Abstract
Aim: Early and intensive low-density lipoprotein (LDL-C)-lowering therapy plays important roles in secondary prevention of acute coronary syndrome (ACS), but the treatment period for further clinical benefit remains undefined. This single-center, retrospective study explored LDL-C trajectory after ACS and its associations with subsequent cardiovascular events (CVE).
Methods: In 831 patients with ACS, we evaluated LDL-C reduction during the first 2 months post-ACS as an index of early intervention and the area over the curve for LDL-C using 70 mg/dl as the threshold in the next 6 months (AOC-70) as a persistent intensity index. Patients were followed for a median of 3.0 (1.1-5.2) years for CVE, defined as the composite of cardiovascular death, non-fatal myocardial infarction, angina pectoris requiring revascularization, cerebral infarction, and coronary bypass grafting.
Results: LDL-C decreased from baseline to 2 months post-ACS (107±38 mg/dl to 78±25 mg/dl,p<0.001) through high-intensity statin prescription (91.8%), while achieving rates of LDL-C <70 mg/dl at 2 months remained only 40.2% with no significant changes thereafter. During the follow-up period, CVE occurred in 200 patients. LDL-C reduction during the first 2 months and AOC-70 in the next 6 months were both associated with subsequent CVE risk (sub-HR [hazard ratio] [95% confidence interval]: 1.48 [1.16-1.89] and 1.22 [1.05-1.44]). Furthermore, early intervention followed by persistently intensive LDL-C-lowering therapy resulted in further CVE risk reduction.
Conclusions: The present study observed that achieving early and intensive LDL-C reduction within the first two months after ACS and maintaining it for the next six months suppressed subsequent CVE risk, suggesting the importance of early, intensive, and persistent LDL-C-lowering therapy in the secondary prevention of ACS.
Keywords: Acute coronary syndrome, Early intervention, High-intensity statins, Intensive LDL-C-lowering therapy, Residual risk
Kozo Okada and Tatsuya Haze contributed equally to this work.
Introduction
Acute coronary syndrome (ACS) remains a leading cause of mortality and morbidity worldwide and continues to be a substantial proportion of the global disease burden 1 , 2) . The primary pathogenesis of ACS is the development and disruption of coronary artery plaque, and low-density lipoprotein cholesterol (LDL-C) plays a central role in the initiation and progression of atherosclerosis 3 - 6) . Lipid-lowering therapy with statins is considered to suppress plaque progression and destabilization and prevent recurrent cardiovascular events (CVE) after ACS 7 - 10) . Multiple large-scale, prospective, randomized, controlled trials (RCTs) have confirmed this and shown the beneficial effects of early and/or aggressive LDL-C-lowering therapy with high-intensity statins on coronary plaques, CVE, and residual risk factors, such as inflammation 11 - 13) . Recent investigations have also suggested the importance of persistently maintaining LDL-C reduction by focusing on the association between cumulative lifetime exposure to LDL-C and the risk of CVE for primary prevention 4) . However, data (especially, real-world clinical data) on the combined effects of the degree of early, intensive, and persistent LDL-C reduction in a specific period after the onset of ACS on the secondary prevention of CVE and associated residual risks is lacking.
Therefore, the present study analyzed longitudinally and in detail the trajectories of LDL-C, focusing on the first two to eight months after the onset of ACS, and explored their associations with subsequent CVE and residual risk factors, using data from a single-center, retrospective, observational cohort.
Methods
Study Population
The present study included patients who were diagnosed with ACS and underwent percutaneous coronary intervention (PCI) with stent implantation at the Yokohama City University Medical Center in Japan between January 2013 and December 2022. Patients treated with balloon angioplasty (including drug-coated balloon) alone or those with severely calcified lesions required for rotational atherectomy were excluded because both patient subsets may relate to increased risks of target lesion failure. Patients with less than two examinations of LDL-C, high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), and hemoglobin A1c (HbA1c) during the six-month period from two to eight months after PCI, those with missing data on covariates (e.g. coronary risk factors, lipid and glucose parameters, and medications), and those with an observational period of less than three months after PCI were excluded.
All clinical data were collected from electronic medical records at baseline (defined as the date of the index PCI) and 2, 4, 6, 8, 10, 12, 24, 36, and 48 months after PCI. Data on high-sensitive C-reactive protein (hsCRP) were collected at baseline and 12 months after PCI. Prescription records were collected at each visit point, and the average daily dose and cumulative dose were tabulated for lipid-lowering drugs, based on the defined daily dose (DDD) set by the World Health Organization 14) . In the present study, atorvastatin, rosuvastatin, and pitavastatin were defined as strong statins, and the other statins were defined as standard statins 11) .
Informed consent was obtained via an opt-out method on the website, and patients who opted out were excluded. The study protocol was approved by the ethics committee of Yokohama City University and followed the principles of the Declaration of Helsinki.
Analyses of LDL-C and Residual Risk Factors and the Primary Endpoint
High-intensity statins were assessed by prescription of strong statins and the DDD. Residual risk factors included the HDL-C, TG, HbA1c, hsCRP, kidney function defined by estimated glomerular function rate (eGFR), uric acid, and history of chronic obstructive pulmonary disease (COPD). Longitudinal management intensity of LDL-C and residual risk factors during the study period was evaluated by referring to target values set by the guidelines 11 - 13) . For each observational time-point from two months to eight months post-PCI, we calculated the area over the curve (AOC) for LDL-C, percent LDL-C to baseline (%LDL-C=LDL-C levels at each visit divided by LDL-C levels at baseline times 100, %), TG and HbA1c, and area under the curve (AUC) for HDL-C using 70 mg/dl, 50%, 175 mg/dl, 7.0%, and 40 mg/dl as guideline-based thresholds for reference 11 - 13) , respectively. The AOC and AUC values served as indicators of the degree of deviation from the target values during the observational period, and smaller values indicated better control ( Fig.1 ) 15) . Early therapeutic intervention for LDL-C was assessed by LDL-C levels and %LDL-C at two months after PCI as well as absolute changes (ΔLDL-C) from baseline to two months. Effects of early, intensive, and persistent LDL-C-lowering therapy during the first two to eight months on residual risks as pleiotropic effects were evaluated by changes in lipid variables other than LDL-C, HbA1c, eGFR, uric acid, and hsCRP from baseline to one year post-PCI.
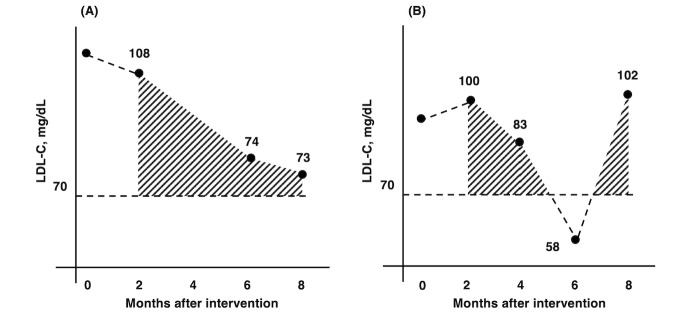
Fig.1. Schematic diagram of the calculation of areas over the curve.
To quantify the degrees of intensity and maintenance of LDL-C-lowering therapy during the six-month period from two to eight months after percutaneous coronary intervention (PCI), excluding the baseline value, we plotted the available LDL-C values from two to eight months after PCI. We then calculated the total trapezoidal area under these linearly connected points above a threshold. LDL-C = low-density lipoprotein cholesterol.
The primary endpoint was composite CVE (cardiovascular death, non-fatal myocardial infarction, angina pectoris requiring revascularization, cerebral infarction, and coronary artery bypass grafting) at two months after the index PCI, as the possibility of pre-planned staged coronary revascularization could not be excluded.
