Abstract
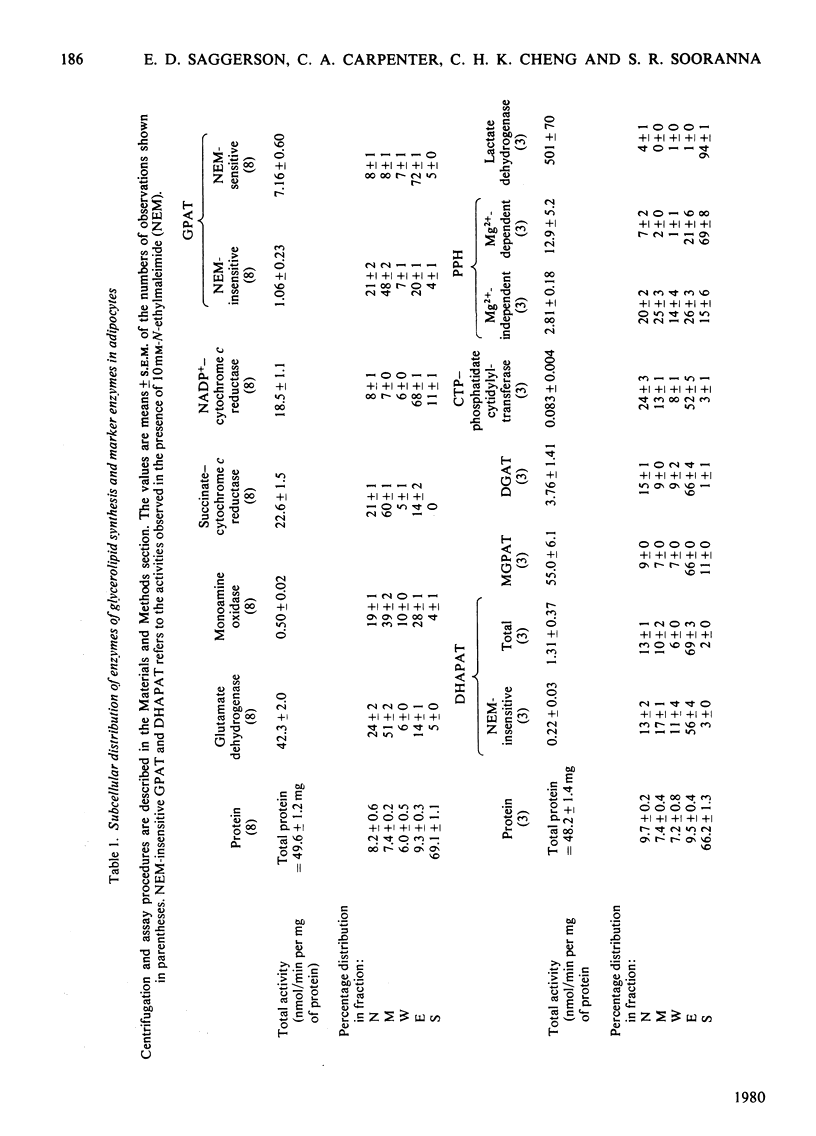
1. Glycerol phosphate acyltransferase (GPAT) activities were measured in subcellular fractions obtained from rat epididymal adipocytes. These contained both N-ethylmaleimide-sensitive and N-ethylmaleimide-insensitive forms of the enzyme. 2. As shown by parallel measurements of marker enzymes, N-ethylmaleimide-insensitive GPAT is most probably a mitochondrial activity, whereas N-ethylmaleimide-sensitive GPAT is the microsomal enzyme. 3. Subcellular distributions are also reported for dihydroxyacetone phosphate acyltransferase (DHAPAT) (assayed with and without N-ethylmaleimide), monoacylglycerol phosphate acyltransferase (MGPAT) and Mg2+-dependent and Mg2+-independent forms of phosphatidate phosphohydrolase (PPH).
