Abstract
Background
Residual malaria transmissions in Africa may be associated with improved coverage of insecticide-treated nets, house features, and livestock husbandry. These human-land use activities may drive the ecology and behaviour of malaria vectors which sustain residual malaria transmission. This study was conducted to assess changes in the ecology and behaviour of Anopheles funestus and Anopheles arabiensis in villages with high coverage of insecticide-treated nets to guide the selection of complementary vector control strategies against residual malaria transmission.
Methods
Mosquitoes were collected using a CDC-light trap, miniaturized double net trap, and Prokopack aspirator from 222 households in three villages (Ebuyu, Chirombora, and Mzelezi) within Kilombero Valley. Anopheles mosquitoes were morphologically identified to their physiological status and species-complex levels. A sub-sample of Anopheles mosquitoes was exposed to laboratory analyses of sibling species, host preference, and sporozoite rates. Additionally, the local houses were geo-referenced using Global Positioning Systems (GPS) devise, and house features were recorded and associated with vector abundance.
Results
The population of An. funestus s.s was abundant with high Plasmodium sporozoite rates inside houses compared to An. arabiensis. However, these vector species equally blood-fed on humans inside houses, but they also flexibly mixed human and animal blood meal. Fewer An. funestus were caught in houses with metal- than grass roofs and houses with and without animals. Contrastingly, fewer An. arabiensis were caught from houses with screened eaves compared to houses with open eaves.
Conclusions
This study confirms that An. funestus dominates residual malaria transmission over An. arabiensis. These vector species exhibit anthropophily and opportunistic blood-feeding behaviour in areas with high coverage of insecticide-treated nets, but they numerically respond differently to local house improvements. These results imply that integrating mosquito-proof houses, improved insecticide-treated nets, and livestock-based interventions could effectively reduce and eventually eliminate residual malaria transmission.
Background
Despite being preventable and curable, malaria continues to pose a persistent global public health challenge which causes 608,000 deaths and 249 million cases worldwide in 2022, a decrease of 11,000 deaths, and an increase of 5 million cases compared to 2021 [1, 2]. The majority of such global malaria burden (i.e. >94%) occurs in sub-Saharan Africa, particularly in children under five years, adolescent girls and pregnant women [3–6]. The protozoan parasites of genus Plasmodium with five known species such as P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi are responsible for causing malaria burden in humans [7]. Plasmodium falciparum is predominantly the deadliest malaria parasite carrying 96% of global malaria infections, particularly prevalent in Africa [8, 9]. Whereas the Plasmodium vivax is the second most important malaria parasite causing almost 2% of global malaria infections, and it is most prevalent/dominant in Southeast Asia and America [8, 10]. The other Plasmodium species are less prevalent and rarely cause deaths including P. malariae, and P. ovale in Africa [11–13], and Southeast Asia [14, 15], and P. knowlesi which causes malaria in humans and monkeys mainly in Southeast Asia [16, 17]. Malaria parasites are transmitted between humans through bites of Anopheles mosquitoes, with An. gambiae s.s, An. arabiensis, An. coluzii, and An. funestus s.s being major African malaria vectors [18, 19]. The variations in ecology and blood feeding behaivour of these major malaria vectors drive malaria transmission intensity [20, 21].
Major malaria vector control strategies in Africa include Insecticide Treated Nets (ITNs) and Indoor Residual Spraying (IRS) [22]. The effectiveness of these intervention exploits the behaviour of malaria vectors inside houses [23]. The scaling-up of these indoor-based interventions has successfully reduced the burden of malaria in Africa from 2000 to 2017 [24, 25]. These interventions effectively target malaria vectors such as An. gambiae s.s and An. funestus s.s which blood feed entirely on humans (anthropophily), inside houses (endophagy), and rests on the wall inside houses (endophily) [26]. However, the progress in reducing the malaria burden has stalled/plateaued with malaria control responses remaining at a cross-road/off track from 2017 to present due to ongoing persistent malaria transmission even in areas with high/absolute coverage of ITNs and IRS, which is known as residual malaria transmission [1, 27]. Malaria vectors evade ITNs and IRS through increased physiological insecticide resistance, behavioural changes, and vector species composition changes [28–31]. These vectors sustain residual malaria transmission in African countries which threatens to achieve the global technical strategy (GTS) goal of eliminating malaria by 2030. Therefore, a clear understanding of the bionomics of primary malaria vectors in areas with high coverage of ITNs is urgently needed to inform the selection of effective vector control intervention packages to complement ITNs and IRS in reducing residual malaria transmission and eventually reaching the global technical strategy goal of malaria elimination in Africa.
The residual malaria transmission may have several drivers in rural villages of African countries. The high coverage of ITNs and IRS inside houses, along with the application of insecticides on livestock and crop production have significantly contributed to increased selection pressure on endophilic and anthropophilic malaria vector populations. The development of widespread physiological insecticide resistance enables these vectors to survive the contact with insecticides on nets and walls to sustain malaria transmission inside houses [32, 33]: Notably, the insecticide resistance mechanisms such as target site insensitivity were observed in An. arabiensis, An. funestus and An. gambiae s.s [34–37], and metabolic insecticide resistance found in An. funestus and An. arabiensis [34, 36–41]. Malaria vectors can also adapt to changing environments of house designs, interventions ITNs/IRS, and the presence of alternative host species through behavioural modifications. These vector behavioural changes enable them to evade ITNs and IRS inside houses through changing biting time (e.g. from night to early morning/evening, daytime, and late night) when people are out of bed nets [33, 42–45], biting and resting location (i.e. from indoors to outdoors) [42, 44–50], and host choice (i.e. from feeding on humans (anthropophilic) to livestock (zoophily or opportunistic/plastic) [42, 48–56] Additionally, the ITNs and IRS could also induce numerical changes in vector populations leading to changes in vector species composition due to disproportionately reduced abundance of one vector species. High coverage of ITNs/IRS have significantly reduced the abundance of An. gambiae s.s or An. funestus s.s to undetectable levels inside and outside houses in most areas, leaving behaviourally resilient An. arabiensis sustaining residual malaria transmission in African countries including Tanzania [50, 52, 57–60].
Some of the physical house features where ITNS and IRS are applied in rural villages may also drive the exposure of humans to malaria vectors. For example, the house features include eaves, windows, doors, and keeping of livestock which are associated with mosquito entry and the abundance of mosquitoes inside houses. The previous studies on the association of households with livestock and the risks of exposing humans to malaria vectors in rural villages generated mixed results: 1) livestock attracted more malaria vectors to inside houses, provided an alternative blood meal source for reproduction, created more breeding habitats; consequently increased increase vector abundance and thus increases malaria risks to humans [53, 61–64] 2) livestock diverted malaria vectors away from humans, and reduced vector abundance inside houses, human mosquito biting rates/preferences, and thus reduced malaria risks to humans [53, 65–67]; and 3) no effect livestock on abundance of vector inside houses and malaria risks- it was as good as not having livestock close to households [68, 69]. These studies suggest that the effect of keeping livestock in/near houses on vector abundance and risk of malaria to humans could be linked with the type of local vector species, their ecology, and behaviour; livestock type and density, the distance between livestock and house, and local house features. Furthermore, previous studies demonstrated that malaria risk in rural villages was higher in poor house designs with open eaves, windows, mud walls, and thatched roofs (i.e., owned by the majority of residents) than those improved households with closed eaves, and metal roofs (i.e., mosquito proof houses) [70–72]. Such mosquito-proof houses may reduce the abundance of mosquitoes inside, but increase outdoor biting malaria vectors similar to ITNs and IRS inside houses. The impact of household features such as ITNs/IRS, livestock husbandry, and house improvements on the abundance, species composition, and behavior of primary malaria vectors is still a complex issue. further investigations these household features on malaria vectors bionomics in different ecological settings are required to inform the selection of complementary vector control strategies to ITNs.
This study aimed to evaluate the variations in the bionomics of primary malaria vectors inside houses and their implications for selecting effective complementary vector control strategies in regions with high ITN coverage (approximately 77.0%) within the Kilombero Valley in southeastern Tanzania. The specific aims of this study were therefore: 1) to determine the variation in abundance and Plasmodium falciparum infections between An. arabiensis and An. funestus, 2) to assess variation in blood feeding preferences between An. arabiensis and An. funestus, 3) to evaluate the impact of house improvements on the abundance of Anopheles arabiensis and Anopheles funestus inside houses.
Materials and methods
Study area
The cross-sectional entomological survey was conducted in three different villages in Ulanga district within Kilombero Valley located in the south-eastern region of Tanzania (Fig 1). Mosquitoes were collected from three villages: Ebuyu (-8.979 S, 36.760 E), Chirombola (-8.926 S, 36.753 E), and Mzelezi (-8.898 S, 36.735 E). Average annual rainfall ranged between 1200 and 1800 mm, and the mean annual temperature was 20–32°C, as reported by Ngowo et al. in 2017 [73]. Most residents in these villages practice subsistence agriculture including rice and maize cultivation; livestock husbandry and fishing as their primary economic activities. The typical local housing structures in this valley have the following features: clay brick walls, open eaves (the space between the roof and walls), and open windows. These human land use activities create favorable environments for breeding, feeding, resting, and survival of main malaria vectors that facilitate high mosquito densities throughout the year. Currently, the major malaria vectors in this valley that sustain malaria transmission include An. arabiensis and An. funestus [54, 74, 75]. These vectors mainly transmit Plasmodium falciparum which causes most of the malaria cases in the area, with An. funestus mediating >90% of malaria transmission in this valley [74, 76]. Another human land use activity in this valley is the use of ITNs against malaria vectors which has now reached approximately 77% of ITN coverage, and 63.3% of ITN usage.