Statistical Analyses
All analyses were performed with the R software program version 4.2.0 (R Foundation for Statistical Computing, Vienna, Austria). Significance was defined as p<0.05. Data are expressed as frequencies and percentages for categorical variables, and as the mean±standard deviation and the median with interquartile range for continuous variables. Categorical comparisons were performed using a chi-square test or Fisher’s exact test. Continuous values were compared using an unpaired t-test or Mann-Whitney U test, as appropriate. Hazard ratios (HRs) were estimated using an extended Cox regression model with LDL-C, HDL-C, log-transformed TG, and log-transformed HbA1c values at each observational point as time-varying covariates 16) . As multivariable models, model 1 was adjusted for the age, sex, and body mass index (BMI). Model 2 was additionally adjusted for hypertension, COPD, the administration of anti-platelet drugs as time-invariant covariates, and the eGFR and log-transformed hsCRP as time-varying covariates. As a non-linear model, we applied penalized spline functions in the Cox proportional hazards model. For the sensitivity analysis, we constructed a piece-wise exponential additive mixed model using penalized spline functions as well 17) .
Survival analyses for composite CVE during the remaining observation period beyond eighth months post-PCI were conducted 15) . The sub-hazard ratio (sub-HR) was calculated using a flexible risk regression with the Fine-Gray model, considering non-CVE-related death as a competing risk 18) . For sensitivity analysis, regression analyses for the absolute risk were conducted 19) . Due to the skewed distribution, we performed Yeo-Johnson transformation on the AOC for %LDL-C >50% to improve normality 20) . Models were adjusted for the age, sex, BMI, AUC for HDL-C <40 mg/dl, AOC for TG >175 mg/dl, and AOC for HbA1c >7.0% in model 1 and additionally included the use of anti-platelet drugs, a history of COPD, hypertension, and the baseline LDL-C, eGFR, and log-transformed hsCRP in model 2. In addition to calculations for an LDL-C of 70 mg/dl, we also calculated the AOC for 55, 40, and 0 mg/dl and created a risk curve predicted for maintaining arbitrary LDL-C levels using the last model (i.e. 0 mg/dl model).
Regarding early therapeutic intervention for LDL-C levels, the relationship between the LDL-C levels at two months after PCI and subsequent CVE was analyzed using the Fine-Gray model. LDL-C levels were incorporated in the models as identical forms of ΔLDL-C or %LDL-C. To assess the combined impacts of early, intensive, and persistent LDL-C-lowering therapy on subsequent CVE, patients were further classified into 4 groups based on the median values of ΔLDL-C in the first 2 months after PCI (21 mg/dl) and AOC for LDL-C >70 mg/dl within the next 6 months (40 mg×month/dl): ‘Early (+ or -)’ and ‘Intensive/Persistent (+ or -)’. CVE risk was predicted for each group utilizing unadjusted Fine-Gray models. Changes in the HDL-C, TG, HbA1c, hsCRP, uric acid, and eGFR values were also compared between the groups with Early (+) and Intensive/Persistent (+) vs. Early (–) and Intensive/Persistent (–) to assess pleiotropic effects of early, intensive, and persistent LDL-C-lowering therapy.
Results
Patient Characteristics and Lipid Status
A total of 1648 cases were evaluated for eligibility, and after excluding 114 cases with missing data, 679 without sufficient observations of lipid and glucose parameters from 2 to 8 months after PCI, and 24 cases with a follow-up period of <3 months, 831 cases were included in the primary analysis (detailed patient flow is shown in Supplementary Fig.1 , and patient characteristics of the largest excluded group of 679 cases are shown in Supplementary Table 1 ).
Supplementary Fig.1. Patient flowchart.
AUC = area under the curve; AOC = area over the curve; LDL-C = low-density lipoprotein cholesterol; PCI = percutaneous coronary intervention.
Supplementary Table 1. Clinical characteristics of patients without sufficient observations of lipid and glucose parameters from two to eight months after PCI.
| Variables |
Patients enrolled in the main analysis (n = 831) |
Patients excluded due to insufficient measurements (n = 679) |
p value |
|---|---|---|---|
| Age, years | 69.4±11.2 | 71.8±12.0 | <0.001 |
| Women, n (%) | 172 (20.7) | 162 (23.9) | 0.14 |
| BMI, kg/m2 | 23.8±3.8 | 23.6±3.9 | 0.26 |
| History of diabetes mellitus, n (%) | 431 (51.9) | 318 (46.8) | 0.05 |
| History of dyslipidemia, n (%) | 720 (86.6) | 522 (76.9) | <0.001 |
| History of hypertension, n (%) | 679 (81.7) | 511 (75.3) | <0.01 |
| History of COPD, n (%) | 51 (6.1) | 54 (8.0) | 0.17 |
| Uses of antihypertensive drugs, n (%) | 565 (68.0) | 470 (69.2) | 0.61 |
| CCB, n (%) | 247 (29.7) | 240 (35.3) | <0.05 |
| ACEi, n (%) | 221 (26.6) | 123 (18.1) | <0.001 |
| ARB, n (%) | 226 (27.2) | 190 (28.0) | 0.73 |
| Thiazides, n (%) | 26 (3.1) | 15 (2.2) | 0.27 |
| Alpha-blockers, n (%) | 15 (1.8) | 15 (2.2) | 0.58 |
| Beta-blockers, n (%) | 371 (44.6) | 279 (41.1) | 0.17 |
| MRB, n (%) | 74 (8.9) | 54 (8.0) | 0.51 |
| eGFR, mL/min/1.73 m2 | 60.5±20.8 | 56.3±24.1 | <0.001 |
| LDL-C, mg/dl | 106.6±38.2 | 102.3±38.7 | <0.05 |
| HDL-C, mg/dl | 46.7±12.2 | 48.0±14.0 | 0.06 |
| TG, mg/dl | 124.0 (86.5, 186.0) | 120.0 (80.0, 176.0) | 0.06 |
| Hemoglobin A1c, % | 6.1 (5.7, 7.0) | 5.9 (5.6, 6.6) | <0.001 |
| Uric acid, mg/dl | 5.9±1.5 | 5.9±1.7 | 0.53 |
| hsCRP, mg/dl | 0.2 (0.1, 0.6) | 0.2 (0.1, 0.6) | 0.40 |
| Uses of lipid-lowering drugs, n (%) | 620 (74.6) | 488 (71.9) | 0.23 |
| Statins, n (%) | 616 (74.1) | 476 (70.1) | 0.08 |
| Fibrates, n (%) | 11 (1.3) | 13 (1.9) | 0.36 |
| Ezetimibe, n (%) | 65 (7.8) | 54 (8.0) | 0.93 |
| PCSK9 inhibitors, n (%) | 1 (0.1) | 1 (0.1) | 0.89 |
| Uses of antidiabetic drugs | 209 (25.2) | 149 (21.9) | 0.15 |
| Insulin, n (%) | 61 (7.3) | 39 (5.7) | 0.21 |
| SGLT2 inhibitors, n (%) | 55 (6.6) | 34 (5.0) | 0.19 |
| Biguanides, n (%) | 21 (2.5) | 18 (2.7) | 0.88 |
Data are expressed as mean±SD for unskewed variables, median (interquartile) for skewed variables, and numbers (%) for categorical variables. The cases excluded excluded due to the insufficient measurements had higher age and lower rates in diabetes mellitus, dyslipidemia, and hypertension compared to the cases enrolled in the primary analysis. ACEi = angiotensin-converting-enzyme inhibitors, ARB = angiotensin receptor blockers, BMI = body mass index, CCB = calcium channel blockers, COPD = chronic obstructive pulmonary disease, hsCRP = high-sensitive C-reactive protein, eGFR = estimated glomerular filtration rate, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, MRB = mineralocorticoid receptor blockers, PCSK9 = proprotein convertase subtilisin/kexin type 9; SD = standard deviation, SGLT2 = sodium-glucose co-transporter 2, TG = triglyceride.
