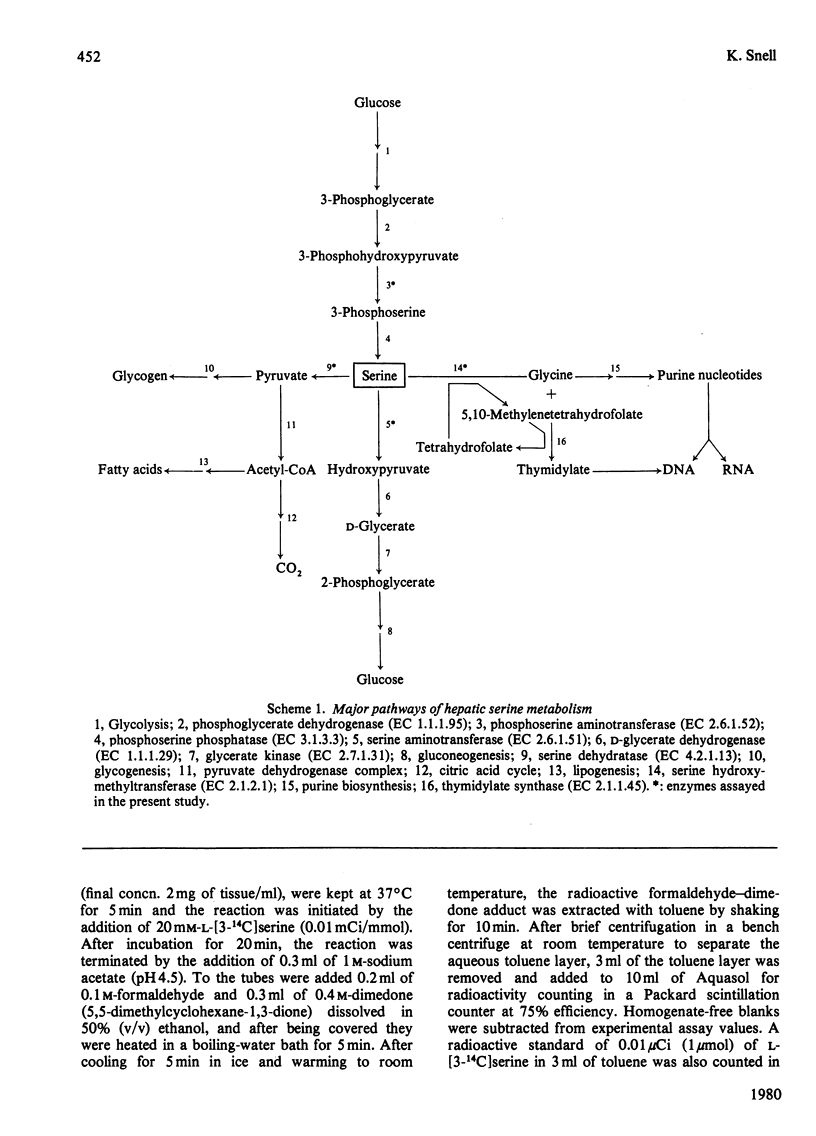
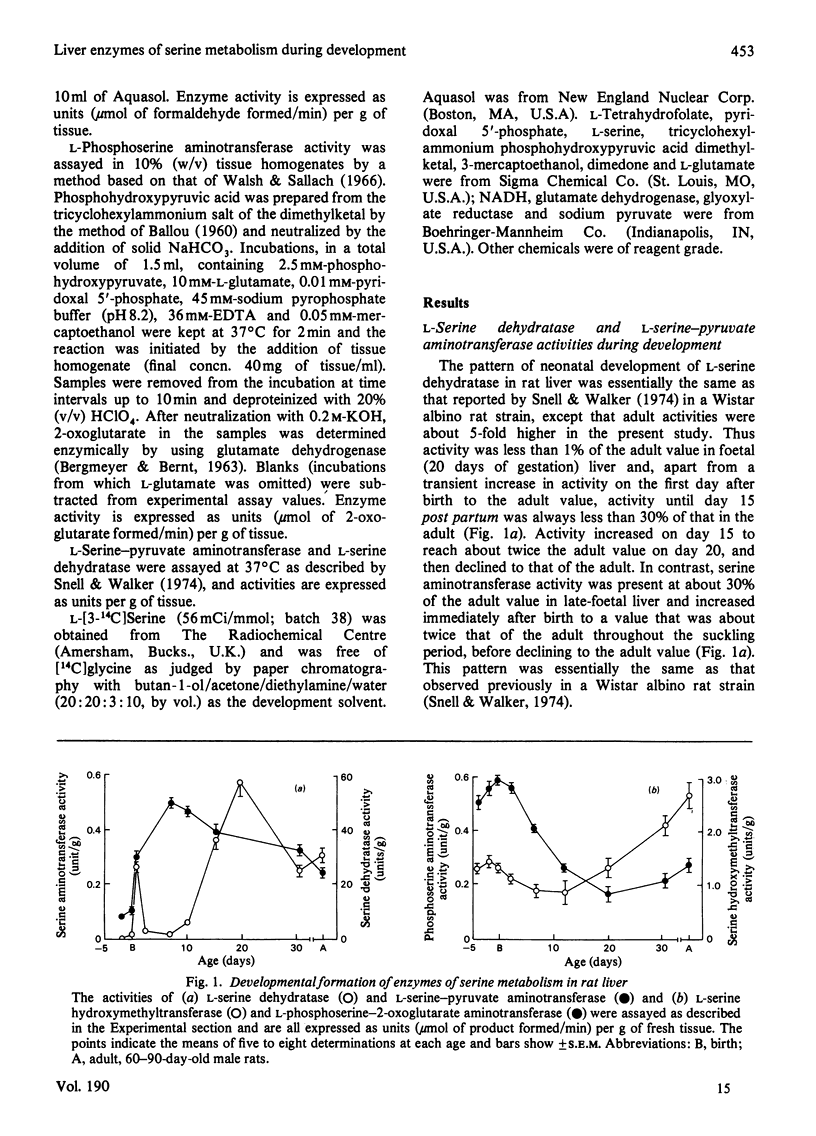
Abstract
The developmental patterns of L-serine hydroxymethyltransferase, L-phosphoserine aminotransferase, L-serine aminotransferase and L-serine dehydratase were determined in rat liver. The results point to an increased capacity for serine biosynthesis de novo in the perinatal period. It is suggested that serine at this time, and also at weaning, may serve as a precursor, via the serine hydroxymethyltransferase reaction, for nucleotide biosynthesis to support the rapid phases of liver growth. The role of the alternative pathways of serine metabolism during neonatal development is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brosnan M. E., Symonds G. W., Hall D. E., Symonds D. L. Polyamine metabolism in liver of young rats. Biochem J. 1978 Sep 15;174(3):727–732. doi: 10.1042/bj1740727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard O., Federman M., Knox W. E. Cytomorphometry of developing rat liver and its application to enzymic differentiation. J Cell Biol. 1972 Feb;52(2):261–272. doi: 10.1083/jcb.52.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON B. E., WALSH D. A., SALLACH H. J. CHANGES IN THE ACTIVITIES OF D-GLYCERATE AND D-3-PHOSPHOGLYCERATE DEHYDROGENASES IN THE DEVELOPING RAT LIVER. Biochim Biophys Acta. 1964 May 4;85:202–205. doi: 10.1016/0926-6569(64)90241-x. [DOI] [PubMed] [Google Scholar]
- Jamdar S. C., Greengard O. Phosphoserine phosphatase: development formation and hormonal regulation in rat tissues. Arch Biochem Biophys. 1969 Oct;134(1):228–232. doi: 10.1016/0003-9861(69)90270-7. [DOI] [PubMed] [Google Scholar]
- Knox W. E., Herzfeld A., Hudson J. Phosphoserine phosphatase distribution in normal and neoplastic rat tissues. Arch Biochem Biophys. 1969 Jul;132(2):397–403. doi: 10.1016/0003-9861(69)90381-6. [DOI] [PubMed] [Google Scholar]
- Knox W. E., Lister-Rosenoer L. M. Timing of gestation in rats by fetal and maternal weights. Growth. 1978 Mar;42(1):43–53. [PubMed] [Google Scholar]
- Krebs H. A., Hems R. The regulation of the degradation of methionine and of the one-carbon units derived from histidine, serine and glycine. Adv Enzyme Regul. 1976;14:493–513. doi: 10.1016/0065-2571(76)90027-3. [DOI] [PubMed] [Google Scholar]
