Abstract
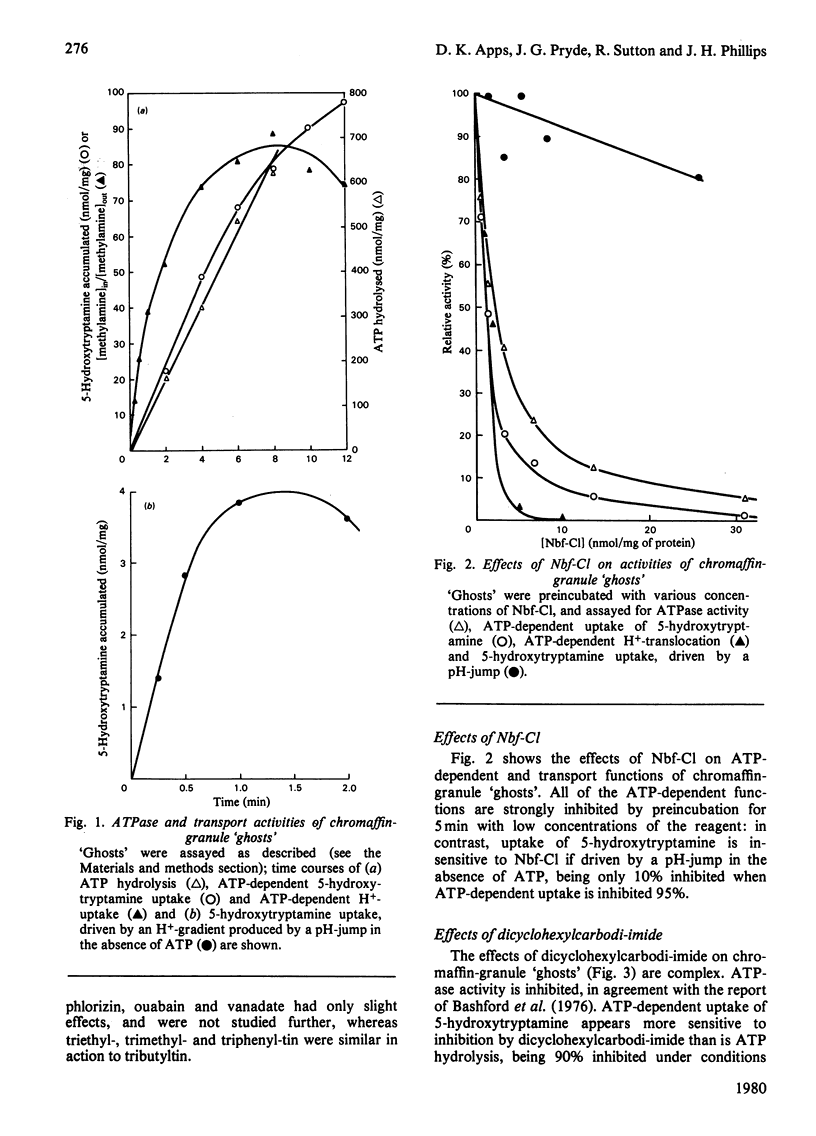
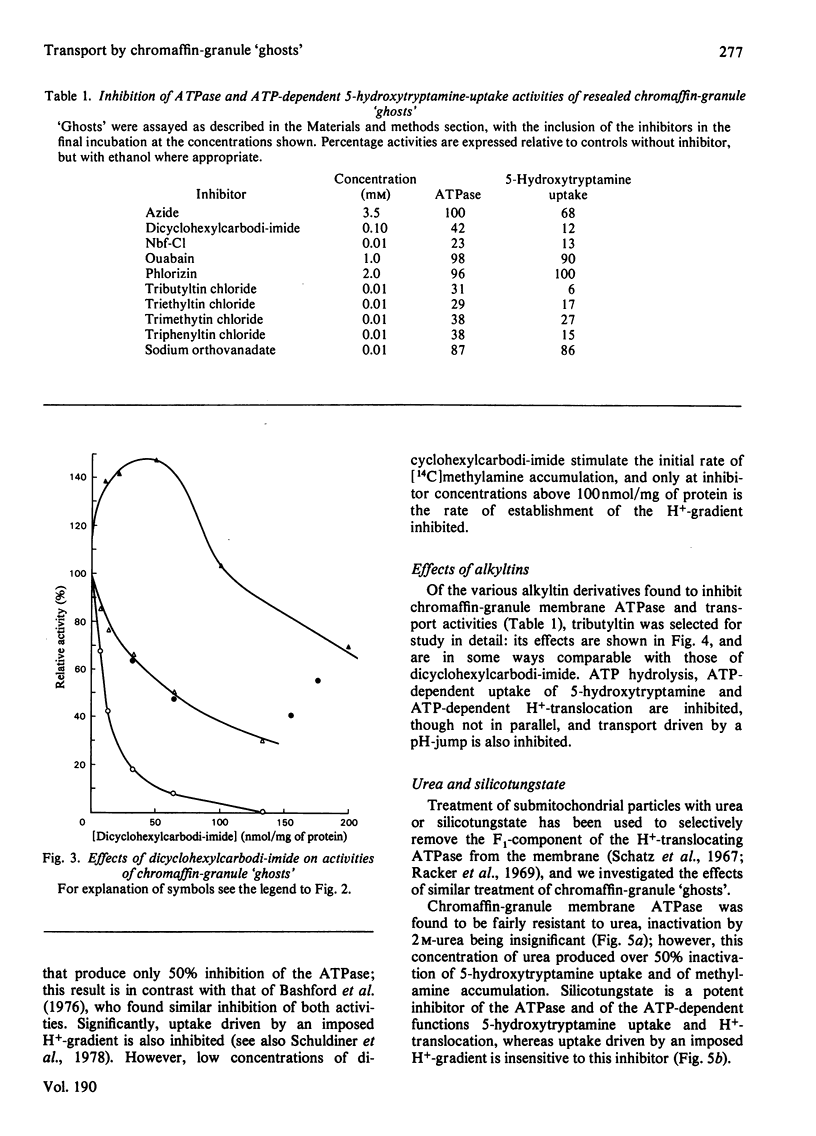
1. Highly purified resealed chromaffin-granule `ghosts' were assayed for ATPase and ATP-driven H+-translocation and 5-hydroxytryptamine-uptake activities, and for 5-hydroxytryptamine uptake driven by an imposed transmembrane H+-gradient. The effects of several inhibitors on these activities were studied. 2. Dicyclohexylcarbodi-imide inhibits all of these activities, but not in parallel; at low concentrations it decreases the permeability of the membrane to protons. 3. 4-Chloro-7-nitrobenzofuran (Nbf-Cl) and silicotungstate inhibit ATP-dependent activities, without effect on 5-hydroxytryptamine uptake driven by an imposed H+-gradient. 4. Tributyltin chloride inhibits all of the activities. 5. Treatment of the `ghosts' with low concentrations of urea inhibits 5-hydroxytryptamine uptake and ATP-dependent generation of a transmembrane H+-gradient, without inhibiting ATPase activity. 6. Nbf-Cl and silicotungstate are without effect on the rate of leakage of 5-hydroxytryptamine from preloaded `ghosts', whereas dicyclohexylcarbodi-imide and tributyltin chloride accelerate the rate of leakage. 7. Treatment of the membranes with 14C-labelled Nbf-Cl labels several proteins; membranes treated with dicyclohexyl[14C]carbodi-imide are labelled predominantly in a protein of low molecular weight, which may be analogous to the mitochondrial H+-conducting proteolipid. 8. It is concluded that Nbf-Cl and silicotungstate inhibit the H+-translocating ATPase of the granule membrane; that dicyclohexylcarbodi-imide inhibits the ATPase, and inhibits 5-hydroxytryptamine accumulation by accelerating leakage of the amine; and that the effects of tributyltin chloride are due to inhibition of the ATPase, and collapse of the transmembrane H+-gradient through OH−-anion exchange.
Full text
PDF


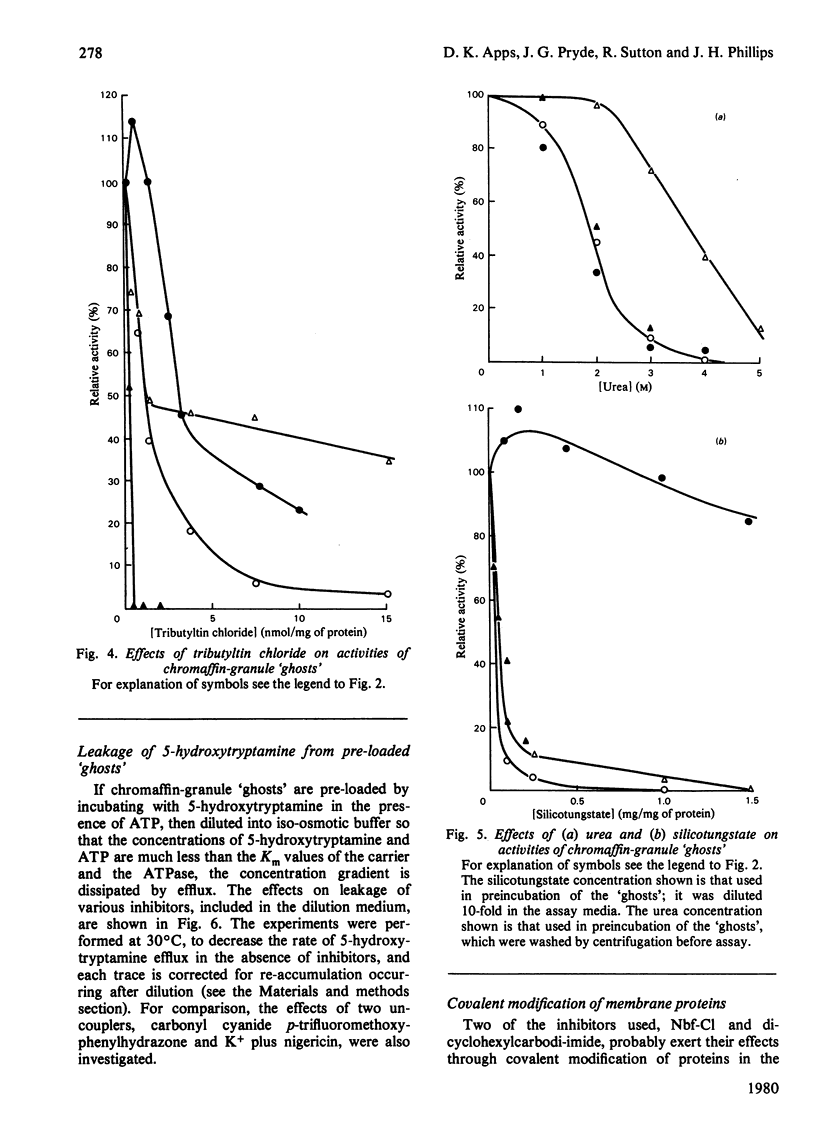



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aberer W., Kostron H., Huber E., Winkler H. A characterization of the nucleotide uptake of chromaffin granules of bovine adrenal medulla. Biochem J. 1978 Jun 15;172(3):353–360. doi: 10.1042/bj1720353b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps D. K., Glover L. A. Isolation and characterization of magnesium adenosinetriphosphatase from the chromaffin granule membrane. FEBS Lett. 1978 Jan 15;85(2):254–258. doi: 10.1016/0014-5793(78)80467-0. [DOI] [PubMed] [Google Scholar]
