Abstract
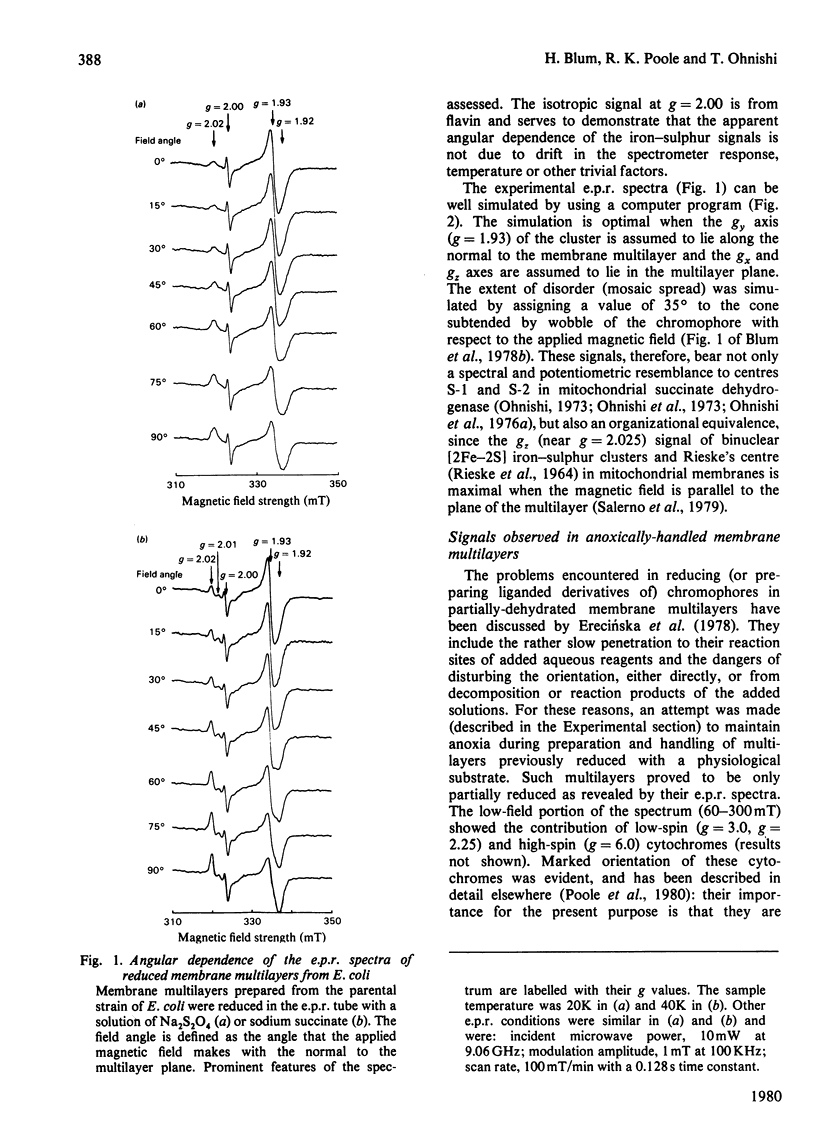
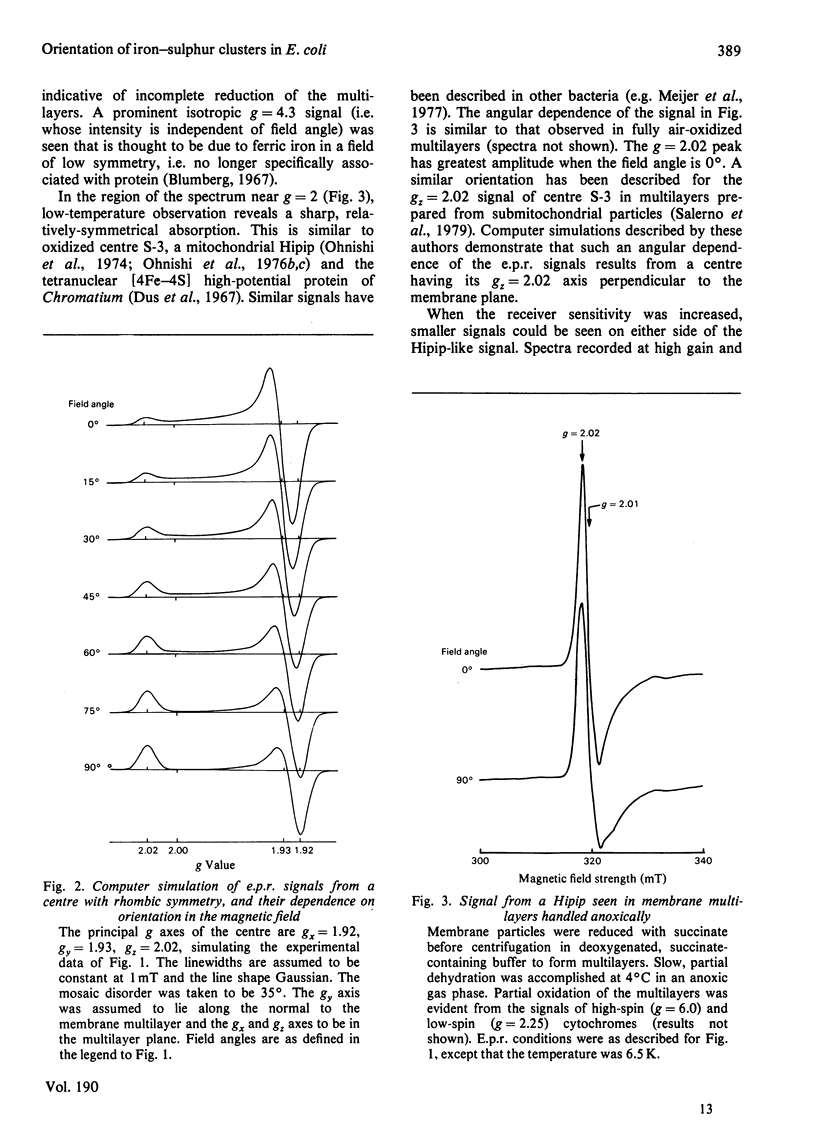
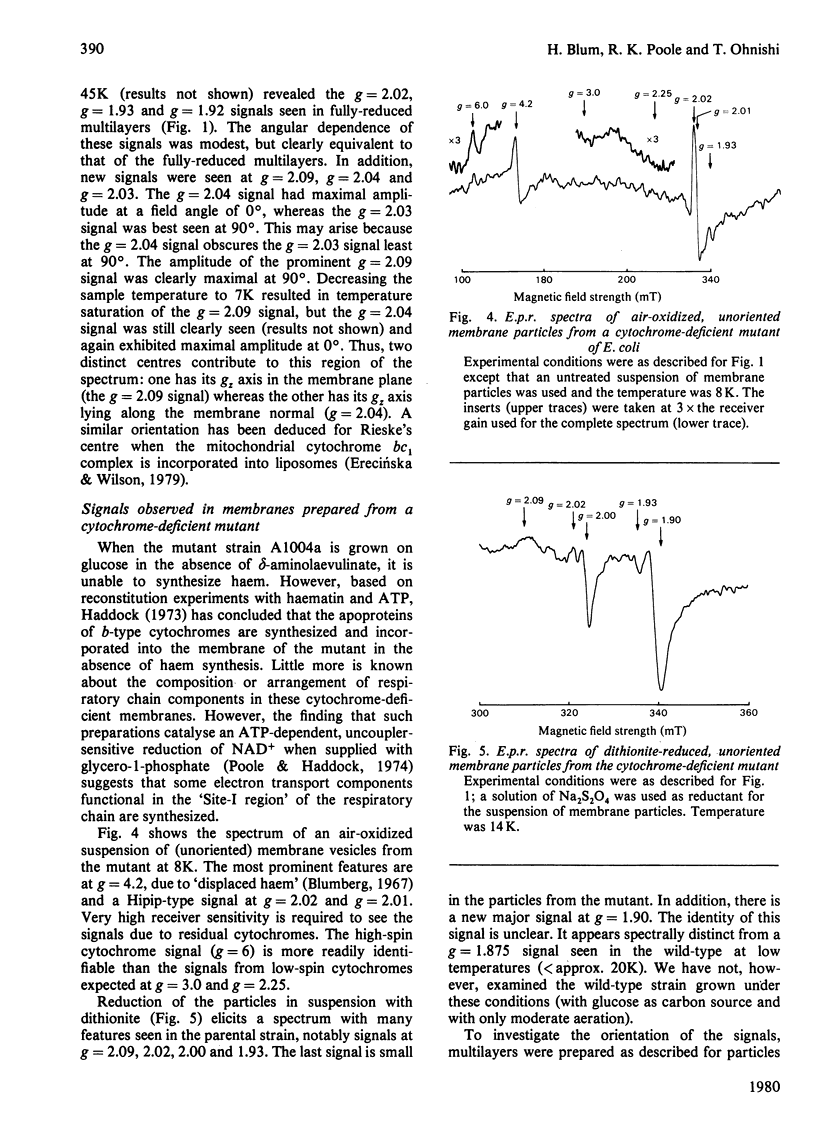
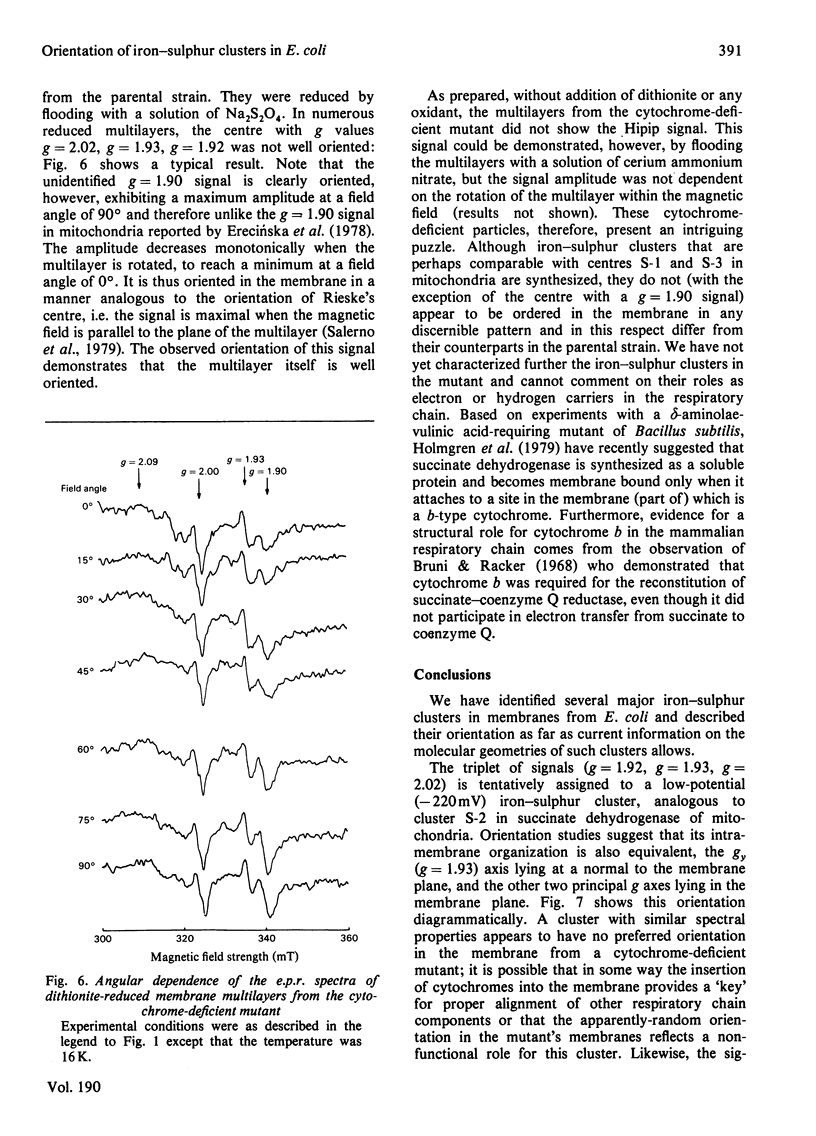
1. Membrane particles prepared from ultrasonically-disrupted, aerobically-grown Escherichia coli were centrifuged on to a plastic film that was supported perpendicular to the centrifugal field to yield oriented membrane multilayers. In such preparations, there is a high degree of orientation of the planes of the membranes such that they lie parallel to each other and to the supporting film. 2. When dithionite- or succinate-reduced multilayers are rotated in the magnetic field of an e.p.r. spectrometer, about an axis lying in the membrane plane, angular-dependent signals from an iron–sulphur cluster at gx=1.92, gy=1.93 and gz=2.02 are seen. The g=1.93 signal has maximal amplitude when the plane of the multilayer is perpendicular to the magnetic field. Conversely, the g=2.02 signal is maximal when the plane of the multilayer is parallel with the magnetic field. 3. Computer simulations of the experimental data show that the cluster lies in the cytoplasmic membrane with the gy axis perpendicular to the membrane plane and with the gx and gz axes lying in the membrane plane. 4. In partially-oxidized multilayers, a signal resembling the mitochondrial high-potential iron–sulphur protein (Hipip) is seen whose gz=2.02 axis may be deduced as lying perpendicular to the membrane plane. 5. Appropriate choice of sample temperature and receiver gain reveals two further signals in partially-reduced multilayers: a g=2.09 signal arises from a cluster with its gz axis in the membrane plane, whereas a g=2.04 signal is from a cluster with the gz axis lying along the membrane normal. 