Fig 1. Map of the study area showing three villages in Ulanga district, south-eastern Tanzania.
Mosquito sampling/collections from indoors and outdoors
The cross-sectional entomological survey was conducted from March 2022 to July 2022, a period that covered the rainy/wet (March ‐ May) and dry (June ‐ Sept) seasons. The abundance of malaria vectors such as An. arabiensis and An. funestus in the villages peaks during wet seasons, but it decreases during dry seasons. However, the abundance of An. funestus persists in some villages due to their preference for permanent/semi-permanent breeding habitats which exist throughout the year. Adult mosquitoes were collected from a total of 222 households in three different villages (i.e., 74 households/village x 3 villages = 222 households) as described previously [77]. Each trap design (i.e., CDC light traps and DN-Mini traps) was randomly assigned to 36 households and 1 household for each trap was used as a sentinel household (i.e. 36 households/trap design x 2 trap designs + 1 sentinel household/trap design x 2 trap designs = 74 households) as described previously [77]. The host-seeking mosquitoes were collected indoors and outdoors by using a double net trap (DN-mini trap) as described previously [78], but it was only from indoors using the CDC light trap as described previously [79]. The resting mosquitoes were also collected indoors and outdoors from sentinel households using a Prokopack aspirator as described elsewhere [80]. The Prokopack aspirator was introduced in houses with the DN-Mini trap and CDC-light trap to collect resting mosquitoes from both indoors and outdoors. These resting mosquitoes collected in Prokopack aspirators were used to understand the species composition and blood feeding behavior of mosquitoes that were not actively seeking hosts at the time of collection. However, the collection of host-seeking mosquitoes by DN-Mini trap and CDC-light trap aimed to capture mosquito host seeking behavior and species diversity within the area. Host-seeking mosquito collections were performed from 6 p.m. to 6 a.m., but resting mosquito collections were conducted from 6.45 a.m. to 7.00 a.m. The CDC light trap with a lid was hung about 1–1.5 m above the ground under an occupied bed net, but the DN-Mini trap was allocated in the sitting room, with male volunteers aged between 18years to 45 years inside acting as bait and collecting mosquitoes after every one-hour interval. A pair of volunteers in DN-Mini traps were rotated between night as described previously by Limwagu et al (2024) [77]. The resting mosquitoes were searched and collected from ceilings, under chairs, cooking pans, hung clothes, and water basins for indoor environments, they were also collected from buckets, clay pots, and car tires around 5metres from sentinel households for outdoor environments. Mosquitoes were collected from each household for three days a week for four months. Each time host-seeking mosquito collections from random households, 2 households were covered per day for 18 days per trap design. The resting mosquitoes were repeatedly collected from sentinel households at a rate of once per week for four months (1 day/week x 4 weeks/month x 4 months = 16 days). Additionally, all the sampled houses were also characterized through the collection of the variables including eave space status, roof type, wall type, window status, presence of animals and poultry inside and outside (i.e., around 100 meters) from each sampled houses, latrine location inside or outside houses. Geographical positioning system (Garming Extrex 20, GPS) device was used to collect GPS coordinates of each sampled house were also collected during household characterization.
Detection of malaria vector sibling species and their Plasmodium infection rates
Female Anopheles mosquitoes collected from inside and outside houses using three different traps were killed each morning using petroleum or alcohol fumes. These mosquitoes were morphologically sorted by taxa and physiological status. While the taxa were recorded as Anopheles gambiae complex or An. funestus group, the abdominal status of each female Anopheles mosquitoes was recorded as unfed, partly-blood fed, fully-blood fed, semi-gravid, or gravid. A sub-sample of An. gambiae complex and An. funestus group was packed individually in 1.5 ml microcentrifuge (Eppendorf®) tubes filled with silica gel and a piece of cotton wool. These mosquito samples were submitted to the Ifakara Health Institute molecular laboratory for sibling species identification using multiplex polymerase chain reaction (PCR). The sibling species of An. gambiae complex was identified using Scott’s protocol [81], but the members of An. funestus group were identified using Koekemoer’s protocol [82]. Additionally, a sub-sample of mosquitoes, consisting of whole bodies with intact body parts, was pooled, with no more than 10 individuals per species, and prepared for Circumsporozoite Protein Enzyme-Linked Immunosorbent Assay (CSP-ELISA). The head and thorax of mosquitoes in these pools were separated from the abdomen and tested for the presence of Plasmodium falciparum circum-sporozoite protein (Pf CSP) in their salivary glands using the enzyme-linked immunosorbent assay (ELISA) method [82]. To prevent detection of false positives, the ELISA lysates were boiled for 10minutes at 100°C to completely eliminate heat labile antigens of non-Plasmodium falciparum.
Detection of blood meal sources in malaria vectors
The blood meal content of Anopheline mosquitoes that were collected from all traps which were placed indoors and outdoors was analyzed using enzyme-linked immunosorbent assay (ELISA). The abdomens of all blood-fed malaria vectors collected were examined to determine their blood meal sources such as from one or a mixture of these host species: humans, goats, dogs, chickens, and bovines. Monoclonal antibodies (Antisera) against humans, cattle, goats, chickens, and dogs immunoglobulin G [IgG] identifiers (KPL, Gaithersburg, MD, USA) were utilized to identify blood meal sources from these different host species following the procedure described by Beier et al., [83].
Assessing the effects of household characteristics on malaria vector density
Mosquitoes were collected from both indoors and outdoors using three different traps (CDC light traps, DN Mini traps, and Prokopack aspirators). The participants also recorded various house characteristics. These details included the house type, construction materials, presence or absence of animals inside and outside around 100 meters from the houses. The overall condition of the house, encompassing factors like the presence of eave spaces, and the status of window and door screening. Additionally, precise coordinates of the house location were collected using a GPS device and concurrently saved in a data collection form. Such data collection form contained more information than what was initially stored in the GPS device. Finally, the effects of these household characteristics on malaria vector density were evaluated.
Data analysis
All data were analyzed using open-source statistical software, R program language version 4.2.11 [84]. The descriptive analysis was performed to summarize data on entomological indices including mosquito abundance, species composition, blood meal sources, and sporozoite rates using frequencies, mean, and proportions. Furthermore, the generalized linear mixed model directly incorporated negative binomial and zero-inflated functions using template model builder (GlmmTMB) was used to analyze mosquito count data [84]. Mosquito counts were modeled as response variables, while house characteristics were modeled as fixed terms. Additionally, the household ID and sampling date were included in the model as random terms to account for any unexplained variations between houses and pseudo-replicates. Significance levels were considered at p<0.05.
Ethical considerations
The study was conducted following the principles of the Declaration of Helsinki. Ethical approval for conducting this study was obtained from the Muhimbili University of Health and Allied Sciences Review Board (MUHAS-REC-12-2021-910). While the permission to publish the work was approved by NIMR (Ref No. BD.242/437/01A/17). Additionally, the permission to conduct this study in three villages within Ulanga district was also secured through the approval of the District Medical Officer of Ulanga district and the cooperation of local government authorities in the chosen villages. Before commencing the execution of the study, meetings were convened with local government leaders, and community members including the head of household to elucidate the objectives, methodologies, risks, and benefits of the study. The research team diligently obtained both verbal and written informed consent from all individual residents of households and the human volunteers engaged in mosquito collection in the mosquito traps. Participants were provided with detailed information regarding the potential benefits and risks associated with their participation, and their voluntary involvement was emphasized and respected. Participants were also assured of their right to withdraw from the study at any point if they wished to do so, without asking for any reasons for facing adverse consequences. Participants were also guaranteed confidentiality and ensured the anonymity of all participants.
Results
Mosquito abundance and their composition
A total of 19851 female mosquitoes were collected indoors and outdoors from March to July 2022 using the CDC-light trap, DN-Mini trap, and Prokopack aspirator. Among all host-seeking mosquitoes, the CDC-light trap captured 45.3% (n = 9008), the DN-Mini trap captured 39.5% (n = 7851) from indoors, but the DN-Mini trap also captured 4.4% (n = 881) from outdoors. Additionally, Prokopack aspirators captured resting mosquitoes at a rate of 8.8% (n = 1747) from indoors, and 1.8% (n = 364) from outdoors. Anopheles mosquitoes collected in traps: An. funestus group. were 6.14% (n = 1219), An. gambiae s.l. were 1.57% (n = 313), and An. coustani was 0.05% (n = 10). On the other hand, Culex species were the majority of mosquitoes collected in traps at a frequency of 92.07% (n = 18268), Mansonia were 0.14% (n = 29), and Aedes species were 0.01% (n = 2).
Malaria vector species composition
A sub-sample of 487 female Anopheles mosquitoes were submitted to the molecular laboratory for sibling species identification. Out of these mosquitoes, 419 individuals were morphologically identified as An. funestus group, and 68 individual mosquitoes as An. gambiae complex. When the PCR was performed on Anopheles mosquito samples, the amplified samples revealed sibling species of An. funestus group were An. funestus s.s (87.59%, n = 367) and An. rivulorum (0.24%, n = 1), while unamplified samples were 12.17% (n = 51). The amplified sibling species of An. gambiae s.l were An. arabiensis (88.24%, n = 60), and An. gambiae s.s (5.88%, n = 4), while the unamplified samples were 5.88% (n = 4).
