Abstract
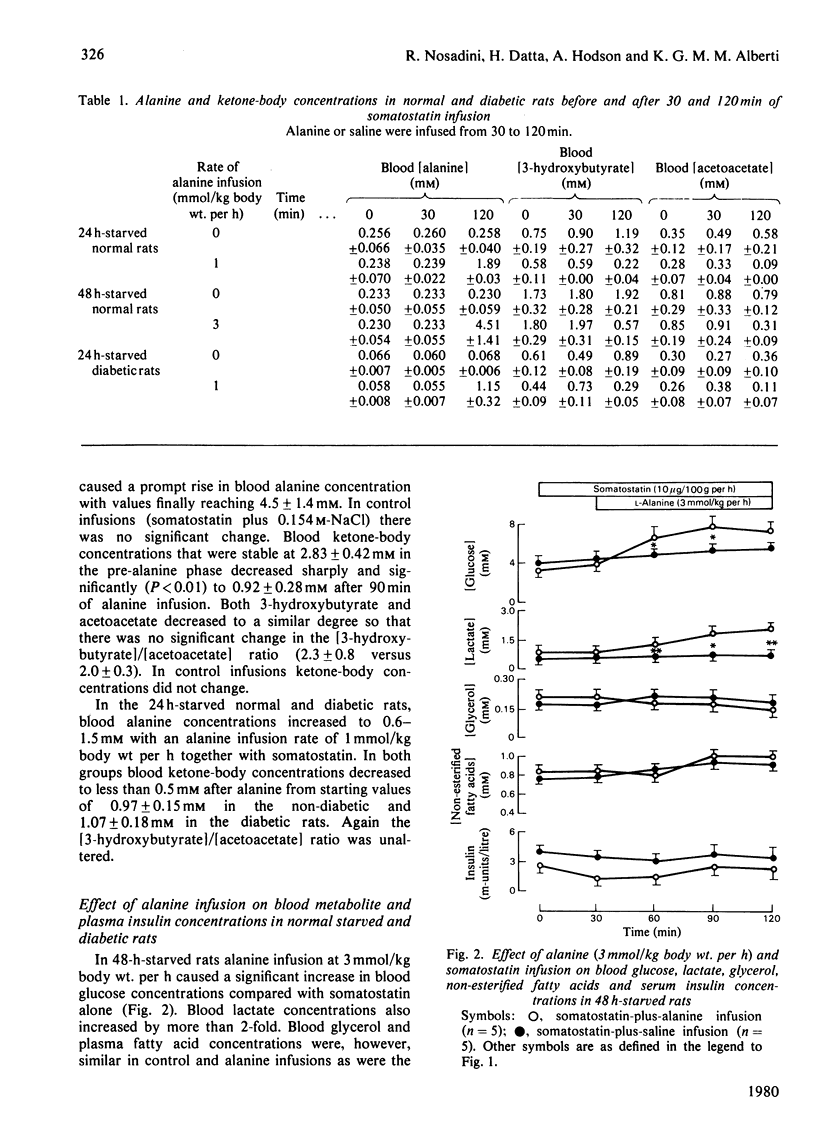
1. The anti-ketogenic effect of alanine has been studied in normal starved and diabetic rats by infusing l-alanine for 90min in the presence of somatostatin (10μg/kg body wt. per h) to suppress endogenous insulin and glucagon secretion. 2. Infusion of alanine at 3mmol/kg body wt. per h caused a 70±11% decrease in [3-hydroxybutyrate] and a 58±9% decrease in [acetoacetate] in 48h-starved rats. [Glucose] and [lactate] increased, but [non-esterified fatty acid], [glycerol] and [3-hydroxybutyrate]/[acetoacetate] were unchanged. 3. Infusion of alanine at 1mmol/kg body wt. per h caused similar decreases in [ketone body] (3-hydroxybutyrate plus acetoacetate) in 24h-starved normal and diabetic rats, but no change in other blood metabolites. 4. Alanine [3mmol/kg body wt. per h] caused a 72±9% decrease in the rate of production of ketone bodies and a 57±8% decrease in disappearance rate as assessed by [3-14C]acetoacetate infusion. Metabolic clearance was unchanged, indicating that the primary effect of alanine was inhibition of hepatic ketogenesis. 5. Aspartate infusion at 6mmol/kg body wt. per h had similar effects on blood ketone-body concentrations in 48h-starved rats. 6. Alanine (3mmol/kg body wt. per h) caused marked increases in hepatic glutamate, aspartate, malate, lactate and citrate, phosphoenolpyruvate, 2-phosphoglycerate and glucose concentrations and highly significant decreases in [3-hydroxybutyrate] and [acetoacetate]. Calculated [oxaloacetate] was increased 75%. 7. Similar changes in hepatic [malate], [aspartate] and [ketone bodies] were found after infusion of 6mmol of aspartate/kg body wt. per h. 8. It is suggested that the anti-ketogenic effect of alanine is secondary to an increase in hepatic oxaloacetate and hence citrate formation with decreased availability of acetyl-CoA for ketogenesis. The reciprocal negative-feedback cycle of alanine and ketone bodies forms an important non-hormonal regulatory system.