- Apps D. K., Pryde J. G., Phillips J. H. Both the transmembrane pH gradient and the membrane potential are important in the accumulation of amines by resealed chromaffin-granule 'ghosts'. FEBS Lett. 1980 Mar 10;111(2):386–390. doi: 10.1016/0014-5793(80)80833-7. [DOI] [PubMed] [Google Scholar]
- Apps D. K., Reid G. A. Adenosine triphosphatase and adenosine diphosphate/adenosine triphosphate isotope-exchange activities of the chromaffin-granule membrane. Biochem J. 1977 Oct 1;167(1):297–300. doi: 10.1042/bj1670297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apps D. K., Schatz G. An adenosine triphosphatase isolated from chromaffin-granulate membranes is closely similar to F1-adenosine triphosphatase of mitochondria. Eur J Biochem. 1979 Oct 15;100(2):411–419. doi: 10.1111/j.1432-1033.1979.tb04184.x. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Casey R. P., Radda G. K., Ritchie G. A. Energy-coupling in adrenal chromaffin granules. Neuroscience. 1976;1(5):399–412. doi: 10.1016/0306-4522(76)90133-0. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Casey R. P., Radda G. K., Ritchie G. A. The effect of uncouplers on catecholamine incorporation by vesicles of chromaffin granules. Biochem J. 1975 Apr;148(1):153–155. doi: 10.1042/bj1480153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman B. J., Mainzer S. E., Allen K. E., Slayman C. W. Effects of inhibitors on the plasma membrane and mitochondrial adenosine triphosphatases of Neurospora crassa. Biochim Biophys Acta. 1978 Sep 11;512(1):13–28. doi: 10.1016/0005-2736(78)90214-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cain K., Partis M. D., Griffiths D. E. Dibutylchloromethyltin chloride, a covalent inhibitor of the adenosine triphosphate synthase complex. Biochem J. 1977 Sep 15;166(3):593–602. doi: 10.1042/bj1660593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell K. J., Lindop C. R., Knight I. G., Beechey R. B. The identification of the site of action of NN'-dicyclohexylcarbodi-imide as a proteolipid in mitochondrial membranes. Biochem J. 1971 Nov;125(1):169–177. doi: 10.1042/bj1250169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhez J., Dufour J. P., Thines D., Goffeau A. Comparison of the properties of plasma membrane-bound and mitochondria-bound ATPases in the yeast Schizosaccharmoyces pombe. Eur J Biochem. 