Plasmodium sporozoite rate malaria vectors
A sub-sample of 487 Anopheles mosquitoes collected using CDC light traps and DN-Mini traps were further analyzed in the molecular laboratory to determine their Plasmodium falciparum infection rates through exposure to ELISA. Out of these Anopheles mosquitoes, 14.0% (n = 68) were morphologically identified as An. gambiae s.l and 86.0% (n = 419) were identified as An. funestus. The Plasmodium falciparum sporozoites rate was 1.4% (n = 1) in An. arabiensis and 3.1% (n = 13) in An. funestus group. Sporozoite infections in An. arabiensis were detected in mosquitoes caught using DN-Mini traps placed indoors in Ebuyu village during the dry season. For An. funestus, all 13 sporozoite infected mosquitoes were detected in samples collected from indoors in three different villages. In Chirombora village, one mosquito from a CDC-light trap and two mosquitoes from DN-Mini traps. In Ebuyu village, one mosquito from CDC-light trap and four from DN-Mini traps. In Mzelezi village, two mosquitoes were from CDC-light traps and three mosquitoes were from DN-Mini traps. Regarding seasonality, nearly all infections in these mosquitoes were detected during the wet season, except for three An. funestus mosquitoes captured in the dry season.
Blood meal sources in malaria vectors
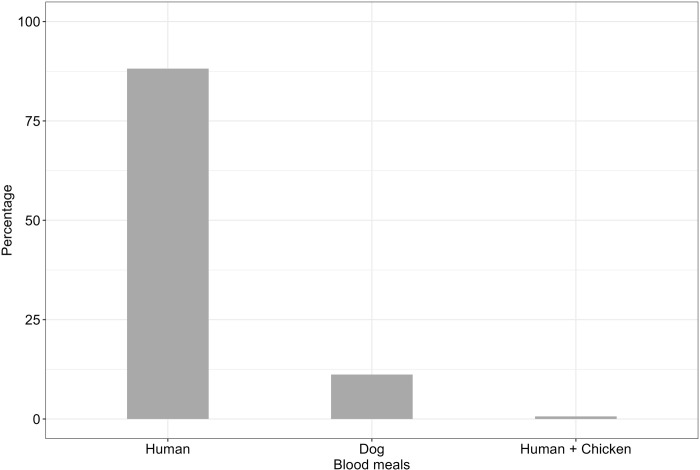
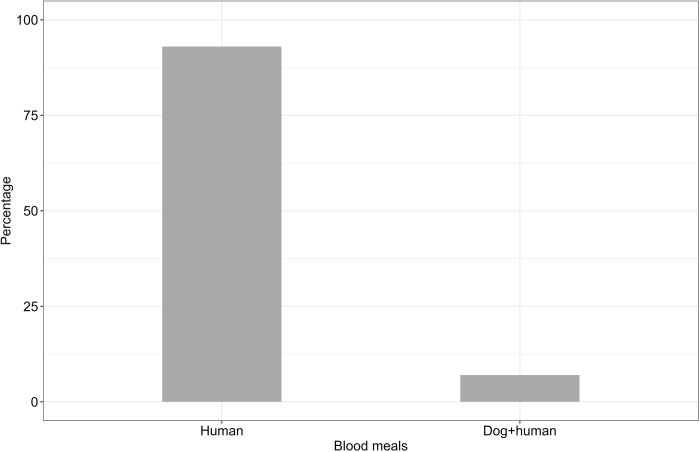
A total of 449 female Anopheline mosquitoes collected using CDC light traps, DN-Mini traps, and Prokopack aspirators were morphologically observed to have ingested a blood meal in their abdomen. The abdominal samples with blood contents were exposed to ELISA to detect blood meal sources. Out of 449 Anopheline mosquitoes, 420 individuals were An. funestus and 29 were An. arabiensis. Out of 449 female Anopheles mosquitoes submitted to molecular laboratory for blood meal-ELISA analysis, mosquitoes with blood meal source identified were 37.2%, (n = 167), and unidentified were 62.8%, (n = 282). Among 167 confirmed blood fed Anopheles mosquitoes: An. funestus were 152, and An. arabiensis were 15. The predominant host blood antigens detected in An. funestus were humans (88.2%), followed by dogs (11.2%), while An. arabiensis had 93.3% for human blood meal (Figs 2 and 3). Additionally, these vector species were observed to mix their blood meals: mixture of blood from humans and dogs (6.7%) for An. arabiensis; and mixture of blood from humans and chickens (0.6%) for An. funestus (Figs 2 and 3).
Fig 2. Blood meal contents of An. funestus from different host species identified by the ELISA method from the sample collected in three different villages.
Fig 3. Blood meal contents of An. arabiensis from different host species identified by the ELISA method from the sample collected in three different villages.
Mosquitoes predominantly fed on humans, with 136 indoor and 11 outdoor. Feeding on dogs and mixed hosts (Dog/Human, Human/Chicken) as observed only indoors. During the wet season, mosquitoes showed a broader range of host feeding, with higher counts for both dog and human blood meals compared to the dry season Table 1.
Table 1. Number of blood meal sources of malaria vectors across different hosts in various location (indoors and outdoors) and seasonality (wet and dry).
| Variables | Host | Total | |
|---|---|---|---|
| Location | Indoor | Dog | 17 |
| Dog/Human | 1 | ||
| Human | 136 | ||
| Human/Chicken | 1 | ||
| Outdoor | Dog | 0 | |
| Dog/Human | 0 | ||
| Human | 11 | ||
| Human/Chicken | 0 | ||
| Season | Dry | Dog | 0 |
| Dog/Human | 0 | ||
| Human | 34 | ||
| Human/Chicken | 0 | ||
| Wet | Dog | 17 | |
| Dog/Human | 1 | ||
| Human | 113 | ||
| Human/Chicken | 1 | ||
Effects of household characteristics on vector density
Mosquito catches from inside houses were influenced by the house characteristics. Notably, fewer An. funestus were caught from inside houses with metal roofs compared to houses with grass roofs (RR = 0.646, p <0.05, Table 2). Similarly, fewer numbers of An. funestus were caught from inside houses with animals than houses without animals (RR = 0.672, p <0.01, Table 2). However, the number of An. funestus inside houses were similar between houses with open versus screened ceilings (RR = 0.780, p = 0.454, Table 2), open versus screened eave spaces (RR = 0.978, p = 0.864, Table 2), or bricks versus mud wall materials (RR = 0.690, p = 0.107, Table 2). Conversely, the catches of An. arabiensis. were significantly lower in houses with screened eaves compared to houses with open eaves (RR = 0.108, p < 0.01, Table 2). However, the number of An. arabiensis caught from inside houses were similar between houses with grass versus metal roofs (RR = 1.600, p = 0.587, Table 2), open versus screened ceilings (RR = 0.526, p = 0.575, Table 2), brick versus mud wall materials (RR = 0.351, p = 0.225, Table 2), and presence versus absence of animals (RR = 0.385, p = 0.254, Table 2).
Table 2. Effect of household characteristics on malaria vector densities inside houses.
| Species | Variable (Household Characteristics) | Total number of mosquitoes indoors | Type | Mean ± 2SE | RR [95% CI] | p-values |
|---|---|---|---|---|---|---|
| Anopheles funestus | Roof Materials | 271 | Grass | 2.24 ± 0.972 | 1 | <0.05 |
| 624 | Metal | 2.10 ± 0.276 | 0.646 [0.418, 0.998] | |||
| Ceiling | 269 | No | 2.12 ± 0.272 | 1 | = 0.454 | |
| 2 | Yes | 2.00 ± 1.20 | 0.780 [0.407, 1.495] | |||
| Eaves | 858 | Open | 2.02 ± 0.314 | 1 | = 0.864 | |
| 324 | Screened | 2.39 ± 0.498 | 0.978 [0.761, 1.258] | |||
| Wall Materials | 201 | Brick | 2.15 ± 0.285 | 1 | = 0.107 | |
| 68 | Mud | 1.76 ± 0.717 | 0.690 [0.439, 1.083] | |||
| Animal (100) | 170 | No | 2.47 ± 0.402 | 1 | <0.01 | |
| 31 | Yes | 1.71 ± 0.331 | 0.672 [0.534, 0.847] | |||
| Anopheles arabiensis | Roof Materials | 81 | Grass | 0.206 ± 0.199 | 1 | = 0.587 |
| 166 | Metal | 0.203 ± 0.083 | 1.600 [0.294, 8.715] | |||
| Ceiling | 81 | Open | 0.203 ± 0.079 | 1 | = 0.575 | |
| 0 | Screened | 0.2 ± 0.392 | 0.526 [0.055, 4.993] | |||
| Eaves | 247 | Open | 0.231 ± 0.096 | 1 | <0.01 | |
| 66 | Screened | 0.120 ± 0.108 | 0.108 [0.022, 0.536] | |||
| Wall Materials | 71 | Brick | 0.206 ± 0.084 | 1 | = 0.225 | |
| 10 | Mud | 0.176 ± 0.154 | 0.351 [0.065, 1.899] | |||
| Animal (100) | 66 | No | 0.115 ± 0.080 | 1 | = 0.254 | |
| 5 | Yes | 0.301 ± 0.135 | 0.385 [0.074, 1.986] |
Discussion
This study demonstrates that Anopheles funestus and Anopheles arabiensis are the main malaria vectors in Kilombero Valley. The former vector species contributing greatly in sustaining residual malaria transmission. Anopheles funestus has higher abundance and Plasmodium falciparum sporozoite rates compared to An. arabiensis. However, An. funestus and An. arabiensis expressed highest degree (i.e., >85%) of blood feeding on humans (anthrophily) inside houses (endophagy). These vector species can also flexibly feed on animals or mix blood meals from humans and animals, but An. arabiensis have high ability of mixing host species than An. funestus. The abundance of An. funestus inside houses was significantly reduced in houses with metal than grass roofing materials, and houses with the presence than the absence of animals. Such abundance of An. funestus was never affected in houses with brick wall materials or screening eave spaces and ceilings. Contrastingly, the abundance of An. arabiensis inside houses was reduced through screening eaves, but it was never reduced through house improvements using brick wall materials, metal roofs, screening ceilings, or the presence of animals outside houses. Overall, the population of An. funestus mediates more residual malaria transmission than An. arabiensis, but some of local house features may reduce the potential of these vectors to transmit malaria in rural villages of Kilombero Valley.