Full text
PDF

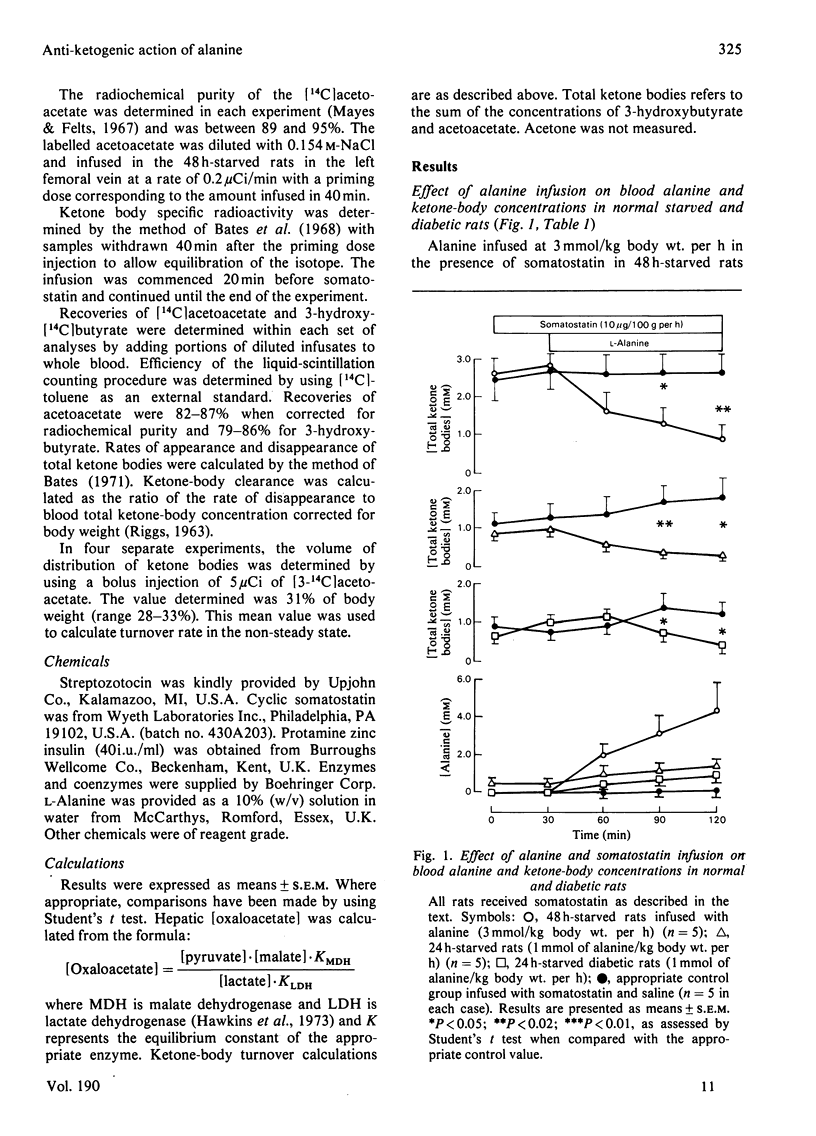
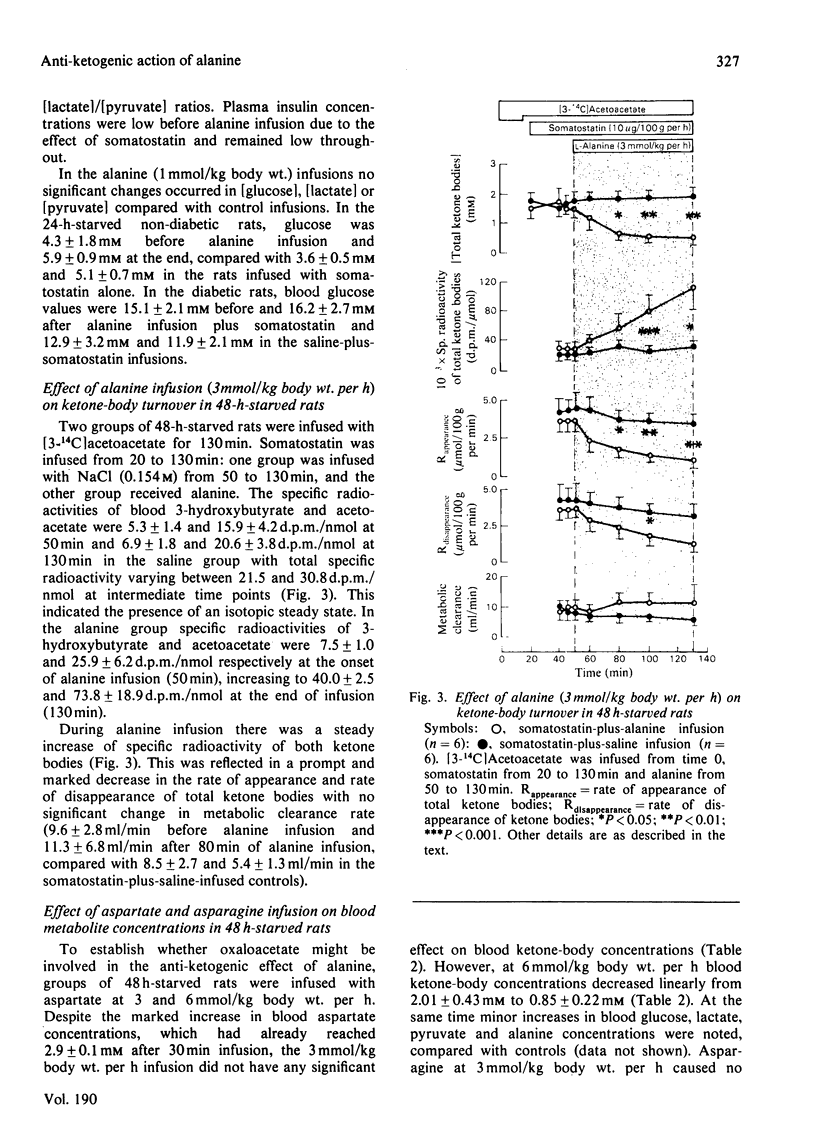



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
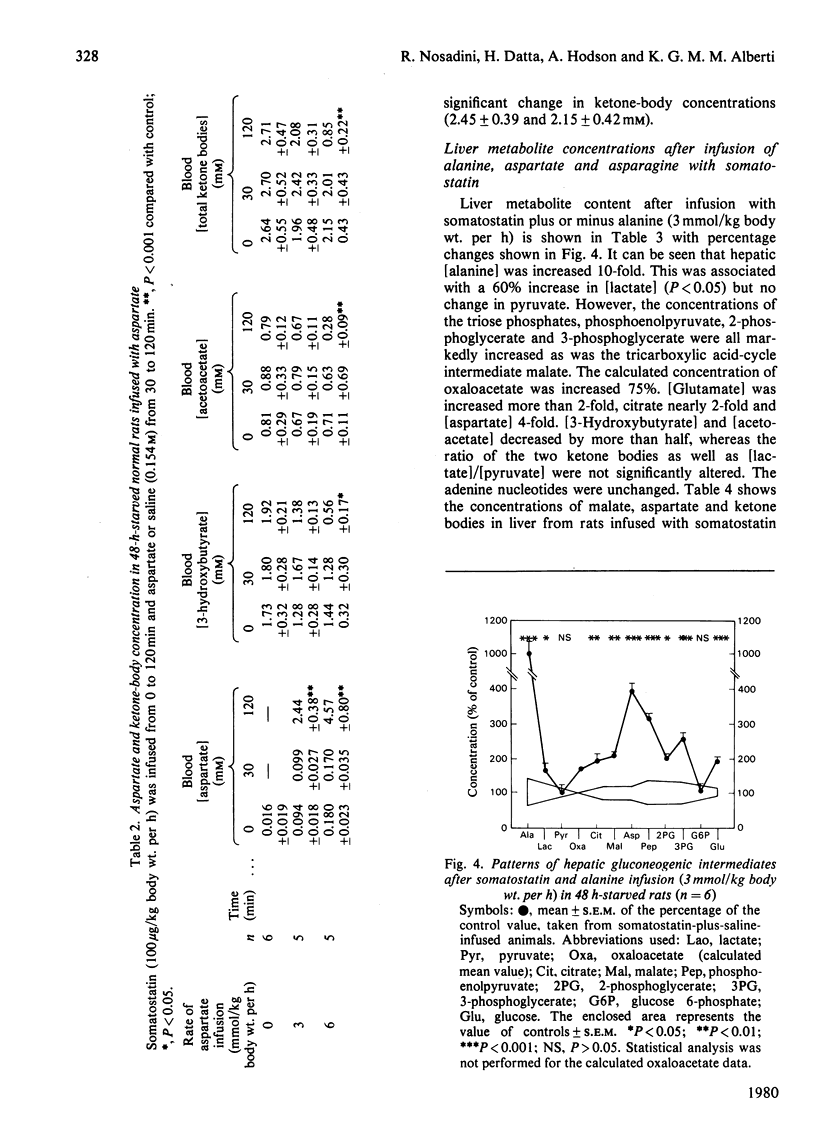
- Alberti K. G., Christensen N. J., Christensen S. E., Hansen A. P., Iversen J., Lundbaek K., Seyer-Hansen K., Orskov H. Inhibition of insulin secretion by somatostatin. Lancet. 1973 Dec 8;2(7841):1299–1301. doi: 10.1016/s0140-6736(73)92873-0. [DOI] [PubMed] [Google Scholar]
- Assan R. La somatostatine : une nouvelle hormone. Diabete Metab. 1976 Sep;2(3):135–146. [PubMed] [Google Scholar]
- Bates M. W., Krebs H. A., Williamson D. H. Turnover rates of ketone bodies in normal, starved and alloxan-diabetic rats. Biochem J. 1968 Dec;110(4):655–661. doi: 10.1042/bj1100655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Alberti K. G. Experimental diabetic ketoacidosis. Sequential changes of metabolic intermediates in blood, liver, cerebrospinal fluid and brain after acute insulin deprivation in the streptozotocin-diabetic rat. Biochem J. 1974 Jan;138(1):107–117. doi: 10.1042/bj1380107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackshear P. J., Holloway P. A., Aberti K. G. The effects of inhibition of gluconeogenesis on ketogenesis in starved and diabetic rats. Biochem J. 1975 Jun;148(3):353–362. doi: 10.1042/bj1480353b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietze G., Wicklmayr M., Mehnert H. Antiketogenic action of fructose in man. Diabetes. 1978 Jul;27(7):709–714. doi: 10.2337/diab.27.7.709. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Corbin J. G., Park C. R. Control of gluconeogenesis in liver. IV. Differential effects of fatty acids and glucagon on ketogenesis and gluconeogenesis in the perfused rat liver. J Biol Chem. 1969 Aug 10;244(15):4095–4102. [PubMed] [Google Scholar]