1977 Sep 15;79(1):319–328. doi: 10.1111/j.1432-1033.1977.tb11812.x. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H. Purification of the carbodiimide-reactive protein component of the ATP energy-transducing system of Escherichia coli. J Biol Chem. 1976 Nov 10;251(21):6630–6637. [PubMed] [Google Scholar]
- Johnson R. G., Scarpa A. Protonmotive force and catecholamine transport in isolated chromaffin granules. J Biol Chem. 1979 May 25;254(10):3750–3760. [PubMed] [Google Scholar]
- Kanner B. I., Fishkes H., Maron R., Sharon I., Schuldiner S. Reserpine as a competitive and reversible inhibitor of the catecholamine transporter of bovine chromaffin granules. FEBS Lett. 1979 Apr 1;100(1):175–178. doi: 10.1016/0014-5793(79)81158-8. [DOI] [PubMed] [Google Scholar]
- Maron R., Fishkes H., Kanner B. I., Schuldiner S. Solubilization and reconstitution of the catecholamine transporter from bovine chromaffin granules. Biochemistry. 1979 Oct 30;18(22):4781–4785. doi: 10.1021/bi00589a003. [DOI] [PubMed] [Google Scholar]
- Mason T. L., Poyton R. O., Wharton D. C., Schatz G. Cytochrome c oxidase from bakers' yeast. I. Isolation and properties. J Biol Chem. 1973 Feb 25;248(4):1346–1354. [PubMed] [Google Scholar]
- Muszbek L., Szabó T., Fésüs L. A high sensitive method for the measurement of ATPase activity. Anal Biochem. 1977 Jan;77(1):286–288. doi: 10.1016/0003-2697(77)90315-3. [DOI] [PubMed] [Google Scholar]
- Nichols J. W., Deamer D. W. Catecholamine uptake and concentration by liposomes maintaining p/ gradients. Biochim Biophys Acta. 1976 Nov 11;455(1):269–271. doi: 10.1016/0005-2736(76)90169-3. [DOI] [PubMed] [Google Scholar]
- Njus D., Radda G. K. A potassium ion diffusion potential causes adrenaline uptake in chromaffin-granule 'ghosts'. Biochem J. 1979 Jun 15;180(3):579–585. doi: 10.1042/bj1800579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njus D., Radda G. K. Bioenergetic processes in chromaffin granules a new perspective on some old problems. Biochim Biophys Acta. 1978 Mar 10;463(3-4):219–244. doi: 10.1016/0304-4173(78)90001-0. [DOI] [PubMed] [Google Scholar]