6. Membrane particles from a glucose-grown, haem-deficient mutant contain dramatically-lowered levels of cytochromes and exhibit, in addition to the iron–sulphur clusters seen in the parental strain, a major signal at g=1.90. 7. Only the latter may be demonstrated to be oriented in multilayer preparations from the mutant. 8. Comparisons are drawn between the orientations of the iron–sulphur proteins in the cytoplasmic membrane of E. coli and those in mitochondrial membranes. The effects of diminished cytochrome content on the properties of the iron–sulphur proteins are discussed.
Full text
PDF


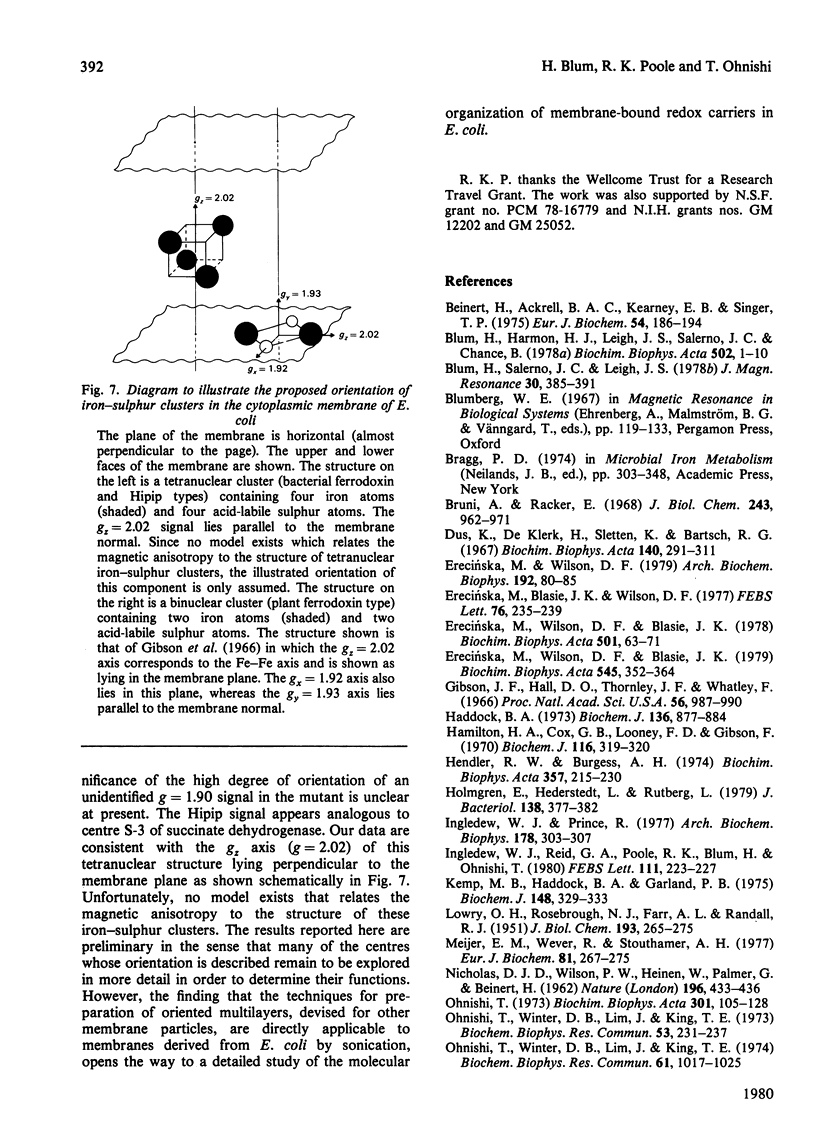

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beinert H., Ackrell B. A., Kearney E. B., Singer T. P. Iron-sulfur components of succinate dehydrogenase: stoichiometry and kinetic behavior in activated preparations. Eur J Biochem. 1975 May;54(1):185–194. doi: 10.1111/j.1432-1033.1975.tb04128.x. [DOI] [PubMed] [Google Scholar]
- Blum H., Harmon H. J., Leigh J. S., Salerno J. C., Chance B. The orientation of a heme of cytochrome c oxidase in submitochondrial particles. Biochim Biophys Acta. 1978 Apr 11;502(1):1–10. doi: 10.1016/0005-2728(78)90125-1. [DOI] [PubMed] [Google Scholar]
- Bruni A., Racker E. Resolution and reconstitution of the mitochondrial electron transport system. I. Reconstitution of the succinate-ubiquinone reductase. J Biol Chem. 1968 Mar 10;243(5):962–971. [PubMed] [Google Scholar]