- Felig P., Kim Y. J., Lynch V., Hendler R. Amino acid metabolism during starvation in human pregnancy. J Clin Invest. 1972 May;51(5):1195–1202. doi: 10.1172/JCI106913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felig P., Marliss E., Ohman J. L., Cahill C. F., Jr Plasma amino acid levels in diabetic ketoacidosis. Diabetes. 1970 Oct;19(10):727–728. doi: 10.2337/diab.19.10.727. [DOI] [PubMed] [Google Scholar]
- Felig P., Owen O. E., Wahren J., Cahill G. F., Jr Amino acid metabolism during prolonged starvation. J Clin Invest. 1969 Mar;48(3):584–594. doi: 10.1172/JCI106017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrichs D., Schoner W. Regulation of gluconeogenesis by alanine. Biochim Biophys Acta. 1974 Apr 22;343(2):341–355. doi: 10.1016/0304-4165(74)90098-1. [DOI] [PubMed] [Google Scholar]
- Genuth S. M., Castro J. Effect of oral alanine on blood beta-hydroxybutyrate and plasma glucose, insulin, free fatty acids, and growth hormone in normal and diabetic subjects. Metabolism. 1974 Apr;23(4):375–386. doi: 10.1016/0026-0495(74)90056-0. [DOI] [PubMed] [Google Scholar]
- Genuth S. M. Effects of oral alanine administration in fasting obese subjects. Metabolism. 1973 Jul;22(7):927–937. doi: 10.1016/0026-0495(73)90065-6. [DOI] [PubMed] [Google Scholar]
- HOHORST H. J., KREUTZ F. H., BUECHER T. [On the metabolite content and the metabolite concentration in the liver of the rat]. Biochem Z. 1959;332:18–46. [PubMed] [Google Scholar]
- Hall S. E., Braaten J. T., McKendry J. B., Bolton T., Foster D., Berman M. Normal alanine-glucose relationships and their changes in diabetic patients before and after insulin treatment. Diabetes. 1979 Aug;28(8):737–745. [PubMed] [Google Scholar]