Our study found that An. funestus has a high abundance and Plasmodium falciparum sporozoite rates than An. arabiensis that enable it to sustain residual malaria transmission in three villages within Kilombero Valley. Possibly, the increasing abundance of An. funestus in the study area could be linked with its greater life-expectancy/daily survival probability/parity rate, and high intensity of pyrethroid resistance (e.g. metabolic resistance) against ITNs [39, 85]. These traits enable them to survive longer, frequently blood feeding on humans, reproduce/breed, acquire and transmit Plasmodium sporozoite infections between humans even in the presence of high coverage of ITNs. For example, most of rural village of Kilombero Valley have 77.0% of ITNs coverage and 63.3% of ITN usage inside the house [86], which suggests that the area experiences residual malaria transmission. An. funestus prefer breeding in permanent and semi-permanent aquatic habitats (e.g., river streams, and ground pools/ponds). These large, permanent breeding habitats for An. funestus persist in both wet and dry seasons throughout the year relative to those temporary small and sunlit breeding habitats of An. arabiensis [87, 88]. Previous field studies demonstrated that An. funestus mediated more malaria transmission in different areas such as Tanzania [48], Burkina Faso [89], Benin [90], Cameroon [91], Malawi [92], Zambia [93], and Kenya [94, 95]. These studies linked the ability of An. funestus to dominate malaria transmission with their high sporozoite rates inside houses. However, some studies in other places have confirmed that both An. funestus and An. arabiensis may have similar high abundance or high sporozoite rates which indicates their equal contributions to malaria transmission [96]. Recent studies confirm that the population of An. funestus is increasing its dominance over An. arabiensis in terms of both abundance and sporozoite rates in certain African settings including Tanzania [57, 92, 97–99]. Furthermore, our study also observed a slight increase in abundance and sporozoite rates of An. arabiensis inside houses almost similar to the population of An. arabiensis in Zambia in an area with high coverage of ITN [100]. This observation suggests that An. arabiensis also increases their potential role in residual malaria transmission.
Malaria transmission intensity may be strongly linked with their blood feeding preference in humans (i.e. anthropophily) of malaria vectors. Our study revealed that both An. funestus and An. arabiensis obtained blood meal mainly from humans (high anthropophily) inside houses. While An. funestus fed on humans at a frequency of > 87.5%, An. arabiensis obtained human blood at a rate of 93% from inside houses in our study area [91]. Furthermore, our study also demonstrated that An. funestus and An. arabiensis can also flexibly blood feed on animals or mixed blood meal from animals and humans with An. arabiensis having highest degree of opportunistic behaviour than An. funestus. Such highest anthropophily behaviour in both An. funestus and An. arabiensis observed in our study area could be linked with the fact that mosquitoes collected from inside houses using CDC light traps and DN-mini traps were most likely fed on humans slept inside houses as their only accessible primary source of blood meal [77]. Both two species An. funestus and An. arabiensis seems to feed on human than on dogs indoors during wet season than dry season, this is consistent with previous observations of indoor feeding behaviour among the An. funestus and An. arabiensis [58]. Additionally, the feeding on humans by mosquitoes collected outdoors by both two methods DN-Mini trap and Prokopack aspirator across seasons, suggests that human hosts remain the primary target outdoors, despite availability of other potential hosts [53].The anthropophily in both vector species could also be linked with their high intensity of pyrethroid resistance that enabled them to contact ITN and continue blood feeding on humans inside houses in presence of ITNs [39, 101]. Additionally, the plasticity in blood feeding behaviour/mixed blood meals in both vector species could be associated with the common practice of most rural communities keeping dogs and chickens inside or near their human dwelling houses which increases chances of malaria vectors blood feeding on available alternative hosts [54, 102, 103]. Such mixed blood meals in mosquitoes are attributed with the low nutritional contents-quality/quantity of first blood meal ingested, host defensive behaviour (e.g. humans protected under ITNs), host movements (i.e. thwarting, skin shaking) and environmental factors (e.g. house modifications to mosquito-proof) which makes vectors to seek additional blood meal from alternative sources [71, 104–106]. Our findings agree with previous studies which demonstrate that An. funestus are highly anthrophilic in most African settings including Tanzania [54, 76, 92–95, 97], and they can also flexibly blood feed on alternative hosts such as dogs and cattle [53, 54, 92, 94, 96, 97]. Additionally, our result also agrees with other field studies which showed that An. arabiensis often exhibit opportunistic/plastic blood-feeding behaviour through obtaining blood from either humans or any available alternative host species such as cattle and dogs [48, 55, 56, 96, 100]. These previous studies suggest that the variations in blood-feeding behaviour patterns and host preferences of An. funestus and An. arabiensis may be linked with the availability and accessibility of their preferred host species such as humans and cattle in rural villages. Although An. arabiensis and An. funestus exhibited a certain degree of plasticity in their blood-feeding preference, they also expressed the highest anthropophily inside houses with ITNs which drives continued malaria transmission.
Our study also found that certain house improvements could reduce the risks of exposing humans to malaria-transmitting mosquitoes. Previous studies revealed that most of households in rural villages have open-eave gaps, unscreened ceilings, windows, and doors that provide the main mosquito entry points into the houses [107], and livestock such as cattle, goats, and chickens near their households which attract mosquitoes [53]. Our study found that while houses with metal roofing materials reduced the abundance of An. funestus inside house than those houses with grass roofing materials, houses with screened eave gaps reduced the abundance of An. arabiensis relative to those houses without screened eave gaps. Additionally, the houses with livestock had reduced the abundance of An. funestus inside houses, but it never reduced the abundance of An. arabiensis inside houses. A possible explanation of our results could be that mosquitoes detect odour plumes emanating from human hosts to the outside via openings like eave gaps. Closing these gaps in houses prevents the flow of odour plumes to the outside of the house that guide mosquitoes towards house entry points. The lack of effect of livestock on abundance of An. arabiensis inside houses could be associated with their generalist host preference. These mosquitoes are equally attracted to and feed on both humans and animals based on host abundance and availability, and they can enter houses through various entry points. Our study supports those previous studies which found high numbers of An. funestus and An. arabiensis inside houses with grass/thatched roofing materials than those houses with metal roofing materials [26, 108]. These previous studies confirm that mosquitoes shift from resting on ceiling to other places including clothing, furniture, and floors due to unfavourable temperature and relative humidity that affect their survival inside houses with metal roofing materials [26]. Contrastingly, the houses with grass roofs provide optimal temperature, humidity environments for mosquito blood feeding, resting, egg production and survival. Also, our findings concur with previous studies which confirm that screened-eave gaps reduce the abundance of mosquitoes inside houses (e.g., An. arabiensis and An. funestus) compared to those houses with open-eave gaps [71, 109–112]. Although house improvement showed reduced vector density and risk of malaria inside houses, no protections were attained in some areas with low seasonal malaria transmission with high coverage of indoor-based interventions [113]. Furthermore, our study supports previous findings that keeping livestock near houses may reduce the abundance of malaria vectors inside houses (e.g., An. arabiensis, An. funestus) than those houses without animals, and it reduces mosquito biting rates on humans and risk of malaria [55, 65, 66, 113]. These previous studies further suggest that most malaria vectors including opportunistic An. arabiensis and anthropophilic vectors (e.g. An. gambiae s.s and An. funestus) are likely to be diverted to blood feed on cattle than humans, and rest inside/nearby cattle shed, which reduces mosquito biting rates on humans and sporozoite rates. Our study also supports those previous studies that demonstrated a similar density of An. arabiensis inside houses between houses with and without livestock [65]. On the contrary, other studies argue that keeping livestock like cattle close to houses may attract more mosquitoes to the houses that increase risks of mosquito entry inside houses, and mosquito biting rates in humans [53, 55, 63, 114]. Therefore, holistic house improvements (i.e. never single house components) and properly locating/positioning livestock houses/sheds could have a potential impact on malaria vector feeding preference, and risks of exposing humans to malaria.