- Dus K., De Klerk H., Sletten K., Bartsch R. G. Chemical characterization of high potential iron proteins from Chromatium and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1967 Jun 27;140(2):291–311. doi: 10.1016/0005-2795(67)90470-9. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Blasie J. K., Wilson D. F. Orientation of the hemes of cytochrome c oxidase and cytochrome c in mitochondria. FEBS Lett. 1977 Apr 15;76(2):235–239. doi: 10.1016/0014-5793(77)80159-2. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F., Blasie J. K. Studies of the orientation of the mitochondrial redox carriers. III. Orientation of the gx and gy axes of the hemes of cytochrome oxidase with respect to the plane of the membrane in oriented membrane multilayers. Biochim Biophys Acta. 1979 Feb 8;545(2):352–364. doi: 10.1016/0005-2728(79)90212-3. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F., Blasie J. K. Studies on the orientations of the mitochondrial redox carriers. II. Orientation of the mitochondrial chromophores with respect to the plane of the membrane in hydrated, oriented mitochondrial multilayers. Biochim Biophys Acta. 1978 Jan 11;501(1):63–71. doi: 10.1016/0005-2728(78)90095-6. [DOI] [PubMed] [Google Scholar]
- Erecińska M., Wilson D. F. Studies on the orientations of the mitochondrial redox carriers. Orientation of the chromophores of cytochrome b--c1 complex with respect to the plane of a cytochrome b--c1 complex--lipid model membrane. Arch Biochem Biophys. 1979 Jan;192(1):80–85. doi: 10.1016/0003-9861(79)90073-0. [DOI] [PubMed] [Google Scholar]
- Gibson J. F., Hall D. O., Thornley J. H., Whatley F. R. The iron complex in spinach ferredoxin. Proc Natl Acad Sci U S A. 1966 Sep;56(3):987–990. doi: 10.1073/pnas.56.