- Hawkins R. A., Houghton C. R., Williamson D. H. Hepatic redox state and gluconeogenesis from lactate in vivo in the rat. Biochem J. 1973 Jan;132(1):19–25. doi: 10.1042/bj1320019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho R. J. Radiochemical assay of long-chain fatty acids using 63Ni as tracer. Anal Biochem. 1970 Jul;36(1):105–113. doi: 10.1016/0003-2697(70)90337-4. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. The regulation of the release of ketone bodies by the liver. Adv Enzyme Regul. 1966;4:339–354. doi: 10.1016/0065-2571(66)90027-6. [DOI] [PubMed] [Google Scholar]
- Lloyd B., Burrin J., Smythe P., Alberti K. G. Enzymic fluorometric continuous-flow assays for blood glucose, lactate, pyruvate, alanine, glycerol, and 3-hydroxybutyrate. Clin Chem. 1978 Oct;24(10):1724–1729. [PubMed] [Google Scholar]
- Lopes-Cardozo M., van den Bergh S. G. Ketogenesis in isolated rat liver mitochondria. I. Relationships with the citric acid cycle and with the mitochondrial energy state. Biochim Biophys Acta. 1972;283(1):1–15. doi: 10.1016/0005-2728(72)90092-8. [DOI] [PubMed] [Google Scholar]
- Mayes P. A., Felts J. M. Determination of 14C-labelled ketone bodies by liquid-scintillation counting. Biochem J. 1967 Jan;102(1):230–235. doi: 10.1042/bj1020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman H. A., Cooney D. A., Young D. M. Role of pancreatic L-asparagine synthetase in homeostasis of L-asparagine. Am J Physiol. 1979 Jun;236(6):E746–E753. doi: 10.1152/ajpendo.1979.236.6.E746. [DOI] [PubMed] [Google Scholar]