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aas M. Organ and subcellular distribution of fatty acid activating enzymes in the rat. Biochim Biophys Acta. 1971 Feb 2;231(1):32–47. doi: 10.1016/0005-2760(71)90253-0. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Saggerson D. A selective decrease in mitochondrial glycerol phosphate acyltransferase activity in livers from streptozotocin-diabetic rats. FEBS Lett. 1977 Dec 15;84(2):229–232. doi: 10.1016/0014-5793(77)80694-7. [DOI] [PubMed] [Google Scholar]
- Bates E. J., Saggerson E. D. A study of the glycerol phosphate acyltransferase and dihydroxyacetone phosphate acyltransferase activities in rat liver mitochondrial and microsomal fractions. Relative distribution in parenchymal and non-parenchymal cells, effects of N-ethylmaleimide, palmitoyl-coenzyme A concentration, starvation, adrenalectomy and anti-insulin serum treatment. Biochem J. 1979 Sep 15;182(3):751–762. doi: 10.1042/bj1820751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E. J., Topping D. L., Sooranna S. P., Saggerson D., Mayes P. A. Acute effects of insulin on glycerol phosphate acyl transferase activity, ketogenesis and serum free fatty acid concentration in perfused rat liver. FEBS Lett. 1977 Dec 15;84(2):225–228. doi: 10.1016/0014-5793(77)80693-5. [DOI] [PubMed] [Google Scholar]
- Bjerve K. S., Daae L. N., Bremer J. Phosphatidic acid biosynthesis in rat liver mitochondria and microsomal fractions. Regulation of fatty acid positional specificity. Biochem J. 1976 Aug 15;158(2):249–254. doi: 10.1042/bj1580249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter J. R., Kennedy E. P. Enzymatic synthesis of cytidine diphosphate diglyceride. J Lipid Res. 1966 Sep;7(5):678–683. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Cheng C. H., Saggerson E. D. Rapid effects of noradrenaline on Mg2+-dependent phosphatidate phosphohydrolase activity in rat adipocytes. FEBS Lett. 1978 Mar 1;87(1):65–68. doi: 10.1016/0014-5793(78)80134-3. [DOI] [PubMed] [Google Scholar]
- Coleman R., Bell R. M. Triacylglycerol synthesis in isolated fat cells. Studies on the microsomal diacylglycerol acyltransferase activity using ethanol-dispersed diacylglycerols. J Biol Chem. 1976 Aug 10;251(15):4537–4543. [PubMed] [Google Scholar]
- Daae L. N., Bremer J. The acylation of glycerophosphate in rat liver. A new assay procedure for glycerophosphate acylation, studies on its subcellular and submitochondrial localization and determination of the reaction products. Biochim Biophys Acta. 1970 Jun 9;210(1):92–104. doi: 10.1016/0005-2760(70)90065-2. [DOI] [PubMed] [Google Scholar]
- Daae L. N. The acylation of glycerol 3 -phosphate in different rat organs and in the liver of different species (including man). Biochim Biophys Acta. 1973 May 24;306(2):186–193. doi: 10.1016/0005-2760(73)90224-5. [DOI] [PubMed] [Google Scholar]
- Daae L. N. The mitochondrial acylation of glycerophosphate in rat liver: fatty acid and positional specificity. Biochim Biophys Acta. 1972 May 23;270(1):23–31. doi: 10.1016/0005-2760(72)90173-7. [DOI] [PubMed] [Google Scholar]
- Denton R. M., Halperin M. L. The control of fatty acid and triglyceride synthesis in rat epididymal adipose tissue. Roles of coenzyme A derivatives, citrate and L-glycerol 3-phosphate. Biochem J. 1968 Nov;110(1):27–38. doi: 10.1042/bj1100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajra A. K. On extraction of acyl and alkyl dihydroxyacetone phosphate from incubation mixtures. Lipids. 1974 Aug;9(8):502–505. doi: 10.1007/BF02532495. [DOI] [PubMed] [Google Scholar]
- Jamdar S. C., Fallon H. J. Glycerolipid synthesis in rat adipose tissue. II. Properties and distribution of phosphatidate phosphatase. J Lipid Res. 1973 Sep;14(5):517–524. [PubMed] [Google Scholar]
- Jones C. L., Hajra A. K. The subcellular distribution of acyl CoA: dihydroxyacetone phosphate acyl transferase in guinea pig liver. Biochem Biophys Res Commun. 1977 Jun 20;76(4):1138–1143. doi: 10.1016/0006-291x(77)90974-3. [DOI] [PubMed] [Google Scholar]
- LANDS W. E., HART P. METABOLISM OF GLYCEROLIPIDS. VI. SPECIFICITIES OF ACYL COENZYME A: PHOSPHOLIPID ACYLTRANSFERASES. J Biol Chem. 1965 May;240:1905–1911. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lamb R. G., Fallon H. J. Glycerolipid formation from sn-glycerol-3-phosphate by rat liver cell fractions. The role of phosphatidate phosphohydrolase. Biochim Biophys Acta. 1974 Apr 26;348(1):166–178. doi: 10.1016/0005-2760(74)90103-9. [DOI] [PubMed] [Google Scholar]
- Lloyd-Davies K. A., Brindley D. N. Palmitate activation and esterification in microsomal fractions of rat liver. Biochem J. 1975 Oct;152(1):39–49. doi: 10.1042/bj1520039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. R., Denton R. M. The intracellular localization of enzymes in white-adipose-tissue fat-cells and permeability properties of fat-cell mitochondria. Transfer of acetyl units and reducing power between mitochondria and cytoplasm. Biochem J. 1970 May;117(5):861–877. doi: 10.1042/bj1170861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroy G., Kelker H. C., Pullman M. E. Partial purification and properties of an acyl coenzyme A:sn-glycerol 3-phosphate acyltransferase from rat liver mitochondria. J Biol Chem. 1973 Apr 25;248(8):2845–2852. [PubMed] [Google Scholar]