- Phillips J. H., Allison V. P. Proton translocation of the bovine chromaffin-granule membrane. Biochem J. 1978 Mar 15;170(3):661–672. doi: 10.1042/bj1700661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H. Hydroxytryptamine transport by the bovine chromaffin-granule membrane. Biochem J. 1978 Mar 15;170(3):673–679. doi: 10.1042/bj1700673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. H. Transport of catecholamines by resealed chromaffin-grnaule "ghosts". Biochem J. 1974 Nov;144(2):311–318. doi: 10.1042/bj1440311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racker E., Horstman L. L., Kling D., Fessenden-Raden J. M. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XXI. Resolution of submitochondrial particles from bovine heart mitochondria with silicotungstate. J Biol Chem. 1969 Dec 25;244(24):6668–6674. [PubMed] [Google Scholar]
- Sachs G., Chang H. H., Rabon E., Schackman R., Lewin M., Saccomani G. A nonelectrogenic H+ pump in plasma membranes of hog stomach. J Biol Chem. 1976 Dec 10;251(23):7690–7698. [PubMed] [Google Scholar]
- Schatz G., Penefsky H. S., Racker E. Partial resolution of the enzymes catalyzing oxidative phosphorylation. XIV. J Biol Chem. 1967 May 25;242(10):2552–2560. [PubMed] [Google Scholar]
- Selwyn M. J., Dawson A. P., Stockdale M., Gains N. Chloride-hydroxide exchange across mitochondrial, erythrocyte and artificial lipid membranes mediated by trialkyl- and triphenyltin compounds. Eur J Biochem. 1970 May 1;14(1):120–126. doi: 10.1111/j.1432-1033.1970.tb00268.x. [DOI] [PubMed] [Google Scholar]
- Sigrist-Nelson K., Sigrist H., Azzi A. Characterization of the dicyclohexylcarbodiimide-binding protein isolated from chloroplast membranes. Eur J Biochem. 1978 Dec 1;92(1):9–14. doi: 10.1111/j.1432-1033.1978.tb12717.x. [DOI] [PubMed] [Google Scholar]
- Toll L., Howard B. D. Role of Mg2+-ATPase and a pH gradient in the storage of catecholamines in synaptic vesicles. Biochemistry. 1978 Jun 27;17(13):2517–2523. doi: 10.1021/bi00606a010. [DOI] [PubMed] [Google Scholar]
- Winkler H. The composition of adrenal chromaffin granules: an assessment of controversial results. Neuroscience. 1976;1(2):65–80. doi: 10.1016/0306-4522(76)90001-4. [DOI] [PubMed] [Google Scholar]