The findings of this study suggest important implications for malaria transmission and effective complementary vector control strategies to ITN against residual malaria transmission. The increasing abundance, persistent anthropophily behaviour, and high sporozoite of An. funestus in areas with high coverage of ITNs suggest that vectors have evolved physiological resistance against ITNs that enable them to survive, blood feed on humans inside houses, reproduce, increase vector density and transmit malaria. For example, the population of An. funestus have exhibited high levels of Plasmodium sporozoite infections, and metabolic insecticide resistance against pyrethroids that enable them to sustain residual malaria transmission in most areas with high coverage of ITNs [39, 98, 115–117]. The improved ITNs including those integrating pyrethroids with Piperonyl Butoxide (ITN-PBO), Chlorfenapyr (ITN-Chlorfenapyr), or Pyriproxyfen (ITN-Pyriproxyfen) could be deployed to effectively counteract metabolic insecticide resistance in An. funestus and An. arabiensis in areas with sustained malaria transmission [22, 118, 119]. These vector species also expressed a certain degree of opportunistic blood feeding on livestock such as cattle and dogs. This vector behaviour enables them to obtain blood meal for their survival and reproduction leading to increased vector density while evading contact with ITNs inside houses to sustain residual malaria transmission. Accordingly, livestock-based interventions such as the treatment of livestock with endoctocides (e.g., ivermectin treatment of cattle–ITC technology) confirmed to reduce the survival and density of malaria vectors feeding on treated animal hosts [120–123]. Therefore, ITC technology could be an appropriate one health approach complementary to ITNs in the management of outdoor biting, animal feeding, and physiologically resistant vectors to reduce residual malaria transmission [23]. Our study also provided evidence that simple improvement of local houses through screened-eave gaps, metal roofs, and livestock husbandry reduces the density of malaria vectors inside houses. Because most houses in rural villages have open eaves, windows, and doors, and keep livestock which increases the abundance of malaria vectors inside houses, and mosquito biting rates on humans, the holistic house improvements, and proper positioning of livestock pens could be complementary vector control tool to ITNs in reducing the risk of residual malaria transmissions [22, 23, 124]. Overall, the improved ITNs, houses, and livestock-based interventions could be integrated to effectively reduce and eventually eliminate residual malaria transmission.
This study has also few limitations. First, the geographical scope of the study, it focused only on three villages of Kilombero Valley. This could restrict the applicability of the findings to other regions with different ecological and socio-economic conditions. Further studies on impact of house feature in vector bionomics could be performed in various ecological and socio-economical settings in the future. Second a significant proportion of blood fed Anopheles mosquitoes (i.e. 63%) were un-identified using ELISA. Such failure to detect host species specific blood proteins (antigens) could be linked to partial digestion of blood proteins (denaturation), or miss-match between antisera used in this assay and the specific proteins (antigen) for host species found in the study area. Future studies in our study area should consider using polymerase chain reactions (PCR) or proteomics such as Maldi-Tof techniques for blood meal analysis in mosquitoes.
Conclusions
The current study demonstrates that variation in abundance, blood-feeding preferences, sporozoites rates of primary malaria vectors inside local houses, and their implications for selecting effective complementary vector control strategies to ITNs in Kilombero Valley. The higher abundance and sporozoite rates of An. funestus than An. arabiensis makes it dominating residual malaria transmission. However, these vector species continue to blood-feed on humans inside houses (anthropophily, endophily), and they can also opportunistically feed on alternative host species such as cattle or dogs in villages with high coverage of ITNs. Moreover, certain local house features significantly reduces the abundance of both vector species in rural villages. Therefore, a comprehensive intervention package that includes improved ITNs, holistic house improvements, and livestock-based measures (e.g., ivermectin treatment of cattle- ITC technology) could enhance the control of An. funestus and An. arabiensis that sustain residual malaria transmission in Kilombero Valley.
Supporting information
(XLS)
Acknowledgments
We extend our heartfelt appreciation to the village leaders and communities of the Ulanga district for their invaluable cooperation and permission, which enabled the successful execution of this study. Our sincere gratitude goes to all the dedicated volunteers who played a crucial role in collecting data during this study. We also thank Dr. Yeromin Mlacha for reviewing the manuscript. We offer special thanks to our project administrator, Rukiya Mohamed, for her exceptional support in logistical and administrative arrangements during the study. Additionally, we acknowledge the diligent efforts of Francis Tumbo for technical support in processing laboratory samples.
List of abbreviations
- CDC
Centre for Disease Control
- CSP
Circumsporozoite protein
- DN
Double Net
- ELISA
Enzyme-linked immunosorbent assays
- EMM
Estimated Marginal Means
- GPS
Geographic Position System
- GLMM
Generalized Linear mixed model
- GTS
Global Technical Strategy
- IRS
Indoor Residual Spray
- ITNs
Insecticide Treated Nets
- ITC
Ivermectin Treated Cattle
- MUHAS
Muhimbili University of Health and Allied Sciences
- NIMR
National Institute for Medical Research
- PBO
Peperonyl Butoxide
- PCR
Polymerase Chain Reaction
- WHO
World Health Organization
Data Availability
All relevant data are within the manuscript and its Supporting Information file
Funding Statement
This study was funded by the Bill and Melinda Gates Foundation, Awarded through the Pan-Africa Mosquito Control Association (PAMCA), Grant recipient Dr. Fredros O. Okumu - Ifakara Health Institute, Grant Number 1214408.
References
- 1.WHO. World malaria report. 2023.
- 2.WHO. World malaria report. 2022.
- 3.Pons-Duran C, Mombo-Ngoma G, Macete E, Desai M, Kakolwa MA, Zoleko-Manego R, et al. Burden of malaria in pregnancy among adolescent girls compared to adult women in 5 sub-Saharan African countries: A secondary individual participant data meta-analysis of 2 clinical trials. PLoS Med. 2022;19: e1004084. doi: 10.1371/journal.pmed.1004084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chilot D, Mondelaers A, Alem AZ, Asres MS, Yimer MA, Toni AT, et al. Pooled prevalence and risk factors of malaria among children aged 6–59 months in 13 sub-Saharan African countries: A multilevel analysis using recent malaria indicator surveys. PLoS One. 2023;18: (5): e0285265. doi: 10.1371/journal.pone.0285265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oshagbemi OA, Lopez-Romero P, Winnips C, Csermak KR, Su G, Aubrun E. Estimated distribution of malaria cases among children in sub-Saharan Africa by specified age categories using data from the Global Burden of Diseases 2019. Malar J. 2023;22: (1):371. doi: 10.1186/s12936-023-04811-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarfo JO, Amoadu M, Kordorwu PY, Adams AK, Gyan TB, Osman AG, et al. Malaria amongst children under five in sub-Saharan Africa: a scoping review of prevalence, risk factors and preventive interventions. Eur J Med Res. 2023;28: 80. doi: 10.1186/s40001-023-01046-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mekonnen SK, Aseffa A, Medhin G, Berhe N, Velavan TP. Re-evaluation of microscopy confirmed Plasmodium falciparum and Plasmodium vivax malaria by nested PCR detection in southern Ethiopia. Malar J. 2014;13: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oboh MA, Oyebola KM, Idowu ET, Badiane AS, Otubanjo OA, Ndiaye D. Rising report of Plasmodium vivax in sub-Saharan Africa: Implications for malaria elimination agenda. Sci Afr. 2020;10: e00596. [Google Scholar]
- 9.Mwesigwa A, Ocan M, Musinguzi B, Nante RW, Nankabirwa JI, Kiwuwa SM, et al. Plasmodium falciparum genetic diversity and multiplicity of infection based on msp-1, msp-2, glurp and microsatellite genetic markers in sub-Saharan Africa: a systematic review and meta-analysis. Malaria J. 2024;23: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howes RE, Reiner RC, Battle KE, Longbottom J, Mappin B, Ordanovich D, et al. Plasmodium vivax Transmission in Africa. PLoS Negl Trop Dis. 2015;9: (11): e0004222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sendor R, Banek K, Kashamuka MM, Mvuama N, Bala JA, Nkalani M, et al. Epidemiology of Plasmodium malariae and Plasmodium ovale spp. in Kinshasa Province, Democratic Republic of Congo. Nat Commun. 2023;14: (1):6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graumans W, Ayo D, van Lieshout N, Lanke K, Bousema T, Arinaitwe E. Plasmodium malariae and Plasmodium ovale—Prevalent and Relevant. Journal of Infectious Diseases. Oxford University Press; 2024. pp. 1239–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yman V, Wandell G, Mutemi DD, Miglar A, Asghar M, Hammar U, et al. Persistent transmission of plasmodium malariae and plasmodium ovale species in an area of declining plasmodium falciparum transmission in Eastern Tanzania. PLoS Negl Trop Dis. 2019;13: (5):e0007414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hawadak J, Dongang Nana RR, Singh V. Global trend of Plasmodium malariae and Plasmodium ovale spp. malaria infections in the last two decades (2000–2020): a systematic review and meta-analysis. Parasit Vectors. 2021;14: 297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li P, Zhao Z, Xing H, Li W, Zhu X, Cao Y, et al. Plasmodium malariae and Plasmodium ovale infections in the China-Myanmar border area. Malar J. 2016;15: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zaw MT, Lin Z. Human Plasmodium knowlesi infections in South-East Asian countries. Journal of Microbiology, Immunology and Infection. 2019;52: 679–684. [DOI] [PubMed] [Google Scholar]
- 17.Lee WC, Cheong FW, Amir A, Lai MY, Tan JH, Phang WK, et al. Plasmodium knowlesi: the game changer for malaria eradication. Malaria Journal. BioMed Central Ltd; 2022. p. 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinka ME, Bangs MJ, Manguin S, Rubio-Palis Y, Chareonviriyaphap T, Coetzee M, et al. A global map of dominant malaria vectors. Parasit Vectors. 2012;5: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Killeen GF, Smith TA, Ferguson HM, Mshinda H, Abdulla S, Lengeler C, et al. Preventing Childhood Malaria in Africa by Protecting Adults from Mosquitoes with Insecticide-Treated Nets. PLoS Med. 2007;4: e229. doi: 10.1371/journal.pmed.0040229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahamba NF, Finda M, Ngowo HS, Msugupakulya BJ, Baldini F, Koekemoer LL, et al. Using ecological observations to improve malaria control in areas where Anopheles funestus is the dominant vector. Malar J. 2022;21: 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forson AO, Hinne IA, Dhikrullahi SB, Sraku IK, Mohammed AR, Attah SK, et al. The resting behavior of malaria vectors in different ecological zones of Ghana and its implications for vector control. Parasit Vectors. 2022;15: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO. Guidelines for malaria, 2023.
