 1995;22(3):820–7. [PubMed] [Google Scholar]
- 73.Schmitz G, Stauffert I, Sippel H, Lepper H, Estler CJ. Toxicity of diclofenac to isolated hepatocytes. J Hepatol. 1992;14(2–3):408–9. doi: 10.1016/0168-8278(92)90196-v [DOI] [PubMed] [Google Scholar]
- 74.Bort R, Ponsoda X, Jover R, Gomez-Lechon MJ, Castell JV. Diclofenac toxicity to hepatocytes: a role for drug metabolism in cell toxicity. J Pharmacol Exp Ther. 1999;288(1):65–72. [PubMed] [Google Scholar]
- 75.Masubuchi Y, Yamada S, Horie T. Possible mechanism of hepatocyte injury induced by diphenylamine and its structurally related nonsteroidal anti-inflammatory drugs. J Pharmacol Exp Ther. 2000;292(3):982–7. [PubMed] [Google Scholar]
- 76.Kretz-Rommel A, Boelsterli UA. Cytotoxic activity of T cells and non-T cells from diclofenac-immunized mice against cultured syngeneic hepatocytes exposed to diclofenac. Hepatology. 1995;22(1):213–22. doi: 10.1002/hep.1840220132 [DOI] [PubMed] [Google Scholar]
- 77.Zabidi MS, Abu Bakar R, Musa N, Mustafa S, Wan Yusuf WN. Population Pharmacokinetics of Colistin Methanesulfonate Sodium and Colistin in Critically Ill Patients: A Systematic Review. Pharmaceuticals (Basel). 2021;14(9). doi: 10.3390/ph14090903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mermelstein F, Hamilton DA, Wright C, Lacouture PG, Ramaiya A, Carr DB. Single-dose and multiple-dose pharmacokinetics and dose proportionality of intravenous and intramuscular HPbetaCD-diclofenac (Dyloject) compared with other diclofenac formulations. Pharmacotherapy. 2013;33(10):1012–21. [DOI] [PubMed] [Google Scholar]
- 79.LoGuidice A, Wallace BD, Bendel L, Redinbo MR, Boelsterli UA. Pharmacologic targeting of bacterial beta-glucuronidase alleviates nonsteroidal anti-inflammatory drug-induced enteropathy in mice. J Pharmacol Exp Ther. 2012;341(2):447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pires S, Peignier A, Seto J, Smyth DS, Parker D. Biological sex influences susceptibility to Acinetobacter baumannii pneumonia in mice. JCI Insight. 2020;5(7). doi: 10.1172/jci.insight.132223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leus IV, Adamiak J, Trinh AN, Smith RD, Smith L, Richardson S, et al. Inactivation of AdeABC and AdeIJK efflux pumps elicits specific nonoverlapping transcriptional and phenotypic responses in Acinetobacter baumannii. Mol Microbiol. 2020;114(6):1049–65. doi: 10.1111/mmi.14594 [DOI] [PubMed] [Google Scholar]
- 82.Leus IV, Weeks JW, Bonifay V, Smith L, Richardson S, Zgurskaya HI. Substrate Specificities and Efflux Efficiencies of RND Efflux Pumps of Acinetobacter baumannii. J Bacteriol. 2018;200(13). doi: 10.1128/JB.00049-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhu Z, Surujon D, Ortiz-Marquez JC, Huo W, Isberg RR, Bento J, et al. Entropy of a bacterial stress response is a generalizable predictor for fitness and antibiotic sensitivity. Nat Commun. 2020;11(1):4365. doi: 10.1038/s41467-020-18134-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–30. doi: 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- 85.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rappsilber J, Ishihama Y, Mann M. Stop and go extraction tips for matrix-assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample pretreatment in proteomics. Anal Chem. 2003;75(3):663–70. doi: 10.1021/ac026117i [DOI] [PubMed] [Google Scholar]
- 87.Rappsilber J, Mann M, Ishihama Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc. 2007;2(8):1896–906. doi: 10.1038/nprot.2007.261 [DOI] [PubMed] [Google Scholar]
- 88.Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26(12):1367–72. doi: 10.1038/nbt.1511 [DOI] [PubMed] [Google Scholar]
- 89.Tyanova S, Temu T, Sinitcyn P, Carlson A, Hein MY, Geiger T, et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat Methods. 2016;13(9):731–40. doi: 10.1038/nmeth.3901 [DOI] [PubMed] [Google Scholar]
- 90.Wattam AR, Abraham D, Dalay O, Disz TL, Driscoll T, Gabbard JL, et al. PATRIC, the bacterial bioinformatics database and analysis resource. Nucleic Acids Res. 2014;42(Database issue):D581–91. doi: 10.1093/nar/gkt1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chirasani SR, Leukel P, Gottfried E, Hochrein J, Stadler K, Neumann B, et al. Diclofenac inhibits lactate formation and efficiently counteracts local immune suppression in a murine glioma model. Int J Cancer. 2013;132(4):843–53. doi: 10.1002/ijc.27712 [DOI] [PubMed] [Google Scholar]
- 92.Ghosh R, Goswami SK, Feitoza L, Hammock B, Gomes AV. Diclofenac induces proteasome and mitochondrial dysfunction in murine cardiomyocytes and hearts. Int J Cardiol. 2016;223:923–35. doi: 10.1016/j.ijcard.2016.08.233 [DOI] [PMC free article] [PubMed] [Google Scholar]