Patient characteristics are shown in Table 1 . The mean age was 69±11 years old, and 20.7% were female. Baseline LDL-C levels at the index PCI were 107±38 mg/dl, which decreased to 78±25 mg/dl at 2 months after PCI (p<0.001) and remained at approximately the same level thereafter ( Table 2 ) . A total of 40.2% (295/733) of patients achieved LDL-C levels of <70 mg/dl (target value according to the guidelines) at 2 months, and 16.8% achieved <50% of baseline LDL-C levels. In contrast, HDL-C, TG, HbA1c, and hsCRP values had remained at approximately the same levels over one year ( Table 2 ) . At the time of PCI, 74.6% of patients were taking lipid-lowering medications, mostly statins, but this rate increased to 91.8% at the 2 months and then gradually declined thereafter ( Table 3 ) . Daily doses of statins slightly increased from 0.62±0.37 at baseline to 0.66±0.37 per DDD at 2 months. The rate of ezetimibe uses also increased from 7.8% at baseline to 10.8% at 2 months.
Table 1. Clinical characteristics.
| Variables | Overall (n= 831) |
|---|---|
| Age, years | 69±11 |
| Women, n (%) | 172 (20.7) |
| BMI, kg/m2 | 23.8±3.8 |
| History of diabetes mellitus, n (%) | 431 (51.9) |
| History of dyslipidemia, n (%) | 720 (86.6) |
| History of hypertension, n (%) | 679 (81.7) |
| History of COPD, n (%) | 51 (6.1) |
| Uses of antihypertensive drugs, n (%) | 565 (68.0) |
| CCB, n (%) | 247 (29.7) |
| ACEi, n (%) | 221 (26.6) |
| ARB, n (%) | 226 (27.2) |
| Thiazides, n (%) | 26 (3.1) |
| Alpha-blockers, n (%) | 15 (1.8) |
| Beta-blockers, n (%) | 371 (44.6) |
| MRB, n (%) | 74 (8.9) |
| eGFR, mL/min/1.73 m2 | 60.5±20.8 |
| LDL-C, mg/dl | 107±38 |
| HDL-C, mg/dl | 47±12 |
| TG, mg/dl | 124 (87 to 186) |
| Hemoglobin A1c, % | 6.1 (5.7 to 7.0) |
| Uric acid, mg/dl | 5.9±1.5 |
| hsCRP, mg/dl | 0.2 (0.1 to 0.6) |
| Uses of lipid-lowering drugs, n (%) | 620 (74.6) |
| Statins, n (%) | 616 (74.1) |
| Fibrates, n (%) | 11 (1.3) |
| Ezetimibe, n (%) | 65 (7.8) |
| PCSK9 inhibitor, n (%) | 1 (0.1) |
| Uses of antidiabetic drugs | 209 (25.2) |
| Insulin, n (%) | 61 (7.3) |
| SGLT2 inhibitors, n (%) | 55 (6.6) |
| Biguanides, n (%) | 21 (2.5) |
Data are expressed as the mean±SD for unskewed variables, median (interquartile) for skewed variables, and numbers (%) for categorical variables. ACEi = angiotensin-converting-enzyme inhibitors, ARB = angiotensin receptor blockers, BMI = body mass index, CCB = calcium channel blockers, COPD = chronic obstructive pulmonary disease, hsCRP = high-sensitive C-reactive protein, eGFR = estimated glomerular filtration rate, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, MRB = mineralocorticoid receptor blockers, PCSK9 = proprotein convertase subtilisin/kexin type 9; SD = standard deviation, SGLT2 = sodium-glucose co-transporter 2, TG = triglyceride.
Table 2. Management statuses of LDL-C and residual risk factors at each visit point.
| Month 0 | Month 2 | Month 4 | Month 6 | Month 8 | Month 10 | Month 12 | |
| LDL-C related values | n=831 | n=733 | n=679 | n=646 | n=606 | n=551 | n=583 |
| LDL-C, mg/dl | 107±38 | 78±25 | 79±25 | 76±23 | 77±23 | 77±25 | 76±23 |
| %LDL-C, % | 100 (100 to 100) | 77.1 (56.1 to 100) | 74.0 (56.1 to 100) | 75.7 (54.9 to 98.8) | 76.1 (55.3 to 98.7) | 75.8 (54.3 to 98.7) | 73.7 (54.7 to 98.0) |
| LDL-C <70 mg/dl, n (%) | 128 (15.4) | 295 (40.2) | 258 (38.0) | 273 (42.3) | 236 (38.9) | 232 (42.1) | 245 (42.0) |
| LDL-C <55 mg/dl, n (%) | 50 (6.0) | 116 (15.8) | 103 (15.2) | 108 (16.7) | 86 (14.2) | 89 (16.2) | 97 (16.6) |
| LDL-C <40 mg/dl, n (%) | 11 (1.3) | 25 (3.4) | 20 (2.9) | 25 (3.9) | 19 (3.1) | 17 (3.1) | 20 (3.4) |
| %LDL-C <50%, n (%) | 0 (0) | 123 (16.8) | 116 (17.1) | 116 (18.0) | 106 (17.5) | 105 (19.1) | 108 (18.5) |
| Residual risk factors | |||||||
| HDL-C, mg/dl | 47±12 (n=831) | 47±11 (n=733) | 47±12 (n=679) | 47±13 (n=645) | 48±12 (n=605) | 49±12 (n=549) | 48±12 (n=582) |
| TG, mg/dl | 124 (87-186)(n=831) | 127 (89-180)(n=737) | 126 (90-177)(n=680) | 127 (90-185)(n=650) | 129 (89-181)(n=606) | 130 (93-181)(n=553) | 126 (90-179)(n=588) |
| HbA1c, % | 6.1 (5.7-7.0)(n=831) | 6.1 (5.7-6.8)(n=721) | 6.2 (5.8-6.8)(n=667) | 6.2 (5.8-6.9)(n=620) | 6.2 (5.8-6.8)(n=598) | 6.2 (5.8-6.8)(n=538) | 6.2 (5.8-6.9)(n=565) |
| hsCRP, mg/dl | 0.2 (0.1-0.6)(n=831) | - | - | - | - | - | 0.1 (0.0-0.2)(n=604) |
Data are expressed as the mean±SD for unskewed variables, median (interquartile range) for skewed variables, and numbers (%) for categorical variables. HbA1c = hemoglobin A1c, HDL-C = high-density lipoprotein cholesterol, hsCRP = high-sensitive C-reactive protein, LDL-C = low- density lipoprotein cholesterol, TG = triglycerides.
