Abstract
The rate of fatty acid synthesis from [6-14C]glucose in mammary tissue remained low until parturition at 22 days of gestation and increased 10-fold at 1 day post partum. Administration of progesterone on days 20 and 21 or removal of pups at parturition abolished this increase. In the latter case, administration of prolactin, corticosterone or oxytocin had no stimulatory effect; tissue from suckled glands in which the ducts had been ligated at parturition also showed no increased rate in 24 h. Foetoplacentectomy on day 18 did not stimulate fatty acid synthesis but subsequent suckling by foster pups did. Whereas lactose synthesis is initiated by withdrawal of progesterone from the circulation, a further stimulus related to removal of milk by suckling is required to initiate fatty acid synthesis.
Full text
PDF
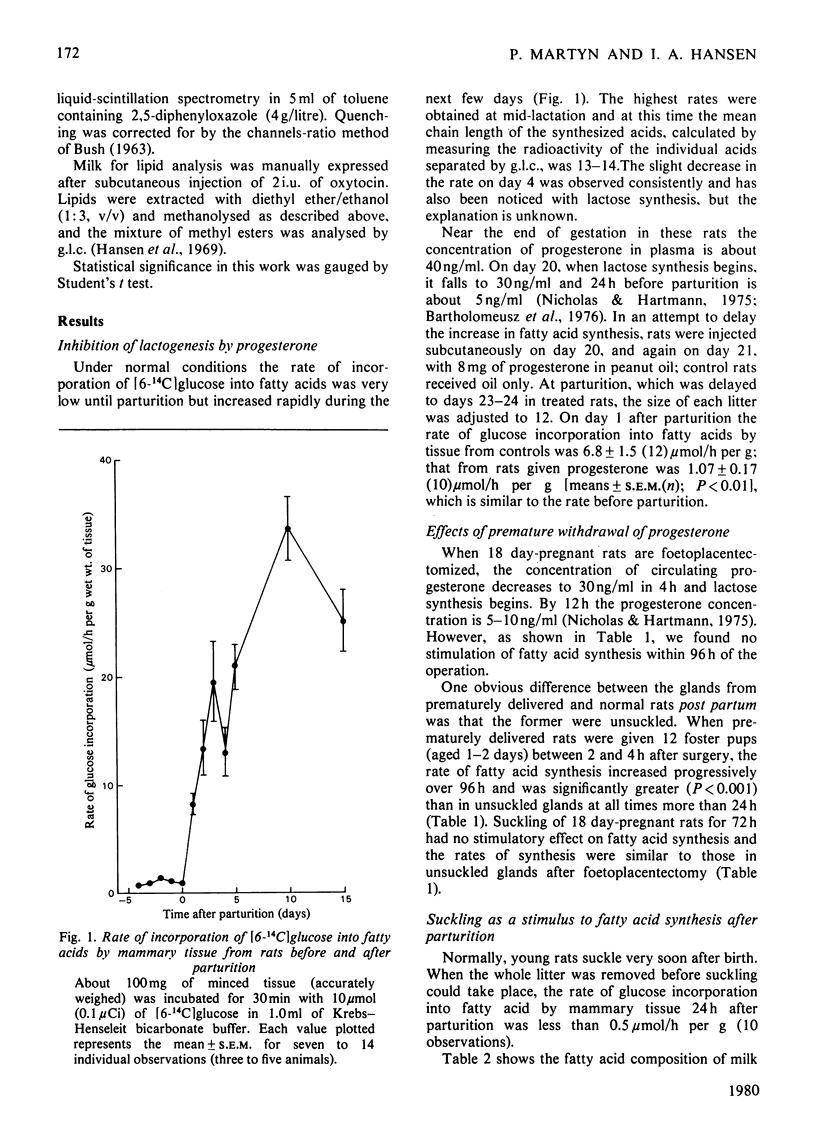
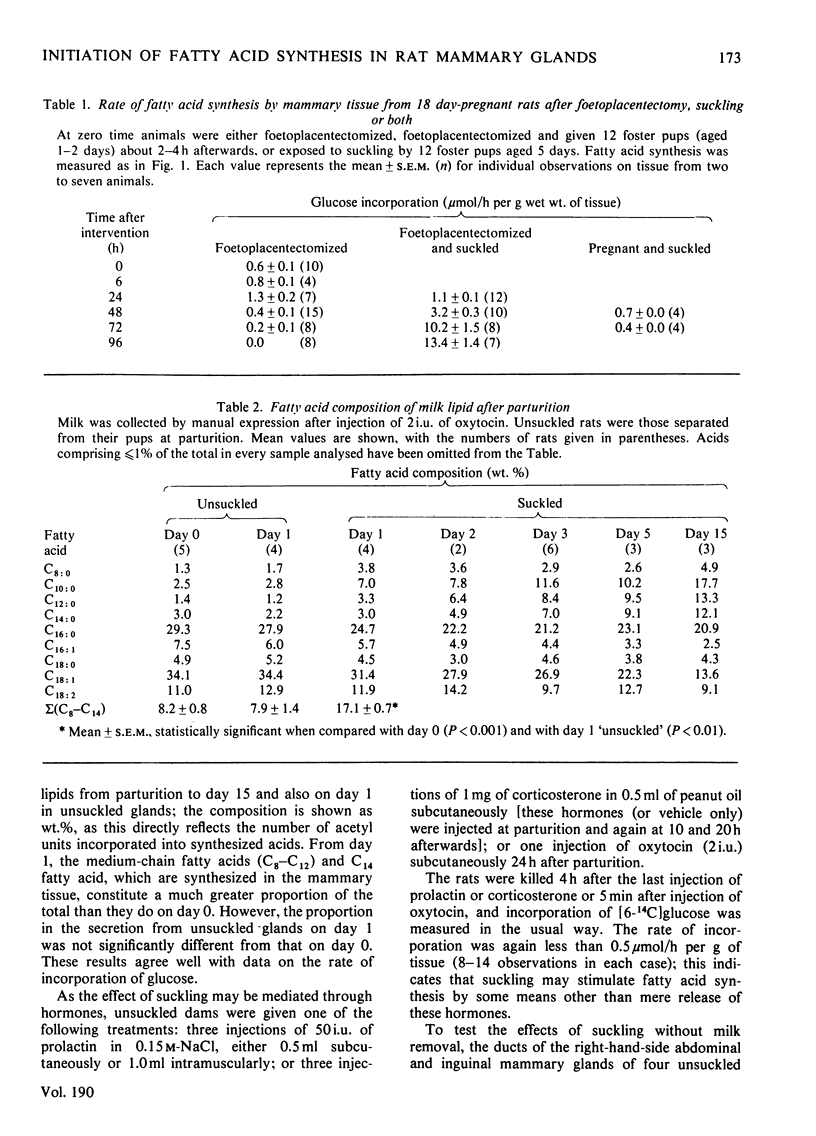


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM S., CHAIKOFF I. L. Glycolytic pathways and lipogenesis in mammary glands of lactating and nonlactating normal rats. J Biol Chem. 1959 Sep;234:2246–2253. [PubMed] [Google Scholar]
- ABRAHAM S., MATTHES K. J., CHAIKOFF I. L. Factors involved in synthesis of fatty acids from acetate by a soluble fraction obtained from lactating rat mammary gland. Biochim Biophys Acta. 1961 May 13;49:268–285. doi: 10.1016/0006-3002(61)90127-5. [DOI] [PubMed] [Google Scholar]