- 23.Takken W, Charlwood D, Lindsay SW. The behaviour of adult Anopheles gambiae, sub-Saharan Africa’s principal malaria vector, and its relevance to malaria control: a review. Malar J. 2024;23: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO. World malaria report 2017.
- 25.Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526: 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Msugupakulya BJ, Kaindoa EW, Ngowo HS, Kihonda JM, Kahamba NF, Msaky DS, et al. Preferred resting surfaces of dominant malaria vectors inside different house types in rural south-eastern Tanzania. Malar J. 2020;19: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO. World malaria report 2020.
- 28.Carnevale P, Manguin S. Review of Issues on Residual Malaria Transmission. J Infect Dis. 2021;223: S61–S80. doi: 10.1093/infdis/jiab084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Namountougou M, Kientega M, Kaboré PDA, Soma DD, Pare Toe L, Sawadogo JME, et al. Residual malaria transmission: Magnitude and drivers of persistent Plasmodium infections despite high coverage of control interventions in Burkina Faso, West Africa. Acta Trop. 2023;242: 106913. [DOI] [PubMed] [Google Scholar]
- 30.Okumu F, Finda M. Key Characteristics of Residual Malaria Transmission in Two Districts in South-Eastern Tanzania ‐ Implications for Improved Control. Journal of Infectious Diseases. 2021;223: S143–S154. doi: 10.1093/infdis/jiaa653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mwesigwa J, Achan J, Di Tanna GL, Affara M, Jawara M, Worwui A, et al. Residual malaria transmission dynamics varies across The Gambia despite high coverage of control interventions. PLoS One. 2017;12: (11): e0187059. doi: 10.1371/journal.pone.0187059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tondossama N, Virgillito C, Coulibaly ZI, Pichler V, Dia I, della Torre A, et al. A High Proportion of Malaria Vector Biting and Resting Indoors despite Extensive LLIN Coverage in Côte d’Ivoire. Insects. 2023;14: (9): 758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nzioki I, Machani MG, Onyango SA, Kabui KK, Githeko AK, Ochomo E, et al. Differences in malaria vector biting behavior and changing vulnerability to malaria transmission in contrasting ecosystems of western Kenya. Parasit Vectors. 2023;16: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mawejje HD, Weetman D, Epstein A, Lynd A, Opigo J, Maiteki-Sebuguzi C, et al. Characterizing pyrethroid resistance and mechanisms in Anopheles gambiae (s.s.) and Anopheles arabiensis from 11 districts in Uganda. Current Research in Parasitology and Vector-Borne Diseases. 2023;3: 100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hemming-Schroeder E, Strahl S, Yang E, Nguyen A, Lo E, Zhong D, et al. Emerging Pyrethroid Resistance among Anopheles arabiensis in Kenya. Am J Trop Med Hyg. 2018;98: 704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menze BD, Riveron JM, Ibrahim SS, Irving H, Antonio-Nkondjio C, Awono-Ambene PH, et al. Multiple Insecticide Resistance in the Malaria Vector Anopheles funestus from Northern Cameroon Is Mediated by Metabolic Resistance Alongside Potential Target Site Insensitivity Mutations. PLoS One. 2016;11: e0163261–e0163261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Odero JO, Nambunga IH, Masalu JP, Mkandawile G, Bwanary H, Hape EE, et al. Genetic markers associated with the widespread insecticide resistance in malaria vector Anopheles funestus populations across Tanzania. Parasit Vectors. 2024;17: 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matowo NS, Munhenga G, Tanner M, Coetzee M, Feringa WF, Ngowo HS, et al. Fine-scale spatial and temporal heterogeneities in insecticide resistance profiles of the malaria vector, Anopheles arabiensis in rural south-eastern Tanzania. Wellcome Open Res. 2017;2: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinda PG, Eichenberger C, Ngowo HS, Msaky DS, Abbasi S, Kihonda J, et al. Comparative assessment of insecticide resistance phenotypes in two major malaria vectors, Anopheles funestus and Anopheles arabiensis in south-eastern Tanzania. Malar J. 2020;19: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wangrawa DW, Odero JO, Baldini F, Okumu F, Badolo A. Distribution and insecticide resistance profile of the major malaria vector Anopheles funestus group across the African. Med Vet Entomol. 2024;38: 119–137. [DOI] [PubMed] [Google Scholar]
- 41.Matowo J, Kulkarni MA, Mosha FW, Oxborough RM, Kitau JA, Tenu F, et al. Biochemical basis of permethrin resistance in Anopheles arabiensis from Lower Moshi, north-eastern Tanzania. 2010;9: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salomé G, Riddin M, Braack L. Species Composition, Seasonal Abundance, and Biting Behavior of Malaria Vectors in Rural Conhane Village, Southern Mozambique. 2023;20: (4):3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cooke MK, Kahindi SC, Oriango RM, Owaga C, Ayoma E, Mabuka D, et al. “A bite before bed”: Exposure to malaria vectors outside the times of net use in the highlands of western Kenya. Malar J. 2015;14: 259. doi: 10.1186/s12936-015-0766-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bedasso AH, Gutto AA, Waldetensai A, Eukubay A, Bokore GE, Kinde S, et al. Malaria vector feeding, peak biting time and resting place preference behaviors in line with Indoor based intervention tools and its implication: scenario from selected sentinel sites of Ethiopia. Heliyon. 2022;8: e12178. doi: 10.1016/j.heliyon.2022.e12178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Degefa T, Githeko AK, Lee MC, Yan G, Yewhalaw D. Patterns of human exposure to early-evening and outdoor biting mosquitoes and residual malaria transmission in Ethiopia. Acta Trop. 2021;216: 105837. doi: 10.1016/j.actatropica.2021.105837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mburu MM, Mzilahowa T, Amoah B, Chifundo D, Phiri KS, van den Berg H, et al. Biting patterns of malaria vectors of the lower Shire valley, southern Malawi. Acta Trop. 2019;197: 105059. doi: 10.1016/j.actatropica.2019.105059 [DOI] [PubMed] [Google Scholar]
- 47.Musiba RM, Tarimo BB, Monroe A, Msaky D, Ngowo H, Mihayo K, et al. Outdoor biting and pyrethroid resistance as potential drivers of persistent malaria transmission in Zanzibar. Malaria J. 2022;21: 1–14. doi: 10.1186/s12936-022-04200-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kreppel KS, Viana M, Main BJ, Johnson PCD, Govella NJ, Lee Y, et al. Emergence of behavioural avoidance strategies of malaria vectors in areas of high LLIN coverage in Tanzania. Sci Rep. 2020;10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gueye A, Ngom EHM, Diagne A, Ndoye BB, Dione ML, Sambe BS, et al. Host feeding preferences of malaria vectors in an area of low malaria transmission. Sci Rep. 2023;13: (1):16410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Musiime AK, Smith DL, Kilama M, Rek J, Arinaitwe E, Nankabirwa JI, et al. Impact of vector control interventions on malaria transmission intensity, outdoor vector biting rates and Anopheles mosquito species composition in Tororo, Uganda. Malar J. 2019;18: 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russell TL, Lwetoijera DW, Maliti D, Chipwaza B, Kihonda J, Charlwood JD, et al. Impact of promoting longer-lasting insecticide treatment of bed nets upon malaria transmission in a rural Tanzanian setting with pre-existing high coverage of untreated nets. Malar J. 2010;9: 187. doi: 10.1186/1475-2875-9-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mutuku FM, King CH, Mungai P, Mbogo C, Mwangangi J, Muchiri EM, et al. Impact of insecticide-treated bed nets on malaria transmission indices on the south coast of Kenya. Malar J. 2011;10: 356. doi: 10.1186/1475-2875-10-356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mwalugelo YA, Muyaga LL, Mahenge HH, Katusi GC, Muhonja F, Omondi Jaramogi Oginga Odinga D, et al. Livestock keeping, mosquitoes and community viewpoints: a mixed methods assessment of relationships between livestock management, malaria vector biting risk and community perspectives in rural Tanzania. 2024;23: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katusi GC, Hermy MRG, Makayula SM, Ignell R, Govella NJ, Hill SR, et al. Seasonal variation in abundance and blood meal sources of primary and secondary malaria vectors within Kilombero Valley, Southern Tanzania. Parasit Vectors. 