- Moellering H., Gruber W. Determination of citrate with citrate lyase. Anal Biochem. 1966 Dec;17(3):369–376. doi: 10.1016/0003-2697(66)90172-2. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. The effect of alanine on glucagon secretion. J Clin Invest. 1971 Oct;50(10):2215–2218. doi: 10.1172/JCI106716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozand P. T., Reed W. D., Girard J., Hawkins R. L., Collins R. M., Jr, Tildon J. T., Cornblath M. Hypoketonaemic effect of L-alamine. Specific decrease in blood concentrations of 3-hydroxybutyrate in the rat. Biochem J. 1977 Jun 15;164(3):557–564. doi: 10.1042/bj1640557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozand P. T., Reed W. D., Hawkins R. L., Stevenson J. H., Tildon J. T., Cornblath M. Effect of L-alanine infusion on gluconeogenesis and ketogenesis in the rat in vivo. Biochem J. 1978 Mar 15;170(3):583–591. doi: 10.1042/bj1700583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliara A. S., Kari I. E., De Vivo D. C., Feigin R. D., Kipnis D. M. Hypoalaninemia: a concomitant of ketotic hypoglycemia. J Clin Invest. 1972 Jun;51(6):1440–1449. doi: 10.1172/JCI106940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prager G. N., Ontko J. A. Direct effects of fructose metabolism on fatty acid oxidation in a recombined rat liver mitochondria-hish speed supernatant system. Biochim Biophys Acta. 1976 Mar 26;424(3):386–395. doi: 10.1016/0005-2760(76)90028-x. [DOI] [PubMed] [Google Scholar]
- Price C. P., Llyod B., Alberti G. M. A kinetic spectrophotometric assay for rapid determination of acetoacetate in blood. Clin Chem. 1977 Oct;23(10):1893–1897. [PubMed] [Google Scholar]
- Sherwin R. S., Hendler R. G., Felig P. Effect of diabetes mellitus and insulin on the turnover and metabolic response to ketones in man. Diabetes. 1976 Sep;25(9):776–784. doi: 10.2337/diab.25.9.776. [DOI] [PubMed] [Google Scholar]