Table 3. Longitudinal changes of prescription statues of lipid-lowering drugs before and after PCI.
| Before intervention |
Month 0 to Month 2 |
Month 2 to Month 4 |
Month 4 to Month 6 |
Month 6 to Month 8 |
Month 8 to Month 10 |
Month 10 to Month 12 |
|
|---|---|---|---|---|---|---|---|
| Lipid-lowering drugs, n (%) |
620 (74.6) (n= 831) |
763 (91.8) (n= 831) |
674 (84.5) (n= 798) |
605 (79.5) (n= 761) |
547 (75.3) (n= 726) |
546 (74.5) (n= 733) |
521 (76.5) (n= 681) |
| Statins, n (%) |
616 (74.1) (n= 831) |
759 (91.3) (n= 831) |
667 (83.6) (n= 798) |
594 (78.1) (n= 761) |
537 (74.0) (n= 726) |
538 (73.4) (n= 733) |
511 (75.0) (n= 681) |
| Statins, per DDD |
0.62±0.37 (n= 616) |
0.66±0.37 (n= 759) |
0.66±0.38 (n= 667) |
0.65±0.37 (n= 594) |
0.66±0.39 (n= 537) |
0.65±0.39 (n= 538) |
0.65±0.38 (n= 511) |
| Fibrates, n (%) |
11 (1.3) (n= 831) |
11 (1.3) (n= 831) |
8 (1.0) (n= 798) |
8 (1.1) (n= 761) |
5 (0.7) (n= 726) |
7 (1.0) (n= 733) |
6 (0.9) (n= 681) |
| Fibrates, per DDD |
0.75±0.50 (n= 11) |
0.65±0.56 (n= 11) |
0.98±0.47 (n= 8) |
0.79±0.31 (n= 8) |
0.81±0.27 (n= 5) |
0.80±0.27 (n= 7) |
0.76±0.28 (n= 6) |
| Ezetimibe, n (%) |
65 (7.8) (n= 831) |
90 (10.8) (n= 831) |
80 (10.0) (n= 798) |
84 (11.0) (n= 761) |
82 (11.3) (n= 726) |
84 (11.5) (n= 733) |
86 (12.6) (n= 681) |
| Ezetimibe, per DDD |
0.78±0.28 (n= 65) |
0.81±0.31 (n= 90) |
0.92±0.19 (n= 80) |
0.97±0.20 (n= 84) |
0.97±0.13 (n= 82) |
0.92±0.20 (n= 84) |
0.93±0.21 (n= 86) |
| PCSK9 inhibitors, n (%) |
1 (0.1) (n= 831) |
0 (0.0) (n= 831) |
1 (0.1) (n= 798) |
3 (0.4) (n= 761) |
3 (0.4) (n= 726) |
3 (0.4) (n= 733) |
3 (0.4) (n= 681) |
Uses and doses of lipid-lowering drugs prescribed in each period before and after percutaneous coronary intervention (PCI) are shown. The mean and standard deviation of the dosage were calculated only for cases that were taking the medication. DDD = defined daily dose. PCSK9 = proprotein convertase subtilisin/kexin type 9.
Associations of Changes in LDL-C and Residual Risk Factors with CVE
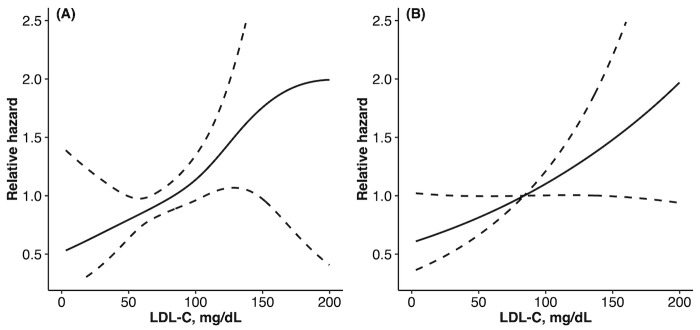
Patients were followed for up to 6 (median 3.0 [1.1-5.2]) years after PCI. During the follow-up period, 200 patients (75.1/1000 person-years) experienced CVE (10 cardiac deaths, 109 non-fatal myocardial infarctions, 63 angina pectoris requiring revascularization [comprising 28 de novo lesions and 35 recurrences at the final treatment sites], 4 coronary artery bypass grafting surgeries, and 14 cerebral infarctions). In the extended COX model as a time-varying analysis, changes in LDL-C levels were associated with CVE even after adjusting for multivariable covariates (HR [95% CI]: 1.23 [1.04-1.46]), while changes in HDL-C, TG, or HbA1c levels were not ( Table 4A ) . The association between the LDL-C management status and increased CVE risk was preserved in the 2 non-linear models (p<0.05 for both), approximating closely linear relationships in both models ( Fig.2 ) .
Table 4. Lipid and glucose management statuses and subsequent CVE risks.
| A | HR (95% CI) | ||
| Unadjusted model | Adjusted model 1 | Adjusted model 2 | |
| LDL-C, mg/dl | 1.20 (1.02 to 1.41)* | 1.25 (1.05 to 1.47)* | 1.23 (1.04 to 1.46)* |
| HDL-C, mg/dl | 0.90 (0.77 to 1.04) | 0.87 (0.74 to 1.03) | 0.90 (0.77 to 1.06) |
| TG, mg/dl | 1.05 (0.91 to 1.21) | 0.97 (0.82 to 1.14) | 0.97 (0.82 to 1.14) |
| Hemoglobin A1c, % | 1.09 (0.95 to 1.26) | 1.06 (0.92 to 1.23) | 1.05 (0.90 to 1.21) |
| B | sub-HR (95% CI) | ||
| Unadjusted model | Adjusted model 1 | Adjusted model 2 | |
| AOC for LDL-C >70 mg/dl | 1.17 (1.01 to 1.35)* | 1.18 (1.02 to 1.37)* | 1.22 (1.05 to 1.44)* |
| AOC for LDL-C >55 mg/dl | 1.18 (1.01 to 1.37)* | 1.19 (1.02 to 1.39)* | 1.25 (1.05 to 1.48)* |
| AOC for LDL-C >40 mg/dl | 1.19 (1.01 to 1.39)* | 1.20 (1.02 to 1.40)* | 1.26 (1.06 to 1.50)† |
| AOC for LDL-C >0 mg/dl | 1.19 (1.02 to 1.39)* | 1.20 (1.02 to 1.41)* | 1.27 (1.07 to 1.52)† |
| AOC for %LDL-C >50% | 1.14 (å0.98 to 1.33) | 1.13 (0.97 to 1.32) | 1.20 (0.97 to 1.47) |
| C | sub-HR (95% CI) | ||
| Unadjusted model | Adjusted model 1 | Adjusted model 2 | |
| AUC for HDL-C <40 mg/dl | 1.19 (1.03 to 1.37)* | 1.23 (1.07 to 1.40)† | 1.20 (1.05 to 1.37)† |
| AOC for TG >175 mg/dl | 1.03 (0.87 to 1.21) | 1.06 (0.92 to 1.23) | 1.08 (0.92 to 1.26) |
| AOC for HbA1c >7.0% | 0.99 (0.83 to 1.19) | 0.97 (0.80 to 1.18) | 0.90 (0.73 to 1.11) |
| D | sub-HR (95% CI) | ||
| Unadjusted model | Adjusted model 1 | Adjusted model 2 | |
| LDL-C at 2 months, mg/dl | 1.19 (1.03 to 1.37)* | 1.21 (1.06 to 1.40)† | 1.29 (1.10 to 1.52)† |
| %LDL-C at 2 months, % | 1.24 (1.06 to 1.44)† | 1.22 (1.05 to 1.43)* | 1.36 (1.10 to 1.68)† |
| ΔLDL-C from baseline to 2 months, mg/dl | 1.29 (1.09 to 1.52)† | 1.26 (1.06 to 1.50)† | 1.48 (1.16 to 1.89)† |
A) Standardized hazard ratios (HR) and 95% confidence interval (CI) were estimated by extended Cox regression models by including low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), log-transformed Triglyceride (TG), and log-transformed hemoglobin A1c (HbA1c) as time-varying covariates. B) and C) Standardized sub-distribution hazard ratios (sub-HR) and 95% CI were estimated by flexible risk regression with Fine-Gray model using areas over/under the curve (AOC/AUC) during the early six months after coronary intervention. D) Sub-HR and 95% CI were estimated by risk regression with Fine-Gray model using LDL-C, %LDL-C, and ΔLDL-C. CVE = cardiovascular event. *p<0.05, †p<0.01.