- Agius L., Robinson A. M., Girard J. R., Williamson D. H. Alterations in the rate of lipogenesis in vivo in maternal liver and adipose tissue on premature weaning of lactating rats: a possible regulatory role of prolactin. Biochem J. 1979 Jun 15;180(3):689–692. doi: 10.1042/bj1800689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomeusz R. K., Bruce N. W., Martin C. E., Hartmann P. E. Serial measurement of arterial plasma progesterone levels throughout gestation and parturition in individual rats. Acta Endocrinol (Copenh) 1976 Jun;82(2):436–443. doi: 10.1530/acta.0.0820436. [DOI] [PubMed] [Google Scholar]
- Bartley J. C., Abraham S. The absolute rate of fatty acid synthesis by mammary gland slices from lactating rats. J Lipid Res. 1976 Sep;17(5):467–477. [PubMed] [Google Scholar]
- Chatterton R. T., Harris J. A., Wynn R. M. Lactogenesis in the rat: an ultrastructural study of the initiation of the secretory process. J Reprod Fertil. 1975 Jun;43(3):479–484. doi: 10.1530/jrf.0.0430479. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gumaa K. A., Greenbaum A. L., McLean P. Adaptive changes in satellite systems related to lipogenesis in rat and sheep mammary gland and in adipose tissue. Eur J Biochem. 1973 Apr 2;34(1):188–198. doi: 10.1111/j.1432-1033.1973.tb02745.x. [DOI] [PubMed] [Google Scholar]
- Hansen I. A., Tang B. K., Edkins E. Erythro-Diols of wax from the uropygial gland of the turkey. J Lipid Res. 1969 May;10(3):267–270. [PubMed] [Google Scholar]
- Howanitz P. J., Levy H. R. Acetyl-CoA carboxylase and citrate cleavage enzyme in the rat mammary gland. Biochim Biophys Acta. 1965 Oct 4;106(2):430–433. doi: 10.1016/0005-2760(65)90056-1. [DOI] [PubMed] [Google Scholar]
- LEVY H. R. THE EFFECTS OF WEANING AND MILK ON MAMMARY FATTY ACID SYNTHESIS. Biochim Biophys Acta. 1964 Jun 15;84:229–238. doi: 10.1016/0926-6542(64)90052-6. [DOI] [PubMed] [Google Scholar]