2022;15: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayagaya VS, Nkwengulila G, Lyimo IN, Kihonda J, Mtambala H. The impact of livestock on the abundance, resting behaviour and sporozoite rate of malaria vectors in southern. 2015;14: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karisa J, Ominde K, Muriu S, Munyao V, Mwikali K, Babu L, et al. Malaria vector bionomics in Taita-Taveta County, coastal Kenya. Parasit Vectors. 2022;15: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Msugupakulya BJ, Urio NH, Jumanne M, Ngowo HS, Selvaraj P, Okumu FO, et al. Changes in contributions of different Anopheles vector species to malaria transmission in east and southern Africa from 2000 to 2022. Parasit Vectors. 2023;16: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lwetoijera D, Harris C, Kiware S, Dongus S, Devine G, McCall P, et al. Increasing role of Anopheles funestus and Anopheles arabiensis in malaria transmission in the Kilombero Valley, Tanzania. Malar J. 2014;13: 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong J, Bayoh N, Olang G, Killeen GF, Hamel MJ, Vulule JM, et al. Standardizing operational vector sampling techniques for measuring malaria transmission intensity: evaluation of six mosquito collection methods in western Kenya. Malar J. 2013;12: 143. doi: 10.1186/1475-2875-12-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mawejje HD, Kilama M, Kigozi SP, Musiime AK, Kamya M, Lines J, et al. Impact of seasonality and malaria control interventions on Anopheles density and species composition from three areas of Uganda with differing malaria endemicity. Malar J. 2021;20: (1):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bouma M, Rowland M. Failure of passive zooprophylaxis: cattle ownership in Pakistan is associated with a higher prevalence of malaria. Trans R Soc Trop Med Hyg. 1995;89: 351–353. doi: 10.1016/0035-9203(95)90004-7 [DOI] [PubMed] [Google Scholar]
- 62.Zeru MA, Zeru MA, Shibru S, Massebo F. Exploring the impact of cattle on human exposure to malaria mosquitoes in the Arba Minch area district of southwest Ethiopia. Parasit Vectors. 2020;13: (1):322. doi: 10.1186/s13071-020-04194-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Okunlola O, Oloja S, Ebiwonjumi A, Oyeyemi O. Vegetation index and livestock practices as predictors of malaria transmission in Nigeria. Sci Rep. 2024;14: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hasyim H, Dhimal M, Bauer J, Montag D, Groneberg DA, Kuch U, et al. Does livestock protect from malaria or facilitate malaria prevalence? A cross-sectional study in endemic rural areas of Indonesia. Malar J. 2018;17: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mburu MM, Zembere K, Mzilahowa T, Terlouw AD, Malenga T, van den Berg H, et al. Impact of cattle on the abundance of indoor and outdoor resting malaria vectors in southern Malawi. Malar J. 2021;20: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loha E. Association between Livestock Ownership and Malaria Incidence in South-Central Ethiopia: A Cohort Study. Am J Trop Med Hyg. 2023;108: 1145–1150. doi: 10.4269/ajtmh.22-0719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morgan JC, Irving H, Okedi LM, Steven A, Wondji CS. Pyrethroid Resistance in an Anopheles funestus Population from Uganda. Rénia L, editor. PLoS One. 2010;5: e11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Begh’ C, Clarke’ SE, Walraven’ GEL, Lindsay’ SW. Zooprophylaxis, artefact or reality? A paired-cohort study of the effect of passive zooprophylaxis on malaria in the Gambia. Trans R Soc Trop Med Hyg. 2002;96: 593–596. doi: 10.1016/s0035-9203(02)90320-2 [DOI] [PubMed] [Google Scholar]
- 69.Chan K, Cano J, Massebo F, Messenger LA. Cattle-related risk factors for malaria in southwest Ethiopia: a cross-sectional study. Malar J. 2022;21: 179. doi: 10.1186/s12936-022-04202-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ondiba IM, Oyieke FA, Ong’amo GO, Olumula MM, Nyamongo IK, Estambale BBA. Malaria vector abundance is associated with house structures in Baringo County, Kenya. PLoS One. 2018;13: (6): e0198970. doi: 10.1371/journal.pone.0198970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaindoa EW, Finda M, Kiplagat J, Mkandawile G, Nyoni A, Coetzee M, et al. Housing gaps, mosquitoes and public viewpoints: a mixed methods assessment of relationships between house characteristics, malaria vector biting risk and community perspectives in rural Tanzania. Malar J. 2018; 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bofu RM, Santos EM, Msugupakulya BJ, Kahamba NF, Swilla JD, Njalambaha R, et al. The needs and opportunities for housing improvement for malaria control in southern Tanzania. Malar J. 2023;22: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ngowo HS, Kaindoa EW, Matthiopoulos J, Ferguson HM, Okumu FO. Variations in household microclimate affect outdoor-biting behaviour of malaria vectors. Wellcome Open Res. 2017;2: 102. doi: 10.12688/wellcomeopenres.12928.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mapua SA, Hape EE, Kihonda J, Bwanary H, Kifungo K, Kilalangongono M, et al. Persistently high proportions of plasmodium-infected Anopheles funestus mosquitoes in two villages in the Kilombero valley, South-Eastern Tanzania. Parasite Epidemiol Control. 2022;18: e00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Katusi GC, Hermy MRG, Makayula SM, Ignell R, Mnyone LL, Hill SR, et al. Effect of non-human hosts on the human biting rate of primary and secondary malaria vectors in Tanzania. Malar J. 2023;22: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaindoa EW, Matowo NS, Ngowo HS, Mkandawile G, Mmbando A, Finda M, et al. Interventions that effectively target Anopheles funestus mosquitoes could significantly improve control of persistent malaria transmission in south-eastern Tanzania. PLoS One. 2017;12: (5): e0177807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Limwagu AJ, Msugupakulya BJ, Kilalangongono MM, Mwalugelo YA, Okumu FO, Lyimo IN, et al. Evaluation of the DN-Mini (miniaturized double net) trap for sampling host-seeking Anopheles mosquitoes in malaria-endemic villages of southern Tanzania. PLoS One. 2024;19: e0294192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Limwagu AJ, Kaindoa EW, Ngowo HS, Hape E, Finda M, Mkandawile G, et al. Using a miniaturized double-net trap (DN-Mini) to assess relationships between indoor–outdoor biting preferences and physiological ages of two malaria vectors, Anopheles arabiensis and Anopheles funestus. Malaria J. 2019;18: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mboera LE, Knols BG, Kihonda J, Braks MA. Short report: Influence of centers for disease control light trap position, relative to a human-baited bed net, on catches of Anopheles gambiae and Culex quinquefasciatus in Tanzania. Am J Trop Med Hyg. 1998;59: 595–596. [DOI] [PubMed] [Google Scholar]
- 80.Maia MF, Robinson A, John A, Mgando J, Simfukwe E, Moore SJ. Comparison of the CDC Backpack aspirator and the Prokopack aspirator for sampling indoor- and outdoor-resting mosquitoes in southern Tanzania. Parasit Vectors. 2011;4: 124. doi: 10.1186/1756-3305-4-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. American Journal of Tropical Medicine and Hygiene. 1993;49: 520–529. [DOI] [PubMed] [Google Scholar]
- 82.Koekemoer LL, Kamau L, Hunt RH, Coetzee M. A cocktail polymerase chain reaction assay to identify members of the Anopheles funestus (Diptera: Culicidae) group. Am J Trop Med Hyg. 2002;66: 804–811. [DOI] [PubMed] [Google Scholar]
- 83.Beier JC, Perkins P V, Wirtz RA, Koros J, Diggs D, Gargan Ii TP, et al. Bloodmeal Identification by Direct Enzyme-Linked Immunosorbent Assay (ELISA), Tested on Anopheles (Diptera: Culicidae) in Kenya 12. J Med Entomol. 1988. [DOI] [PubMed] [Google Scholar]
- 84.R Development Core Team R. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. 2011.
- 85.Ntabaliba W, Vavassori L, Stica C, Makungwa N, Odufuwa OG, Swai JK, et al. Life expectancy of Anopheles funestus is double that of Anopheles arabiensis in southeast Tanzania based on mark-release-recapture method. Scientific Reports 2023 13:1. 2023;13: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.2022 TD and HS and MIS. Tanzania Demographic and Health Survey and Malaria Indicator Survey. 2022; 1–23.