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A. The reconstitution of oxidase activity in membranes derived from a 5-aminolaevulinic acid-requiring mutant of Escherichia coli. Biochem J. 1973 Dec;136(4):877–884. doi: 10.1042/bj1360877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton J. A., Cox G. B., Looney F. D., Gibson F. Ubisemiquinone in membranes from Escherichia coli. Biochem J. 1970 Jan;116(2):319–320. doi: 10.1042/bj1160319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendler R. W., Burgess A. H. Fractionation of the electron-transport chain of Escherichia coli. Biochim Biophys Acta. 1974 Aug 23;357(2):215–230. doi: 10.1016/0005-2728(74)90062-0. [DOI] [PubMed] [Google Scholar]
- Holmgren E., Hederstedt L., Rutberg L. Role of heme in synthesis and membrane binding of succinic dehydrogenase in Bacillus subtilis. J Bacteriol. 1979 May;138(2):377–382. doi: 10.1128/jb.138.2.377-382.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingledew W. J., Prince R. C. Thermodynamic resolution of the iron-sulfur centers of the succinic dehydrogenase of Rhodopseudomonas sphaeroides. Arch Biochem Biophys. 1977 Jan 15;178(1):303–307. doi: 10.1016/0003-9861(77)90195-3. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J., Reid G. A., Poole R. K., Blum H., Ohnishi T. The iron-sulphur centres of aerobically-grown Escherichia coli K12. FEBS Lett. 1980 Feb 25;111(1):223–227. doi: 10.1016/0014-5793(80)80798-8. [DOI] [PubMed] [Google Scholar]
- Meijer E. M., Wever R., Stouthamer A. H. The role of iron-sulfur center 2 in electron transport and energy conservation in the NADH-ubiquinone segment of the respiratory chain in Paracoccus denitrificans. Eur J Biochem. 1977 Dec 1;81(2):267–275. doi: 10.1111/j.1432-1033.1977.tb11948.x. [DOI] [PubMed] [Google Scholar]
