Abstract
Direct measurement of N tau-methylhistidine turnover in skeletal muscle, skin and gastrointestinal muscle indicates that these three tissues contribute only 24.9, 6.8 and 9.8% of the total urinary excretion. Measurement of the decay rate of radioactively labelled N tau-methylhistidine in urine indicates that skeletal muscle accounts for 74.5% of the urinary excretion and this is probably an overestimate. These results suggest that the common assumption, that N tau-methylhistidine in urine originates almost entirely from skeletal muscle, may be wrong.
Full text
PDF
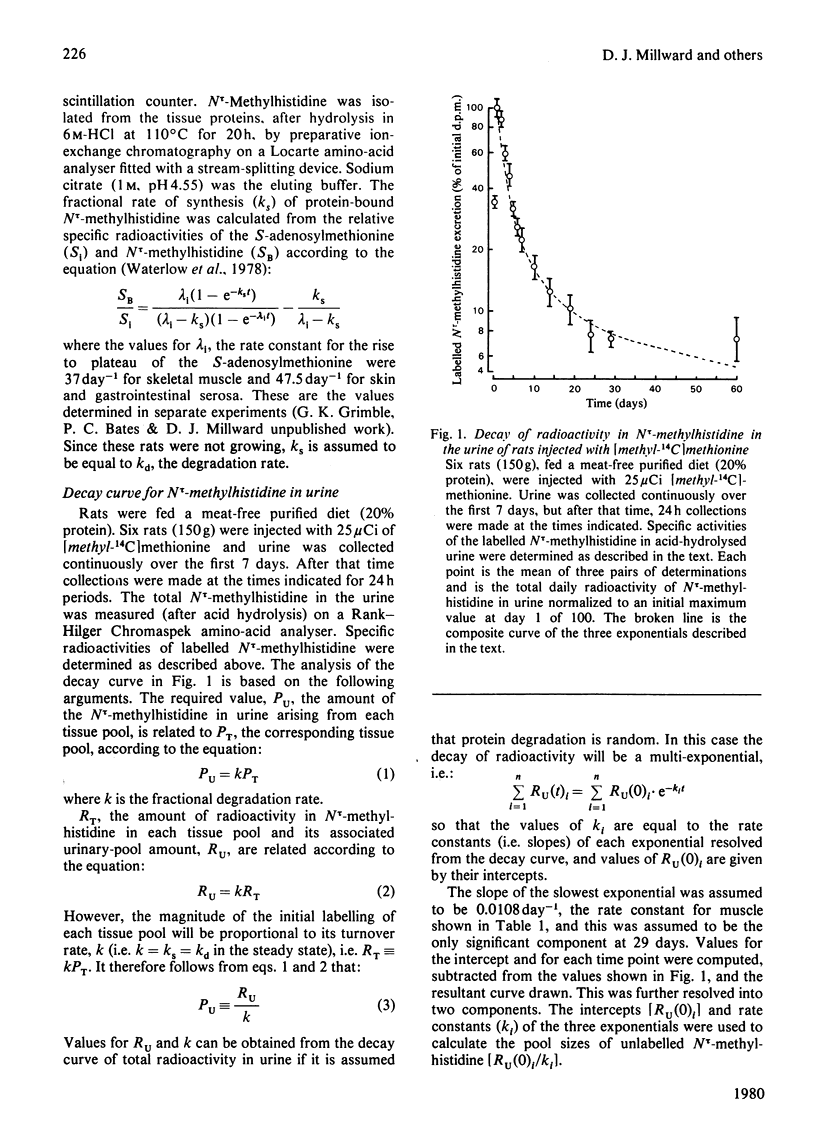
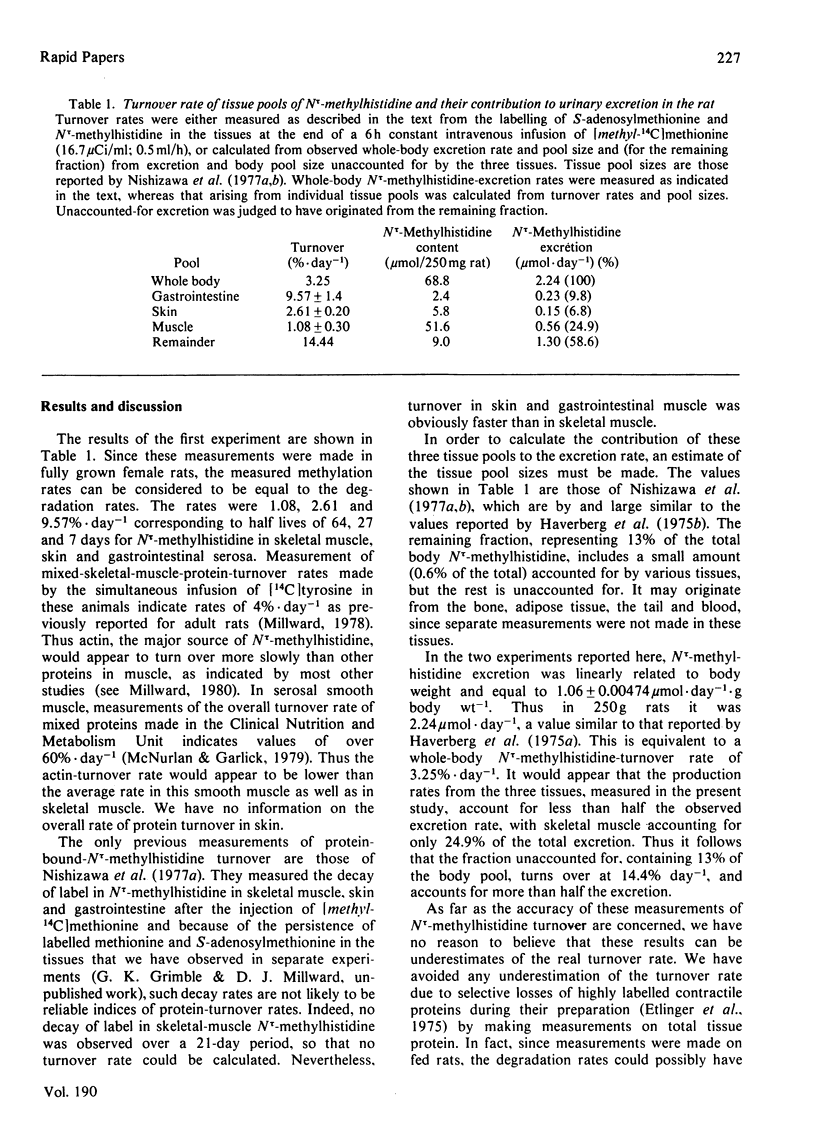

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Etlinger J. D., Zak R., Fischman D. A., Rabinowitz M. Isolation of newly synthesised myosin filaments from skeletal muscle homogenates and myofibrils. Nature. 1975 May 15;255(5505):259–261. doi: 10.1038/255259a0. [DOI] [PubMed] [Google Scholar]
- Grimble G. K., Millward D. J. The measurement of ribosomal ribonucleic acid synthesis in rat liver and skeletal muscle in vivo [proceedings]. Biochem Soc Trans. 1977;5(4):913–916. doi: 10.1042/bst0050913. [DOI] [PubMed] [Google Scholar]
- Haverberg L. N., Deckelbaum L., Bilmazes C., Munro H. N., Young V. R. Myofibrillar protein turnover and urinary N-tau-methylhistidine output. Response to dietary supply of protein and energy. Biochem J. 1975 Dec;152(3):503–510. doi: 10.1042/bj1520503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haverberg L. N., Omstedt P. T., Munro H. N., Young V. R. Ntau-methylhistidine content of mixed proteins in various rat tissues. Biochim Biophys Acta. 1975 Sep 9;405(1):67–71. doi: 10.1016/0005-2795(75)90315-3. [DOI] [PubMed] [Google Scholar]