Fig.2. Association between LDL-C and CVE in the non-linear models.
Partial estimates and 95% confidence intervals for LDL-C expressed as an exponential form in (A) a Cox proportional hazard model with splines and (B) piece-wise exponential additive mixed model with splines. Both models were adjusted for the age, sex, body mass index, estimated glomerular filtration rate, high-density lipoprotein cholesterol, triglyceride, hemoglobin A1c, history of chronic obstructive pulmonary disease, hypertension, use of anti-platelet drugs, and high-sensitive C-reactive protein. CVE = cardiovascular event; LDL-C = low-density lipoprotein cholesterol.
Regarding residual risk factors, a history of COPD (HR [95% CI]: 1.82 [1.13-2.93]) and hsCRP (HR [95% CI]: 1.25 [1.08-1.44]) were also associated with CVE.
Longitudinal Management Statuses of LDL-C and Residual Risk Factors and CVE
The analyses included 801 patients, with a median follow-up period of 2.8 years counting from 8 months after PCI (i.e. after calculating the AOC during the first 2 to 8 months post-PCI). During this period, 164 CVE occurred (73.1/1000 person-years). The AOC for LDL-C >70 mg/dl during the early 6 months after PCI was statistically associated with subsequent CVE in both the unadjusted and adjusted models (adjusted sub-HR [95% CI]: 1.22 [1.05-1.44]) ( Table 4B ) . Similarly, the AOC for LDL >55 mg/dl, >40 mg/dl, and even >0 mg/dl were all associated with an increased CVE risk. If we assumed that LDL-C levels were sustained at a specific value over six months after PCI, subsequent CVE risks were predicted as in Fig.3 . When computing the AOC with a cut-off value of >50% reduction from baseline, a similar trend was observed with no statistical significance (adjusted sub-HR [95% CI]: 1.20 [0.97-1.47], p=0.09). Regarding management statuses of residual risk factors, AUC for HDL <40 mg/dl was associated with subsequent CVE (adjusted sub-HR [95% CI]: 1.20 [1.05-1.37]), whereas AOC for HbA1c >7.0% or TG >175 mg/dl was not ( Table 4C ) . Similar results were also seen in the analyses of absolute risk regression (data not shown).
Fig.3. Impacts of longitudinal LDL-C management during the early six months after PCI on CVE.
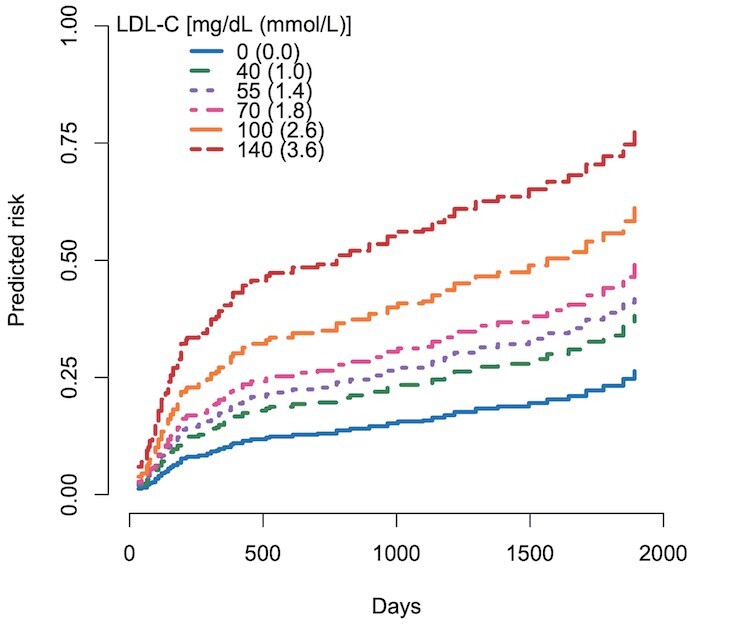
Predicted risk curves for cardiovascular events (CVE) were estimated by a multi-covariate-adjusted model (model 2) assuming that each LDL-C value continues during the first six months after percutaneous coronary intervention (PCI).
Combined Impacts of Early, Intensive, Persistent LDL-C-Lowering Therapy on CVE
LDL-C levels and %LDL-C at two months after PCI and ΔLDL-C from baseline to two months were all associated with subsequent CVE ( Table 4D ) . Significantly greater cumulative DDD of statins, especially of strong statins, within the first 2 months were seen in patients with vs. without LDL-C <70 mg/dl at 2 months post-PCI ( Table 5 ) . Patients who could achieve and maintain early, intensive, persistent LDL-C- reduction (Early [+] and Intensive/Persistent [+] group) showed further lower CVE risk compared with those who did not (Early [–] and Intensive/Persistent [–] group) ( Fig.4 ) . Compared to the reference group of achieving early, intensive, and persistent LDL-C reduction, groups that failed to achieve these components showed sub-HR [95% CI] values of 1.90 [1.20-3.01] for Early (-) and Intensive/Persistent (-), 1.20 [0.70-2.07] for Early (+) and Intensive/Persistent (-), and 1.57 [0.94-2.65] for Early (-) and Intensive/Persistent (+), respectively ( Fig.4 ) . In addition, patients in the Early (+) and Intensive/Persistent (+) group showed significantly decreased hsCRP (-0.1 [–0.5 to –0.0] vs. 0.0 [-0.2 to 0.1] mg/dl, p<0.001) and TG (-7.5 [-52.2 to 21.2] vs. 6.0 [-43.0 to 49] mg/dl, p<0.05) levels from baseline to one-year post-PCI, and suppressed eGFR reduction during 1 year (-0.6 [-7.3 to 5.7] vs. -3.7 [-8.7 to 1.1] ml/min/1.73 m2, p<0.05) compared with those of the Early (–) and Intensive/Persistent (–) group.
Table 5. Comparison of clinical characteristics between LDL-C ≥ 70 vs. LDL-C <70 mg/dl at 2 months among patients who had not taken any lipid-lowering medications at baseline.