- Sherwin R. S., Hendler R. G., Felig P. Effect of ketone infusions on amino acid and nitrogen metabolism in man. J Clin Invest. 1975 Jun;55(6):1382–1390. doi: 10.1172/JCI108057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Walker D. G. Gluconeogenesis in the newborn rat: the substrates and their quantitative significance. Enzyme. 1973;15(1):40–81. [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D. H., MELLANBY J., KREBS H. A. Enzymic determination of D(-)-beta-hydroxybutyric acid and acetoacetic acid in blood. Biochem J. 1962 Jan;82:90–96. doi: 10.1042/bj0820090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLLENBERGER A., RISTAU O., SCHOFFA G. [A simple technic for extremely rapid freezing of large pieces of tissue]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1960;270:399–412. [PubMed] [Google Scholar]
- Wieland O., Weiss L., Eger-Neufeldt I. Enzymatic regulation of liver acetyl-CoA metabolism in relation to ketogenesis. Adv Enzyme Regul. 1964;2:85–99. doi: 10.1016/s0065-2571(64)80007-8. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lopes-Vieira O., Walker B. Concentrations of free glucogenic amino acids in livers of rats subjected to various metabolic stresses. Biochem J. 1967 Aug;104(2):497–502. doi: 10.1042/bj1040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson D. H., Veloso D., Ellington E. V., Krebs H. A. Changes in the concentrations of hepatic metabolites on administration of dihydroxyacetone or glycerol to starved rats and their relationship to the control of ketogenesis. Biochem J. 1969 Sep;114(3):575–584. doi: 10.1042/bj1140575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. R., Browning E. T., Scholz R. Control mechanisms of gluconeogenesis and ketogenesis. I. Effects of oleate on gluconeogenesis in perfused rat liver. J Biol Chem. 1969 Sep 10;244(17):4607–4616. [PubMed] [Google Scholar]