- Machovich R., Greengard O. Thymidine kinase in rat tissues during growth and differentiation. Biochim Biophys Acta. 1972 Dec 29;286(2):375–381. doi: 10.1016/0304-4165(72)90273-5. [DOI] [PubMed] [Google Scholar]
- Rowsell E. V., al-Tai A. H., Carnie J. A. Increased liver L-serine-pyruvate aminotransferase activity under gluconeogenic conditions. Biochem J. 1973 May;134(1):349–351. doi: 10.1042/bj1340349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Knox W. E. Enzymes of serine metabolism in normal and neoplastic tissues [proceedings]. Biochem Soc Trans. 1979 Oct;7(5):1048–1050. doi: 10.1042/bst0071048. [DOI] [PubMed] [Google Scholar]
- Snell K. Mitochondrial-cytosolic interrelationships involved in gluconeogenesis from serine in rat liver. FEBS Lett. 1975 Jul 15;55(1):202–205. doi: 10.1016/0014-5793(75)80992-6. [DOI] [PubMed] [Google Scholar]
- Snell K. Pathways of gluconeogenesis from L-serine in the neonatal rat. Biochem J. 1974 Aug;142(2):433–436. doi: 10.1042/bj1420433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K., Walker D. G. Gluconeogenesis in the newborn rat: the substrates and their quantitative significance. Enzyme. 1973;15(1):40–81. [PubMed] [Google Scholar]
- Snell K., Walker D. G. Regulation of hepatic L-serine dehydratase and L-serine-pyruvate aminotransferase in the developing neonatal rat. Biochem J. 1974 Dec;144(3):519–531. doi: 10.1042/bj1440519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturman J. A., Gaull G. E., Räihä N. C. DNA synthesis from the beta-carbon of serine by fetal and mature human liver. Biol Neonate. 1975;27(1-2):17–22. doi: 10.1159/000240755. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Sallach H. J. Comparative studies on the pathways for serine biosynthesis in animal tissues. J Biol Chem. 1966 Sep 10;241(17):4068–4076. [PubMed] [Google Scholar]
- Yeoh G., Oliver I. T. A stimulatory effect of glucagon on DNA synthesis in neonatal rat liver. Comp Biochem Physiol A Comp Physiol. 1971 Aug 1;39(4):723–733. doi: 10.1016/0300-9629(71)90195-2. [DOI] [PubMed] [Google Scholar]