- Monroy G., Rola F. H., Pullman M. E. A substrate- and position-specific acylation of sn-glycerol 3-phosphate by rat liver mitochondria. J Biol Chem. 1972 Nov 10;247(21):6884–6894. [PubMed] [Google Scholar]
- Nimmo H. G. Evidence for the existence of isoenzymes of glycerol phosphate acyltransferase. Biochem J. 1979 Jan 1;177(1):283–288. doi: 10.1042/bj1770283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama H., Eibl H., Lands W. E. Acyl coenzyme A:2-acyl-sn-glycerol-3-phosphate acyltransferase activity in rat liver microsomes. Biochim Biophys Acta. 1971 Nov 5;248(2):263–273. doi: 10.1016/0005-2760(71)90014-2. [DOI] [PubMed] [Google Scholar]
- PHILLIPS A. H., LANGDON R. G. Hepatic triphosphopyridine nucleotide-cytochrome c reductase: isolation, characterization, and kinetic studies. J Biol Chem. 1962 Aug;237:2652–2660. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Saggerson E. D., Greenbaum A. L. The regulation of triglyceride synthesis and fatty acid synthesis in rat epididymal adipose tissue. Biochem J. 1970 Sep;119(2):193–219. doi: 10.1042/bj1190193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. Lipogenesis in rat and guinea-pig isolated epididymal fat-cells. Biochem J. 1974 May;140(2):211–224. doi: 10.1042/bj1400211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D. The regulation of glyceride synthesis in isolated white-fat cells. The effects of palmitate and lipolytic agents. Biochem J. 1972 Aug;128(5):1057–1067. doi: 10.1042/bj1281057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Tomassi G. The regulation of glyceride synthesis from pyruvate in isolated fat cells. The effects of palmitate and alteration of dietary status. Eur J Biochem. 1971 Nov 11;23(1):109–117. doi: 10.1111/j.1432-1033.1971.tb01597.x. [DOI] [PubMed] [Google Scholar]
- Sarzala M. G., Van Golde L. M., De Kruyff B., Van Deenen L. L. The intramitochondrial distribution of some enzymes involved in the biosynthesis of rat-liver phospholipids. Biochim Biophys Acta. 1970 Feb 10;202(1):106–119. doi: 10.1016/0005-2760(70)90222-5. [DOI] [PubMed] [Google Scholar]
- Schlossman D. M., Bell R. M. Triacylglycerol synthesis in isolated fat cells. Evidence that the sn-glycerol-3-phosphate and dihydroxyacetone phosphate acyltransferase activities are dual catalytic functions of a single microsomal enzyme. J Biol Chem. 1976 Sep 25;251(18):5738–5744. [PubMed] [Google Scholar]
- Shephard E. H., Hübscher G. Phosphatidate biosynthesis in mitochondrial subfractions of rat liver. Biochem J. 1969 Jun;113(2):429–440. doi: 10.1042/bj1130429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sooranna S. R., Saggerson E. D. Interactions of insulin and adrenaline with glycerol phosphate acylation processes in fat-cells from rat. FEBS Lett. 1976 Apr 15;64(1):36–39. doi: 10.1016/0014-5793(76)80242-6. [DOI] [PubMed] [Google Scholar]
- Sooranna S. R., Saggerson E. D. Studies of the effect of adrenaline on glycerol phosphate acyltransferase activity in rat adipocytes. FEBS Lett. 1978 Jun 1;90(1):141–144. doi: 10.1016/0014-5793(78)80316-0. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W., Schiefer H. G. Biosynthesis and composition of phosphatides in outer and inner mitochondrial membranes. Hoppe Seylers Z Physiol Chem. 1968 Aug;349(8):1017–1026. doi: 10.1515/bchm2.1968.349.2.1017. [DOI] [PubMed] [Google Scholar]
- Sánchez M., Nicholls D. G., Brindley D. N. [The relationship between palmitoyl-coenzyme A synthetase activity and esterification of sn-glycerol 3-phosphate in rat liver mitochondria]. Biochem J. 1973 Apr;132(4):697–706. doi: 10.1042/bj1320697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K., Okuyama H. Selectivity of diacylglycerophosphate synthesis in subcellular fractions of rat liver. Arch Biochem Biophys. 1978 Oct;190(2):409–420. doi: 10.1016/0003-9861(78)90294-1. [DOI] [PubMed] [Google Scholar]
- Yamashita S., Hosaka K., Taketo M., Numa S. Distribution of glycerolipid-synthesizing enzymes in the subfractions of rat liver microsomes. FEBS Lett. 1973 Feb 1;29(3):235–238. doi: 10.1016/0014-5793(73)80027-4. [DOI] [PubMed] [Google Scholar]
- Zborowski J., Wojtczak L. Phospholipid synthesis in rat liver mitochondria. Biochim Biophys Acta. 1969 Jul 29;187(1):73–84. doi: 10.1016/0005-2760(69)90134-9. [DOI] [PubMed] [Google Scholar]
- van Tol A. The effect of fasting on the acylation of carnitine and glycerophosphate in rat liver subcellular fractions. Biochim Biophys Acta. 1974 Jul 25;357(1):14–23. doi: 10.1016/0005-2728(74)90107-8. [DOI] [PubMed] [Google Scholar]
- van den Bosch H. Phosphoglyceride metabolism. Annu Rev Biochem. 1974;43(0):243–277. doi: 10.1146/annurev.bi.43.070174.001331. [DOI] [PubMed] [Google Scholar]