- Long C. L., Haverberg L. N., Young V. R., Kinney J. M., Munro H. N., Geiger J. W. Metabolism of 3-methylhistidine in man. Metabolism. 1975 Aug;24(8):929–935. doi: 10.1016/0026-0495(75)90084-0. [DOI] [PubMed] [Google Scholar]
- Millward D. J. The regulation of muscle-protein turnover in growth and development. Biochem Soc Trans. 1978;6(3):494–499. doi: 10.1042/bst0060494. [DOI] [PubMed] [Google Scholar]
- Nishizawa N., Noguchi T., Hareyama S., Funabiki R. Fractional flux rates of Nt-methylhistidine in skin and gastrointestine: the contribution of these tissues to urinary excretion of Nt-methylhistidine in the rat. Br J Nutr. 1977 Jul;38(1):149–151. doi: 10.1079/bjn19770072. [DOI] [PubMed] [Google Scholar]
- Nishizawa N., Shimbo M., Hareyama S., Funabiki R. Fractional catabolic rates of myosin and actin estimated by urinary excretion of Ntau-methylhistidine: the effect of dietary protein level on catabolic rates under conditions of restricted food intake. Br J Nutr. 1977 May;37(3):345–353. doi: 10.1079/bjn19770038. [DOI] [PubMed] [Google Scholar]
- Ward L. C., Buttery P. J. The kinetics of myofibrillar protein breakdown in perfused rat skeletal muscle. Biochim Biophys Acta. 1979 Oct 18;587(3):415–423. doi: 10.1016/0304-4165(79)90445-8. [DOI] [PubMed] [Google Scholar]
- Willocks J. The assessment of foetal growth. Proc Nutr Soc. 1977 May;36(1):1–7. doi: 10.1079/pns19770002. [DOI] [PubMed] [Google Scholar]
- Young V. R., Alexis S. D., Baliga B. S., Munro H. N., Muecke W. Metabolism of administered 3-methylhistidine. Lack of muscle transfer ribonucleic acid charging and quantitative excretion as 3-methylhistidine and its N-acetyl derivative. J Biol Chem. 1972 Jun 10;247(11):3592–3600. [PubMed] [Google Scholar]
- Young V. R., Munro H. N. Ntau-methylhistidine (3-methylhistidine) and muscle protein turnover: an overview. Fed Proc. 1978 Jul;37(9):2291–2300. [PubMed] [Google Scholar]