- Lincoln D. W., Hill A., Wakerley J. B. The milk-ejection reflex of the rat: an intermittent function not abolished by surgical levels of anaesthesia. J Endocrinol. 1973 Jun;57(3):459–476. doi: 10.1677/joe.0.0570459. [DOI] [PubMed] [Google Scholar]
- Mackall J. C., Lane M. D. Changes in mammary-gland acetyl-coenzyme A carboxylase associated with lactogenic differentiation. Biochem J. 1977 Mar 15;162(3):635–642. doi: 10.1042/bj1620635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellenberger R. W., Bauman D. E. Metabolic adaptations during lactogenesis. Lactose synthesis in rabbit mammary tissue during pregnancy and lactation. Biochem J. 1974 Sep;142(3):659–665. doi: 10.1042/bj1420659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad T. M. Ultrastructural study of rat mammary gland during pregnancy. Anat Rec. 1970 May;167(1):17–35. doi: 10.1002/ar.1091670104. [DOI] [PubMed] [Google Scholar]
- Shyamala G., McBlain W. A. Distinction between progestin- and glucocorticoid-binding sites in mammary glands. Apparent lack of cytoplasmic progesterone receptors in lactating mammary glands. Biochem J. 1979 Feb 15;178(2):345–352. doi: 10.1042/bj1780345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong C. R., Dils R. Fatty acid biosynthesis in rabbit mammary gland during pregnancy and early lactation. Biochem J. 1972 Aug;128(5):1303–1309. doi: 10.1042/bj1281303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian M. G., Reece R. P. Anterior pituitary and plasma prolactin in rats after 2 to 90 minutes of suckling. Proc Soc Exp Biol Med. 1975 Jul;149(3):754–756. doi: 10.3181/00379727-149-38892. [DOI] [PubMed] [Google Scholar]