- 87.Nambunga IH, Ngowo HS, Mapua SA, Hape EE, Msugupakulya BJ, Msaky DS, et al. Aquatic habitats of the malaria vector Anopheles funestus in rural south-eastern Tanzania. Malar J. 2020;19: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kahamba NF, Okumu FO, Jumanne M, Kifungo K, Odero JO, Baldini F, et al. Geospatial modelling of dry season habitats of the malaria vector, Anopheles funestus, in south-eastern Tanzania. Parasit Vectors. 2024;17: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dabiré KR, Diabaté A, Paré-Toé L, Rouamba J, Ouari A, Fontenille D, et al. Year to year and seasonal variations in vector bionomics and malaria transmission in a humid savannah village in west Burkina Faso. Journal of Vector Ecology. 2008;33: 70–75. doi: 10.3376/1081-1710(2008)33[70:ytyasv]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- 90.Djouaka R, Akoton RI, Tchigossou GM, Atoyebi SM, Irving H, Kusimo MO, et al. Mapping the distribution of Anopheles funestus across Benin highlights a sharp contrast of susceptibility to insecticides and infection rate to Plasmodium between southern and northern populations. Wellcome Open Res. 2017;1: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Djamouko-Djonkam L, Nkahe DL, Kopya E, Talipouo A, Ngadjeu CS, Doumbe-Belisse P, et al. Implication of Anopheles funestus in malaria transmission in the city of Yaoundé, Cameroon. Parasite. 2020;27: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mbewe RB, Keven JB, Mzilahowa T, Mathanga D, Wilson M, Cohee L, et al. Blood-feeding patterns of Anopheles vectors of human malaria in Malawi: implications for malaria transmission and effectiveness of LLIN interventions. Malar J. 2022;21: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hamwata WP, Muleba M, Daka V, Shimaponda-Mataa NM. Implication of Anopheles gambiae and Anopheles funestus coexistence on malaria elimination efforts in a peri-urban setting in Ndola district, Zambia. 2024;17: 216. [Google Scholar]
- 94.Ogola EO, Fillinger U, Ondiba IM, Villinger J, Masiga DK, Torto B, et al. Insights into malaria transmission among Anopheles funestus mosquitoes, Kenya. Parasit Vectors. 2018;11: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Degefa T, Yewhalaw D, Zhou G, Lee MC, Atieli H, Githeko AK, et al. Indoor and outdoor malaria vector surveillance in western Kenya: Implications for better understanding of residual transmission. Malar J. 2017;16: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ogola E, Villinger J, Mabuka D, Omondi D, Orindi B, Mutunga J, et al. Composition of Anopheles mosquitoes, their blood-meal hosts, and Plasmodium falciparum infection rates in three islands with disparate bed net coverage in Lake Victoria, Kenya. Malar J. 2017;16: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Natchema S. Fonkou B, Tchouakui M, Menze BD, Mugenzi LMJ, Fofie D, Nguiffo-Nguete D, et al. Entomological longitudinal surveys in two contrasted eco-climatic settings in Cameroon reveal a high malaria transmission from Anopheles funestus associated with GSTe2 metabolic resistance. BMC Infect Dis. 2023;23: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Matowo NS, Martin J, Kulkarni MA, Mosha JF, Lukole E, Isaya G, et al. An increasing role of pyrethroid-resistant Anopheles funestus in malaria transmission in the Lake Zone, Tanzania. 2021;11: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stevenson JC, Pinchoff J, Muleba M, Lupiya J, Chilusu H, Mwelwa I, et al. Spatio-temporal heterogeneity of malaria vectors in northern Zambia: Implications for vector control. Parasit Vectors. 2016;9: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gebhardt ME, Searle KM, Kobayashi T, Shields TM, Hamapumbu H, Simubali L, et al. Understudied Anophelines Contribute to Malaria Transmission in a Low-Transmission Setting in the Choma District, Southern Province, Zambia. Am J Trop Med Hyg. 2022;106: 1406–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Urio NH, Pinda PG, Ngonzi AJ, Muyaga LL, Msugupakulya BJ, Finda M, et al. Effects of agricultural pesticides on the susceptibility and fitness of malaria vectors in rural south-eastern Tanzania. Parasit Vectors. 2022;15: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Takken W, Verhulst NO. Host Preferences of Blood-Feeding Mosquitoes. Annu Rev Entomol. 2013;58. doi: 10.1146/annurev-ento-120811-153618 [DOI] [PubMed] [Google Scholar]
- 103.Lyimo IN, Ferguson HM. Ecological and evolutionary determinants of host species choice in mosquito vectors. Trends in Parasitology. 2009. pp. 189–196. doi: 10.1016/j.pt.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 104.Lyimo IN, Haydon DT, Mbina KF, Daraja AA, Mbehela EM, Reeve R, et al. The fitness of African malaria vectors in the presence and limitation of host behaviour. Malar J. 2012;11: 425. doi: 10.1186/1475-2875-11-425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lyimo IN, Haydon DT, Russell TL, Mbina KF, Daraja AA, Mbehela EM, et al. The impact of host species and vector control measures on the fitness of African malaria vectors. Proceedings of the Royal Society B: Biological Sciences. 2013;280: 1–10. doi: 10.1098/rspb.2012.2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lyimo IN, Keegan SP, Ranford-Cartwright LC, Ferguson HM. The impact of uniform and mixed species blood meals on the fitness of the mosquito vector Anopheles gambiae s.s: Does a specialist pay for diversifying its host species diet? J Evol Biol. 2012;25: 452–460. [DOI] [PubMed] [Google Scholar]
- 107.Mburu MM, Juurlink M, Spitzen J, Moraga P, Hiscox A, Mzilahowa T, et al. Impact of partially and fully closed eaves on house entry rates by mosquitoes. Parasit Vectors. 2018;11: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lindsay SW, Jawara M, Mwesigwa J, Achan J, Bayoh N, Bradley J, et al. Reduced mosquito survival in metal-roof houses may contribute to a decline in malaria transmission in sub-Saharan Africa. Sci Rep. 2019;9: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Abong’o B, Gimnig JE, Omoke D, Ochomo E, Walker ED. Screening eaves of houses reduces indoor mosquito density in rural, western Kenya. Malar J. 2022;21: 377. doi: 10.1186/s12936-022-04397-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jatta E, Jawara M, Bradley J, Jeffries D, Kandeh B, Knudsen JB, et al. How house design affects malaria mosquito density, temperature, and relative humidity: an experimental study in rural Gambia. Lancet Planet Health. 2018;2: e498–e508. doi: 10.1016/S2542-5196(18)30234-1 [DOI] [PubMed] [Google Scholar]
- 111.Msoffe R, Hewitt M, Masalu JP, Finda M, Kavishe DR, Okumu FO, et al. Participatory development of practical, affordable, insecticide-treated mosquito proofing for a range of housing designs in rural southern Tanzania. Malar J. 2022;21: 318. doi: 10.1186/s12936-022-04333-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ng’Ang’A PN, Okoyo C, Mbogo C, Mutero CM. Evaluating effectiveness of screening house eaves as a potential intervention for reducing indoor vector densities and malaria prevalence in Nyabondo, western Kenya. Malar J. 2020;19: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pinder M, Bradley J, Jawara M, Affara M, Conteh L, Correa S, et al. Improved housing versus usual practice for additional protection against clinical malaria in The Gambia (RooPfs): a household-randomised controlled trial. Lancet Planet Health. 2021;5: e220–e229. doi: 10.1016/S2542-5196(21)00002-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morgan CE, Topazian HM, Brandt K, Mitchell C, Kashamuka MM, Muwonga J, et al. Association between domesticated animal ownership and Plasmodium falciparum parasite prevalence in the Democratic Republic of the Congo: a national cross-sectional study. Lancet Microbe. 2023;4: e516–e523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Riveron JM, Huijben S, Tchapga W, Tchouakui M, Wondji MJ, Tchoupo M, et al. Escalation of Pyrethroid Resistance in the Malaria Vector Anopheles funestus Induces a Loss of Efficacy of Piperonyl Butoxide-Based Insecticide-Treated Nets in Mozambique. Journal of Infectious Diseases. 2019;220: 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chanda J, Saili K, Phiri F, Stevenson JC, Mwenda M, Chishimba S, et al. Pyrethroid and carbamate resistance in Anopheles funestus Giles along Lake Kariba in Southern Zambia. American Journal of Tropical Medicine and Hygiene. 2020;103: 90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sued RM, Ng’habi K, Kidima W, Philbert A. Resistance to pyrethroids in Anopheles gambiae s.l. from the Kilombero Valley, Tanzania: synergists, oxidases and susceptibility to malaria parasites (Plasmodium falciparum). Aust Entomol. 2023;62: 96–105. [Google Scholar]
- 118.Sih C, Protopopoff N, Koffi AA, Ahoua Alou LP, Dangbenon E, Messenger LA, et al. Efficacy of chlorfenapyr-pyrethroid and piperonyl butoxide-pyrethroid long-lasting insecticidal nets (LLINs) compared to pyrethroid-only LLINs for malaria control in Côte d’Ivoire: a three group, cluster randomised trial. Trials. 2024;25: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mosha JF, Kulkarni MA, Lukole E, Matowo NS, Pitt C, Messenger LA, et al. Effectiveness and cost-effectiveness against malaria of three types of dual-active-ingredient long-lasting insecticidal nets (LLINs) compared with pyrethroid-only LLINs in Tanzania: a four-arm, cluster-randomised trial. Lancet. 2022;399: 1227. doi: 10.1016/S0140-6736(21)02499-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Damene E, Massebo F. Administration of ivermectin to cattle induced mortality, reduced fecundity and survivorship of Anopheles arabiensis in Ethiopia: an implication for expansion of vector control toolbox. Trop Med Health. 2024;52: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sagna AB, Zéla L, Ouedraogo COW, Pooda SH, Porciani A, Furnival-Adams J, et al. Ivermectin as a novel malaria control tool: Getting ahead of the resistance curse. Acta Trop. 2023;245: 106973. doi: 10.1016/j.actatropica.2023.106973 [DOI] [PubMed] [Google Scholar]
- 122.Pooda SH, Moiroux N, Porciani A, Courjaud AL, Roberge C, Gaudriault G, et al. Proof-of-concept study for a long-acting formulation of ivermectin injected in cattle as a complementary malaria vector control tool. Parasit Vectors. 2023;16: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lyimo IN, Kessy ST, Mbina KF, Daraja AA, Mnyone LL. Ivermectin-treated cattle reduces blood digestion, egg production and survival of a free-living population of Anopheles arabiensis under semi-field condition in south-eastern Tanzania. Malar J. 2017;16: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tusting LS, Ippolito MM, Willey BA, Kleinschmidt I, Dorsey G, Gosling RD, et al. The evidence for improving housing to reduce malaria: A systematic review and meta-analysis. Malar J. 2015;14: 209. doi: 10.1186/s12936-015-0724-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information file