| LDL-C ≥70 mg/dl at 2 months (n= 102) | LDL-C <70 mg/dl at 2 months (n= 76) | p value | |
|---|---|---|---|
| Age, years | 68±12 | 70±12 | 0.40 |
| Women, n (%) | 20 (19.6) | 11 (14.5) | 0.43 |
| BMI, kg/m2 | 24.0±4.4 | 23.7±3.5 | 0.68 |
| History of diabetes mellitus, n (%) | 44 (43.1) | 30 (39.5) | 0.65 |
| History of dyslipidemia, n (%) | 71 (69.6) | 56 (73.7) | 0.62 |
| History of hypertension, n (%) | 76 (74.5) | 58 (76.3) | 0.86 |
| History of COPD, n (%) | 7 (6.9) | 4 (5.3) | 0.76 |
| Use of anti-hypertension drugs, n (%) | 24 (23.5) | 15 (19.7) | 0.59 |
| Baseline eGFR, ml/min/1.73 m2 | 61.3±24.2 | 63.6±23.0 | 0.52 |
| Baseline LDL-C, mg/dl | 126±38 | 110±32 | <0.01† |
| Baseline HDL-C, mg/dl | 44±11 | 45±12 | 0.48 |
| Baseline TG, mg/dl | 118 (69 to 198) | 121 (74 to 223) | 0.59 |
| Baseline HbA1c, % | 6.1 (5.7 to 6.7) | 6.0 (5.7 to 6.6) | 0.58 |
| Baseline uric acid, mg/dl | 6.3±1.6 | 5.8±1.7 | 0.09 |
| Baseline high-sensitive C-reactive protein, mg/dl | 0.2 (0.1-0.4) | 0.2 (0.1-0.4) | 0.83 |
| Baseline use of lipid-lowering drugs, n (%) | 0 (0.0) | 0 (0.0) | NA |
| Baseline use of antidiabetic drugs | 9 (8.8) | 6 (7.9) | 1.00 |
| Use of lipid-lowering drugs during M0 to M2, n (%) | 83 (81.4) | 69 (90.8) | 0.09 |
| Cumulative DDD of statins during M0 to M2 | 31.3±23.6 | 40.5±22.8 | <0.01 |
| Cumulative DDD of standard statins during M0 to M2 | 0.3±2.2 | 0.3±1.8 | 0.98 |
| Cumulative DDD of strong statins during M0 to M2 | 31.0±23.9 | 40.2±23.2 | <0.05 |
| Cumulative DDD of fibrates during M0 to M2 | 0.0±0.0 | 0.0±0.0 | NA |
| Cumulative DDD of ezetimibe during M0 to M2 | 1.8±9.4 | 4.1±13.1 | 0.18 |
| Cumulative DDD of other lipid-lowering drugs during M0 to M2 | 0.0±0.4 | 0.0±0.0 | 0.32 |
Data are expressed as the mean±SD for unskewed variables, median (interquartile range) for skewed variables, and counts (%) for countable variables. BMI = body mass index, COPD = chronic obstructive pulmonary disease, DDD = defined daily dose, eGFR = estimated glomerular filtration rate, HDL-C = high-density lipoprotein cholesterol, LDL-C = low-density lipoprotein cholesterol, TG = triglyceride. M0 = baseline, M2= visit point two months after the baseline. NA = not available.
Fig.4. Combined impact of early and intensive LDL-C-lowering therapy on CVE.
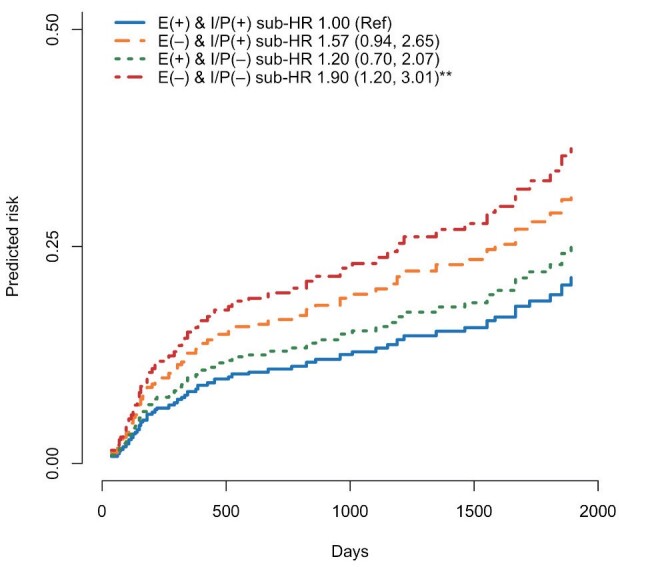
Predicted risk curves for cardiovascular events (CVE) were estimated by the Fine-Gray model. Patients who achieved early, intensive, and persistent LDL-C-lowering therapy showed the lowest CVE risk. **p<0.01 compared with the E(+) & IP(+) group. E = early, IP = intensive and persistent.
Discussion
The present study was an important one suggesting the synergetic benefits for secondary prevention of comprehensively achieving and maintaining an early, intensive, and persistent LDL-C reduction for the first eight months after ACS.
High-Intensity Statins for Achieving Target LDL-C Levels
A number of RCTs have demonstrated the benefit of high-intensity statins, and guidelines recommend strict and early control of LDL-C to <70 mg/dl with the use of high-intensity statins in patients with ACS 3 , 7 - 13) . The present study also confirmed the efficacy of high-intensity statins using real-world clinical data over a decade from a specialized CAD treatment center and observed that the cumulative dose of strong statins was a statistically significant determinant of achievements of LDL-C <70 mg/dl at 2 months after PCI, especially in patients who were statin-naïve at baseline. In contrast, the present study reaffirmed another issue, as reported in previous studies 21 , 22) , that the achievement rates of LDL-C <70 mg/dl at 2 months was insufficient (approximately 40%), with no significant changes seen afterward. The present results underscore the importance of using high-dose strong statins immediately after intervention, as well as the intensification of statin therapy, including combination therapy with the other lipid-lowering agents, without delay when target LDL-C levels are not achieved at least 2 months after the intervention. Indeed, the present study found that the dose of statins at 2 months was not adequate, being limited to around 0.6 to 0.7 per DDD despite a high prescription rate of strong statins, and the administration rate of ezetimibe was also small about 10%. It was also revealed that, as reported in a previous study 21) , LDL-C levels did not decrease significantly after the first two months, suggesting clinical inertia as well as the importance of early therapeutic intervention with LDL-C-lowering therapy.
Combination of Early, Intensive, and Persistent LDL-C-Lowering Therapy
Previous studies have reported the clinical benefits of early and intensive LDL-C-lowering therapy immediately after the onset of ACS 3 , 23 - 25) . Multiple studies have shown that early and intensive LDL-C-lowering therapy with high-intensity statins alone, statins plus ezetimibe, or statins plus proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors improved long-term clinical outcomes compared with standard statin therapy 25 - 27) . Comprehensive studies of intracoronary imaging have reported that intensive LDL-C-lowering therapy starting from early after the onset of ACS resulted in plaque regression and/or increased fibrous cap thickness at one year compared to standard therapy 28 , 29) . The present study also identified that greater LDL-C reduction during the first two months after PCI was associated with a subsequent reduction in CVE risk, contributing to the cumulative evidence.
However, the clinically important question of the minimum duration of continuing such early and intensive LDL-C-lowering therapy to achieve further clinical benefit remains unanswered. The present study used quantitative methods of AOC/AUC during the early six months (two to eight months) post-PCI to demonstrate that maintaining an LDL-C below guideline-recommended target levels of 70, 55, and 40 mg/dl 11 - 13) for as long as possible during this 6-month period was associated with further CVE risk reduction. Notably, the correlation between the longitudinal intensity of LDL-C-lowering therapy during the early six months and subsequent CVE risks appeared almost linear, with no minimum threshold for LDL-C target values. In addition, such persistently intensive LDL-C-lowering, when combined with early intervention, was observed to have synergetic clinical benefits for both CVE and residual risk factors (e.g. inflammation, TG, kidney function), building on the findings of previous studies. The results not only supported the concept of “strike early, strike strong (the lower, the better)” 10 - 13) but also indicated the importance of maintaining the concept for more than six months, at least for the secondary prevention of ACS.
Clinical Implications
Despite the growing body of evidence regarding early and intensive LDL-C-lowering therapy, it is not easy to implement and sustain it in the clinical setting, for various reasons, including medical costs, adherence, and clinical inertia. The present study showed that rapidly lowering the LDL-C level in the first two months and then maintaining it for six months (eight months total) could reduce the risk of recurrence of CVE, findings that may prove useful for physicians. Indeed, it was reported that most of the cumulative number of recurrent CVE after ACS occur within the first year after the onset of ACS 2) . In contrast, previous studies have shown that coronary plaque stabilization and regression can be seen after high-intensity statin therapy of several weeks to months 30 - 32) . A previous case report showed that reduction in plaque volume and its lipid components with intensive LDL-C-lowering therapy using a PCSK9 inhibitor for eight months was maintained at one year after discontinuing the PCSK9 inhibitor, suggesting a legacy effect 33) . These previous studies partially support the suggestion that our results would be clinically applicable.