- NICHOLAS D. J., WILSON P. W., HEINEN W., PALMER G., BEINERT H. Use of electron paramagnetic resonance spectroscopy in investigations of functional metal components in micro-organisms. Nature. 1962 Nov 3;196:433–436. doi: 10.1038/196433a0. [DOI] [PubMed] [Google Scholar]
- Ohnishi T., Ingledew W. J., Shiraishi S. Resolution and functional characterization of two mitochondrial iron-sulphur centres of the 'high-potential iron-sulphur protein' type. Biochem J. 1976 Jan 1;153(1):39–48. doi: 10.1042/bj1530039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi T., Lim J., Winter D. B., King T. E. Thermodynamic and EPR characteristics of a HiPIP-type iron-sulfur center in the succinate dehydrogenase of the respiratory chain. J Biol Chem. 1976 Apr 10;251(7):2105–2109. [PubMed] [Google Scholar]
- Ohnishi T., Salerno J. C. Thermodynamic and EPR characteristics of two ferredoxin-type iron-sulfur centers in the succinate-ubiquinone reductase segment of the respiratory chain. J Biol Chem. 1976 Apr 10;251(7):2094–2104. [PubMed] [Google Scholar]
- Onishi T. Mechanism of electron transport and energy conservation in the site I region of the respiratory chain. Biochim Biophys Acta. 1973 Dec 7;301(2):105–128. [PubMed] [Google Scholar]
- Onishi T., Winter D. B., Lim J., King T. E. EPR studies on a Hipip type iron-sulfur center in the succinate dehydrogenase segment of the respiratory chain. Biochem Biophys Res Commun. 1974 Dec 11;61(3):1017–1025. doi: 10.1016/0006-291x(74)90257-5. [DOI] [PubMed] [Google Scholar]
- Onishi T., Winter D. B., Lim J., King T. E. Low temperature electron paramagnetic resonance studies on two iron-sulfur centers in cardiac succinate dehydrogenase. Biochem Biophys Res Commun. 1973 Jul 2;53(1):231–237. doi: 10.1016/0006-291x(73)91424-1. [DOI] [PubMed] [Google Scholar]