The present study may also offer a treatment option in combination with the interfering ribonucleic acid (siRNA) therapeutic inclisiran, which reduces hepatic production of PCSK9 and results in sustained LDL-C reduction (effects last for six months with a single dose) 34) . Although medical cost remains an issue, introduction of inclisiran early after ACS onset followed by additional dosing three months later per protocol could help achieve early, intensive, and persistent (at least nine months) LDL-C reduction, thus overcoming the issues of poor adherence and clinical inertia and improving clinical outcomes.
The present study suggested not only the prognostic and pleiotropic effects of early, intensive, persistent LDL-C-lowering therapy but also the importance of managing residual risk factors, such as low HDL-C levels, COPD, and increased inflammation, as in previous studies 11 - 13 , 35) . Although not all of these factors could be analyzed in detail due to the retrospective nature of the study, our study reaffirmed the clinical relevance of a comprehensive lipid-lowering strategy considering high-intensity Statins, maintenance of intensive lipid-Lowering, Early therapeutic intervention, Residual risks, and Pleiotropic effects (SLERP components) in the secondary prevention of ACS. Further studies are required to investigate synergetic benefits and the associated mechanisms of comprehensively achieving SLERP components.
Limitations
Several limitations should be noted. First, as this was a single-center, relatively large-scale analysis with homogeneous data, our results will need external validation with a larger sample size and heterogeneous population. The patients included in the analysis were those who visited our hospital over a long period and underwent frequent testing. This may have introduced a selection bias. Second, the prescription data collected in this study were based on electronical medical records of our hospital, so we may not have accurately extracted information on medications prescribed by their family doctors. Third, due to the nature of our tertiary medical institution, some patients had been referred to their family doctors in the middle of the study period. This may have affected clinical inertia with lipid-lowering therapy (i.e. possibility of not being aggressive enough to consider intensifying statin doses and combination therapy with ezetimibe and PCSK9 inhibitors when insufficient). Indeed, the use of PCSK9 inhibitors was very low in this study cohort. Fourth, the present study was not designed to assess residual confounders, such as changes in blood pressure, preferences (e.g. smoking), and dietary habits. Fifth, due to the limited sample size, we could not perform detailed analyses based on the causes and mechanisms of CVE (such as de novo lesions, restenosis, and stent thrombosis, etc.), risk scores and baseline LDL-C values, which warrants future studies. Finally, the combined impact of pre-treatment risk scores and post-treatment strategy of early, intensive, and persistent LDL-lowering on the reduced CVE risks, as well as their mechanisms, fell outside the scope of the present study, necessitating further investigation.
Conclusions
Using real-world data obtained over a decade in a specialized treatment center for CAD, the present study observed that achieving early and intensive LDL-C reduction within two months after the onset of ACS and maintaining it for the next six months suppressed subsequent CVE risk, suggesting the importance of early, intensive, and persistent LDL-C-lowering therapy in the secondary prevention of ACS. Further investigations are warranted to evaluate the clinical benefits of treatment strategies designed by incorporating our results.
Acknowledgements
None.
Funding
None.
Conflicts of Interest
All authors have nothing to disclose.
References
- 1).Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, V Elkind MS, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang NY, Yaffe K, Martin SS. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation, 2022; 145: e153-e639 [DOI] [PubMed] [Google Scholar]
- 2).Daida H, Miyauchi K, Ogawa H, Yokoi H, Matsumoto M, Kitakaze M, Kimura T, Matsubara T, Ikari Y, Kimura K, Tsukahara K, Origasa H, Morino Y, Tsutsui H, Kobayashi M, Isshiki T, on behalf of the PACIFIC investigators. Management and two-year long-term clinical outcome of acute coronary syndrome in Japan: prevention of atherothrombotic incidents following ischemic coronary attack (PACIFIC) registry. Circ J, 2013; 77: 934-943 [DOI] [PubMed] [Google Scholar]
- 3).Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive vs. moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med, 2004; 350: 1495-1504 [DOI] [PubMed] [Google Scholar]
- 4).Ference BA, Graham I, Tokgozoglu L, Catapano AL. Impact of Lipids on Cardiovascular Health: JACC Health Promotion Series. J Am Coll Cardiol, 2018; 72: 1141-1156 [DOI] [PubMed] [Google Scholar]
- 5).Niccoli G, Montone RA, Di Vito L, Gramegna M, Refaat H, Scalone G, Leone AM, Trani C, Burzotta F, Porto I, Aurigemma C, Prati F, Crea F. Plaque rupture and intact fibrous cap assessed by optical coherence tomography portend different outcomes in patients with acute coronary syndrome. Eur Heart J, 2015; 36: 1377-1384 [DOI] [PubMed] [Google Scholar]
- 6).Okada K, Hibi K, Kikuchi S, Kirigaya H, Hanajima Y, Sato R, Nakahashi H, Minamimoto Y, Matsuzawa Y, Maejima N, Iwahashi N, Kosuge M, Ebina T, Tamura K, Kimura K. Culprit Lesion Morphology of Rapidly Progressive and Extensive Anterior-Wall ST-Segment Elevation Myocardial Infarction. Circ Cardiovasc Imaging, 2022; 15: e014497 [DOI] [PubMed] [Google Scholar]
- 7).Ahmadi A, Argulian E, Leipsic J, Newby DE, Narula J. From Subclinical Atherosclerosis to Plaque Progression and Acute Coronary Events: JACC State-of-the-Art Review. J Am Coll Cardiol, 2019; 74: 1608-1617 [DOI] [PubMed] [Google Scholar]
- 8).Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, Davignon J, Erbel R, Fruchart JC, Tardif JC, Schoenhagen P, Crowe T, Cain V, Wolski K, Goormastic M, Tuzcu EM, ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA, 2006; 295: 1556-1565 [DOI] [PubMed] [Google Scholar]
- 9).Silverman MG, Ference BA, Im K, Wiviott SD, Giugliano RP, Grundy SM, Braunwald E, Sabatine MS. Association Between Lowering LDL-C and Cardiovascular Risk Reduction Among Different Therapeutic Interventions: A Systematic Review and Meta-analysis. JAMA, 2016; 316: 1289-1297 [DOI] [PubMed] [Google Scholar]
- 10).White HD. Value of expert opinion in recommending early intensive lipid lowering in patients with ACS. Eur Heart J Acute Cardiovasc Care, 2022; 11: 936-938 [DOI] [PubMed] [Google Scholar]
- 11).Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, Ferranti Sd, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation, 2019; 139: e1082-e1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Kimura K, Kimura T, Ishihara M, Nakagawa Y, Nakao K, Miyauchi K, Sakamoto T, Tsujita K, Hagiwara N, Miyazaki S, Ako J, Arai H, Ishii H, Origuchi H, Shimizu W, Takemura H, Tahara Y, Morino Y, Iino K, Itoh T, Iwanaga Y, Uchida K, Endo H, Kongoji K, Sakamoto K, Shiomi H, Shimohama T, Suzuki A, Takahashi J, Takeuchi I, Tanaka A, Tamura T, Nakashima T, Noguchi T, Fukamachi D, Mizuno T, Yamaguchi J, Yodogawa K, Kosuge M, Kohsaka S, Yoshino H, Yasuda S, Shimokawa H, Hirayama A, Akasaka T, Haze K, Ogawa H, Tsutsui H, Yamazaki T, on behalf of the Japanese Circulation Society Joint Working Group. JCS 2018 Guideline on Diagnosis and Treatment of Acute Coronary Syndrome. Circ J, 2019; 83: 1085-1196 [DOI] [PubMed] [Google Scholar]