- Poole R. K., Haddock B. A. Effects of sulphate-limited growth in continuous culture on the electron-transport chain and energy conservation in Escherichia coli K12. Biochem J. 1975 Dec;152(3):537–546. doi: 10.1042/bj1520537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K., Haddock B. A. Energy-linked reduction of nicotinamide--adenine dinucleotide in membranes derived from normal and various respiratory-deficient mutant strains of Escherichia coli K12. Biochem J. 1974 Oct;144(1):77–85. doi: 10.1042/bj1440077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole R. K., Waring A. J., Chance B. The reaction of cytochrome omicron in Escherichia coli with oxygen. Low-temperature kinetic and spectral studies. Biochem J. 1979 Nov 15;184(2):379–389. doi: 10.1042/bj1840379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno J. C., Blum H., Ohnishi T. The orientation of iron-sulfur clusters and a spin-coupled ubiquinone pair in the mitochondrial membrane. Biochim Biophys Acta. 1979 Aug 14;547(2):270–281. doi: 10.1016/0005-2728(79)90010-0. [DOI] [PubMed] [Google Scholar]
- Synthesis and sideedness of membrane-bound respiratory nitrate reductase (EC1.7.99.4) in Escherichia coli lacking cytochromes. Biochem J. 1975 May;148(2):329–333. [PMC free article] [PubMed] [Google Scholar]
- Tiede D. M., Leigh J. S., Dutton P. L. Structural organization of the Chromatium vinosum reaction center associated c-cytochromes. Biochim Biophys Acta. 1978 Sep 7;503(3):524–544. doi: 10.1016/0005-2728(78)90151-2. [DOI] [PubMed] [Google Scholar]