- 13).Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J, 2020; 41: 111-188 [DOI] [PubMed] [Google Scholar]
- 14).Haze T, Hirawa N, Yano Y, Tamura K, Kurihara I, Kobayashi H, Tsuiki M, Ichijo T, Wada N, Katabami T, Yamamoto K, Oki K, Inagaki N, Okamura S, Kai T, Izawa S, Yamada M, Chiba Y, Tanabe A, Naruse M. Association of aldosterone and blood pressure with the risk for cardiovascular events after treatments in primary aldosteronism. Atherosclerosis, 2021; 324: 84-90 [DOI] [PubMed] [Google Scholar]
- 15).Lopes MB, Karaboyas A, Bieber B, Pisoni RL, Walpen S, Fukagawa M, Christensson A, Evenepoel P, Pegoraro M, Robinson BM, Pecoits-Filho R. Impact of longer term phosphorus control on cardiovascular mortality in hemodialysis patients using an area under the curve approach: results from the DOPPS. Nephrol Dial Transplant, 2020; 35: 1794-1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Zhang Z, Reinikainen J, Adeleke KA, Pieterse ME, Groothuis-Oudshoorn CGM. Time-varying covariates and coefficients in Cox regression models. Ann Transl Med, 2018; 6: 121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Bender A, Groll A, Scheipl F. A generalized additive model approach to time-to-event analysis. Statistical Modelling, 2018; 18: 299-321 [Google Scholar]
- 18).Scheike TH, Zhang MJ. Flexible competing risks regression modeling and goodness-of-fit. Lifetime Data Anal, 2008; 14: 464-483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Gerds TA, Scheike TH, Andersen PK. Absolute risk regression for competing risks: interpretation, link functions, and prediction. Stat Med, 2012; 31: 3921-3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Yeo I, Johnson RA. A new family of power transformations to improve normality or symmetry. Biometrika, 2000; 87: 954-959 [Google Scholar]
- 21).Nakamura M, Ako J, Arai H, Hirayama A, Nohara A, Murakami Y, Ozaki A, Harada-Shiba M. Lipid Management and 2-Year Clinical Outcomes in Japanese Patients with Acute Coronary Syndrome: EXPLORE-J. J Atheroscler Thromb, 2021; 28: 1307-1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22).Sachdeva A, Cannon CP, Deedwania PC, LaBresh KA, Smith SC, Dai D, Hernandez A, Fonarow GC. Lipid levels in patients hospitalized with coronary artery disease: an analysis of 136,905 hospitalizations in Get With The Guidelines. Am Heart J, 2009; 157: 111-117 e2 [DOI] [PubMed] [Google Scholar]
- 23).Hulten E, Jackson JL, Douglas K, George S, Villines TC. The effect of early, intensive statin therapy on acute coronary syndrome: a meta-analysis of randomized controlled trials. Arch Intern Med, 2006; 166: 1814-1821 [DOI] [PubMed] [Google Scholar]
- 24).Dohi T, Miyauchi K, Okazaki S, Yokoyama T, Yanagisawa N, Tamura H, Kojima T, Yokoyama K, Kurata T, Daida H. Early intensive statin treatment for six months improves long-term clinical outcomes in patients with acute coronary syndrome (Extended-ESTABLISH trial): a follow-up study. Atherosclerosis, 2010; 210: 497-502 [DOI] [PubMed] [Google Scholar]
- 25).Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White Harvey D, Zeiher AM. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med, 2018; 379: 2097-2107 [DOI] [PubMed] [Google Scholar]
- 26).Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, Ferrari GMD, Ruzyllo W, Lucca PD, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM. Ezetimibe Added to Statin Therapy after Acute Coronary Syndromes. N Engl J Med, 2015; 372: 2387-97 [DOI] [PubMed] [Google Scholar]
- 27).Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, Waters D, Zeiher A, Chaitman BR, Leslie S, Stern T, for the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering Study Investigators. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA, 2001; 285: 1711-1718 [DOI] [PubMed] [Google Scholar]
- 28).Nicholls SJ, Kataoka Y, Nissen SE, Prati F, Windecker S, Puri R, Hucko T, Aradi D, Herrman JPR, Hermanides RS, Wang B, Wang H, Butters J, Di Giovanni G, Jones S, Pompili G, Psaltis PJ. Effect of Evolocumab on Coronary Plaque Phenotype and Burden in Statin-Treated Patients Following Myocardial Infarction. JACC Cardiovasc Imaging, 2022; 15: 1308-1321 [DOI] [PubMed] [Google Scholar]
- 29).Tsujita K, Sugiyama S, Sumida H, Shimomura H, Yamashita T, Yamanaga K, Komura N, Sakamoto K, Oka H, Nakao K, Nakamura S, Ishihara M, Matsui K, Sakaino N, Nakamura N, Yamamoto N, Koide S, Matsumura T, Fujimoto K, Tsunoda R, Morikami Y, Matsuyama K, Oshima S, Kaikita K, Hokimoto S, Ogawa H. Impact of Dual Lipid-Lowering Strategy With Ezetimibe and Atorvastatin on Coronary Plaque Regression in Patients With Percutaneous Coronary Intervention: The Multicenter Randomized Controlled PRECISE-IVUS Trial. J Am Coll Cardiol, 2015; 66: 495-507 [DOI] [PubMed] [Google Scholar]
- 30).Kini AS, Baber U, Kovacic JC , Limaye A, Ali ZA, Sweeny J, Maehara A, Mehran R, Dangas G, Mintz GS, Fuster V, Narula J, Sharma SK, Moreno PR. Changes in plaque lipid content after short-term intensive vs. standard statin therapy: the YELLOW trial (reduction in yellow plaque by aggressive lipid-lowering therapy). J Am Coll Cardiol, 2013; 62: 21-29 [DOI] [PubMed] [Google Scholar]
- 31).Nishiguchi T, Kubo T, Tanimoto , Ino Y, Matsuo Y, Yamano T, Terada K, Emori H, Katayama Y, Taruya A, Ozaki Y, Shiono Y, Shimamura K, Kameyama T, Kitabata H, Yamaguchi T, Tanaka A, Hozumi T, Akasaka T. Effect of Early Pitavastatin Therapy on Coronary Fibrous-Cap Thickness Assessed by Optical Coherence Tomography in Patients With Acute Coronary Syndrome: The ESCORT Study. JACC Cardiovasc Imaging, 2018; 11: 829-838 [DOI] [PubMed] [Google Scholar]
- 32).Okazaki S, Yokoyama T, Miyauchi K, Shimada K, Kurata T, Sato H, Daida H. Early statin treatment in patients with acute coronary syndrome: demonstration of the beneficial effect on atherosclerotic lesions by serial volumetric intravascular ultrasound analysis during half a year after coronary event: the ESTABLISH Study. Circulation, 2004; 110: 1061-1068 [DOI] [PubMed] [Google Scholar]
- 33).Omori H, Ota H, Mizukami T , Kawase Y, Tanigaki T, Hirata T, Okubo M, Kawasaki M, Matsuo H. How Do Coronary Lipid-Rich Plaques Change After Cessation of Proprotein Convertase Subtilisin/Kexin Type 9 Inhibitors? - Serial Assessment Using Near-Infrared Spectroscopy. Circ J, 2021; 85: 1404 [DOI] [PubMed] [Google Scholar]
- 34).Ray KK, Troquay RPT, Visseren FLJ , Leiter LA, Scott Wright R, Vikarunnessa S, Talloczy Z, Zang X, Maheux P, Lesogor A, Landmesser U. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol, 2023; 11: 109-119 [DOI] [PubMed] [Google Scholar]
- 35).Jansen H, Samani NJ, Schunkert H. Mendelian randomization studies in coronary artery disease. Eur Heart J, 2014; 35: 1917-1924 [DOI] [PubMed] [Google Scholar]